Abstract
APOBEC1 is a cytidine deaminase involved in cholesterol metabolism that has been linked to retrovirus restriction, analogous to the evolutionarily-related APOBEC3 proteins. In particular, murine APOBEC1 was shown to inhibit Friend retrovirus (FV) in vitro, generating high levels of C-to-T and G-to-A mutations. These observations raised the possibility that FV infection might be altered in APOBEC1-null mice. To examine this question directly, we infected wild-type and APOBEC1-null mice with FV complex and evaluated acute infection levels. Surprisingly, APOBEC1-null mice exhibited similar cellular infection levels and plasma viremia relative to wild-type mice. Moreover, next-generation sequencing analyses revealed that in contrast to APOBEC3, APOBEC1 did not enhance retroviral C-to-T and G-to-A mutational frequencies in genomic DNA. Thus, APOBEC1 neither inhibited nor significantly drove the molecular evolution of FV in vivo. Our findings reinforce that not all retrovirus restriction factors characterized as potent in vitro may be functionally relevant in vivo.
Keywords: APOBEC1, APOBEC3, deaminase, Friend retrovirus, HIV-1, hypermutation, restriction factor, innate immunity
INTRODUCTION
Incessant cycles of genetic conflict between mammalian hosts and retroviruses resulted in the emergence of host gene products that can directly inhibit retroviruses including HIV-1 (Duggal and Emerman, 2012). Among these ‘retrovirus restriction factors’ are the apolipoprotein B mRNA editing enzyme catalytic polypeptide-3 (APOBEC3 or A3) deaminases (Malim, 2009). Rodents encode a single APOBEC3 gene (mA3 in mice), whereas humans and rhesus macaques encode 7 APOBEC3 genes (A3A, B, C, D, F, G and H) (Hultquist et al., 2011; Santiago and Greene, 2008; Schmitt et al., 2011). In the absence of the HIV-1 protein Vif, which can shunt APOBEC3 proteins for proteasome-mediated degradation, several APOBEC3 proteins get encapsidated into HIV-1ΔVif virions and inhibit viral replication in the next target cell. Inhibition of retrovirus replication can occur through direct physical blockade of reverse transcription (Bishop et al., 2008), or through induction of lethal G→A hypermutation in the retroviral plus strand by deaminating deoxycytidines into deoxyuridines in reverse transcripts (Mangeat et al., 2003; Yu et al., 2004). The APOBEC3 proteins preferentially deaminate deoxycytidines in the 5′-YC-3′ context (where Y=pyrimidine; the underlined C is the deaminated deoxycytidine), with subtle variations between different members. Human A3G preferentially mutates in the CC context, whereas A3F mutates in the TC context (Beale et al., 2004; Langlois et al., 2005). Mutations that fit these dinucleotide ‘hotspot’ contexts have been used to track APOBEC3 activity both in vitro and in vivo [for example, see (Krisko et al., 2013; Vartanian et al., 2008)].
The status of APOBEC3 as an evolutionarily-conserved retrovirus restriction factor was galvanized by a series of studies utilizing mA3 knock-out (KO) mice. Mouse mammary tumor virus, Friend retrovirus (FV) complex, Moloney murine leukemia virus (Mo-MLV), CasFrKP MLV and LP-BM5 retrovirus complex all replicated to significantly higher levels during the acute phase of infection in mA3 KO mice compared to wild-type (WT) mice (Jones et al., 2012; Kolokithas et al., 2010; Low et al., 2009; Okeoma et al., 2007; Santiago et al., 2008; Takeda et al., 2008). Furthermore, mA3 from C57BL/6 (B6) mice was shown to influence the FV-specific neutralizing antibody (NAb) response (Santiago et al., 2008; Tsuji-Kawahara et al., 2010). Recently, we obtained evidence that mA3 could directly edit FV-specific immunoglobulin (Ig) genes (Halemano et al., 2014). This novel biological activity of mA3 is reminiscent of activation-induced deaminase (AID), which critically drives antibody class-switch recombination and Ig somatic hypermutation (Muramatsu et al., 2000; Revy et al., 2000). APOBEC3 and AID proteins are evolutionarily related, and may have originated from a single primordial deaminase that diverged during vertebrate evolution (Conticello et al., 2005). However, since mA3 exhibits detectable enzymatic activity against Ig genes, the functional divergence between APOBEC3 and AID may have been incomplete. This theory prompted us to investigate if there are functional redundancies within the greater family of (deoxy)cytidine deaminases.
Prior to the identification of AID and APOBEC3, another evolutionarily-related enzyme, APOBEC1, was discovered as the protein responsible for generating functional diversity among the 2 major isoforms of apolipoprotein B (apoB): apoB100 and apoB48 (Navaratnam et al., 1993; Teng et al., 1993). ApoB100 is a major component of circulating low-density lipoproteins (LDL), where it mediates LDL particle clearance in the liver by binding to the LDL receptor (LDLR). ApoB48 is a truncated form of apoB100 that is found primarily in chylomicrons in the small intestine where it can be cleared through LDLR-independent mechanisms (Ishibashi et al., 1994; Rohlmann et al., 1998). APOBEC1 introduces C→U mutations at position 6666 and 20 other downstream sites in apoB mRNA, resulting in a stop codon and the translation of apoB48 (Blanc et al., 2012). Human APOBEC1 (hA1) exhibits restricted expression in the small intestine, but interestingly, murine APOBEC1 (mA1) is also highly expressed in the liver, spleen, bone marrow and lymph nodes (Blanc and Davidson, 2010; Shay and Kang, 2013). Hepatic expression of mA1 explained why LDLR KO mice only have a mild elevation in cholesterol levels whereas humans with LDLR mutations develop severe hypercholesterolemia (Brown and Goldstein, 1974; Goldstein et al., 1983). Since mA1 is expressed in the liver, apoB48+ lipoprotein particles can be cleared through LDLR-independent mechanisms. Confirming this notion, LDLR/mA1 double-KO mice – which express only apoB100+ particles that cannot be cleared due to LDLR deletion – exhibited severe hypercholesterolemia and developed atherosclerotic lesions (Powell-Braxton et al., 1998).
The expanded tissue expression profile of mA1 hinted at the existence of additional functions and targets unrelated to apoB RNA editing. Indeed, recent studies (Blanc et al., 2014; Rosenberg et al., 2011) have demonstrated that more than 50 such RNA targets exist in the mouse small intestine. In addition, and notwithstanding its original description as an RNA editing enzyme, APOBEC1 has been shown to edit single stranded DNA substrates in vitro (Beale et al., 2004; Harris et al., 2002; Petersen-Mahrt and Neuberger, 2003). Several co-transfection studies demonstrated that APOBEC1 can significantly restrict and/or deaminate HIV-1, SIV, MLV, L1 retroelements, feline immunodeficiency virus and even hepatitis B virus (Bishop et al., 2004a; Bishop et al., 2004b; Gonzalez et al., 2009; Ikeda et al., 2011; Ikeda et al., 2008; Renard et al., 2010). Thus, it is formally possible that APOBEC1 may also function as a retrovirus restriction factor, analogous to the evolutionarily-related APOBEC3 proteins. Most relevant to this work, mA1 was shown to induce unprecedented levels of C→T and G→A mutations in Friend MLV transcripts, suggesting that mA1 can edit both viral RNA and DNA (Petit et al., 2009). In the Petit et al. 2009 study, mA1 preferably mutated in the TC context whereas mA3 primarily mutated in the CC dinucleotide context in vitro. Using a low-denaturation PCR strategy (3D-PCR) to preferentially amplify hypermutated transcripts, Friend MLV sequences were obtained from spleens of infected B6 WT mice. The observed high levels of TC mutations were taken as evidence that mA1 exerts significant antiviral and mutagenic activity in vivo. However, our group and others showed that mA3 could also preferentially edit in the TC context (Halemano et al., 2014; Jern et al., 2007; Langlois et al., 2009; MacMillan et al., 2013). Thus, without infection data in mA1 KO mice, it would be difficult to ascribe Friend MLV TC mutations to mA1 and conclude a significant impact on retroviral replication in vivo. Given these uncertainties, we reassessed the impact of mA1 on Friend retrovirus (FV) replication in vivo by performing experimental infections in WT versus mA1 KO mice.
RESULTS
APOBEC1 deficiency does not impact acute FV replication in vivo
To investigate if mA1 could inhibit FV replication in vivo, B6 WT and mA1 KO mice (Hirano et al., 1996) were infected intravenously with FV complex. FV complex consists of two retroviruses, a replication-competent Friend MLV (F-MuLV) and replication-defective spleen-focus forming virus (SFFV) (Hasenkrug and Chesebro, 1997). SFFV encodes an altered form of the Env protein that activates the erythropoeitin receptor, resulting in rapid proliferation of erythroblast precursors, leading to splenomegaly in mice strains encoding the Fv2 susceptibility gene (Hoatlin et al., 1990; Nishigaki et al., 2005; Persons et al., 1999). Although FV disease is mainly due to SFFV, most detection assays for FV infection are specific to F-MuLV as it is critical for replication and is present at >100-fold levels than SFFV (He et al., 2008; Lilly and Steeves, 1973). Of note, earlier FV stocks also harbor lactate-dehydrogenase elevating virus (LDV), an RNA virus that can augment type I interferon (IFN) responses and exacerbate FV disease by promoting immune activation (Marques et al., 2008; Robertson et al., 2008). As restriction factors may be activated by type I IFNs (Harper et al., 2013; Schoggins et al., 2011), we evaluated the impact of mA1 on acute FV replication in both FV and FV/LDV infections.
Consistent with the Fv2 resistance of B6 mice, FV and FV/LDV infection of B6 WT and mA1 KO mice did not result in significant splenomegaly (Fig. 1A). The absolute numbers of Ter119+ erythroblasts, which increase during FV disease, were not significantly different between genotypes (Fig. 1B). We next quantified FV infection levels by flow cytometry using mAb 34, a monoclonal antibody against the F-MuLV glyco-gag protein (Chesebro et al., 1983). As outlined in Table 1, mA1 KO mice did not exhibit significantly higher FV infection levels compared to WT mice in both the bone marrow and spleen. In fact, in LDV-free FV infection, mA1 KO mice had lower %FV infection levels in the spleen, in part due to decreased %FV infection levels in erythroblasts (Table 1, asterisks). However, these percentages did not translate to significantly lower absolute numbers of FV+ splenic erythroblasts (Mann-Whitney U test, p=0.1049). Moreover, FV infection levels in multiple cell subpopulations were not significantly different between B6 WT and mA1 KO mice (Table 1). This includes myeloid cells and B cells, which express high levels of mA1 based on gene expression surveys of various immune cell subsets (Shay and Kang, 2013) (www.immgen.org). Plasma viral RNA levels, as measured by quantitative real-time PCR (qPCR) (Harper et al., 2013; Li et al., 2013), were not significantly different between B6 WT and mA1 KO mice (Fig. 1C). We also determined if plasma virus were less infectious, as we previously observed in mA3 KO mice (Harper et al., 2013; Smith et al., 2011). No difference in infectious titers was observed between WT and mA1 KO mice (Fig. 1D). Thus, mA1 did not function as an innate restriction factor against FV infection in vivo.
Figure 1. APOBEC1 deficiency does not impact acute FV replication in vivo.
B6 WT and mA3 KO mice were infected with 10,000 SFFU of either FV/LDV or FV only. Samples were analyzed at 7 days post-infection. (A) Splenomegaly. A normal, uninfected mouse has about 100 mg spleen mass. (B) Erythroblast numbers. The absolute numbers of erythroblasts were calculated by multiplying the absolute number of splenocytes with the %Ter119+ cells as determined by flow cytometry. To estimate the number of cells, a factor of 108 splenocytes/100 mg spleen was used. (C) Plasma viral load. Viral RNA copies were computed using qPCR. Data were log-transformed. (D) Infectious viremia. Plasma (5 μl) were inoculated into Mus dunni cells and after 2 days, proviral DNA levels were evaluated by qPCR and normalized against total DNA input. Dotted lines correspond to the assay limit of detection. For all panels, each dot corresponds to data from a single mouse. Solid lines correspond to median values and p values following a 2-tailed Mann-Whitney U test are shown. All comparisons were not significant (p>0.05).
Table 1.
FV+ cell subpopulations in infected WT versus mA1 KO micea.
| FV/LDV | FV | |||||
|---|---|---|---|---|---|---|
| B6 WT (n=12) | B6 mA1 KO (n=12) | p-valueb | B6 WT (n=8) | B6 mA1 KO (n=8) | p-valueb | |
| Bone marrowc | ||||||
| Total | 13.0 ± 4.7 | 13.4 ± 5.5 | 0.848 | 9.28 ± 4.25 | 8.76 ± 6.03 | 0.844 |
| Erythroblast | 17.2 ± 7.2 | 20.3 ± 10.6 | 0.400 | 12.6 ± 6.5 | 11.6 ± 9.5 | 0.801 |
| B cells | 12.3 ± 4.2 | 13.7 ± 5.9 | 0.502 | 7.63 ± 3.31 | 8.42 ± 5.66 | 0.742 |
| Myeloid | 7.82 ± 1.74 | 8.30 ± 4.17 | 0.713 | 6.67 ± 1.28 | 6.42 ± 3.54 | 0.853 |
| DCs | 7.57 ± 3.82 | 8.09 ± 5.25 | 0.786 | 8.38 ± 3.59 | 8.49 ± 4.15 | 0.957 |
| Spleenc | ||||||
| Total | 6.66 ± 2.06 | 6.38 ± 2.38 | 0.763 | 7.57 ± 1.98 | 5.14 ± 1.90 | 0.0249* |
| Erythroblast | 7.97 ± 2.82 | 7.18 ± 2.61 | 0.485 | 10.2 ± 3.7 | 6.16 ± 2.79 | 0.0282* |
| B cells | 5.19 ± 1.30 | 5.58 ± 1.50 | 0.513 | 4.46 ± 0.76 | 3.99 ± 0.97 | 0.301 |
| Myeloid | 8.74 ± 2.62 | 8.93 ± 2.71 | 0.863 | 6.66 ± 2.27 | 4.96 ± 1.92 | 0.129 |
| DCs | 9.05 ± 2.91 | 9.46 ± 3.30 | 0.749 | 7.62 ± 2.20 | 6.34 ± 2.40 | 0.286 |
| T cells | 4.12 ± 1.10 | 4.45 ± 1.04 | 0.461 | 4.95 ± 0.46 | 4.63 ± 0.93 | 0.397 |
FV infection levels were determined by flow cytometry using an anti-glycogag antibody. Mean values are shown ± SD.
Difference between means were evaluated using a 2-tailed Student’s t test.
p<0.05.
Cell subpopulations analyzed included Ter119+ erythroblasts, CD19+ B cells, CD11b+ myeloid cells, CD11c+ dendritic cells and CD3+ T cells.
APOBEC1 deficiency does not induce high FV DNA deamination mutations in vivo
In co-transfection studies, mA1 induced high levels of C→T and G→A mutations against F-MuLV (Petit et al., 2009). However, these sequences were obtained using 3D-PCR to enrich for hypermutated (A/T-biased) sequences. The 3D-PCR method may not accurately quantify true mutational rates, as it would miss templates with only a few deaminations. Moreover, 3D-PCR requires the use of Taq polymerases without proofreading functions. Thus, assessing quasispecies diversity may require more robust methods. Next-generation sequencing (NGS) has been used to quantify HIV-1 deamination profiles (Knoepfel et al., 2011), and we have adopted these methods to quantify deamination frequencies in Ig genes (Halemano et al., 2014). In contrast to 3D-PCR, NGS methods could capture high numbers of sequence reads for comprehensive mutational analyses in an unbiased manner, and are compatible with high-fidelity Taq polymerases. We therefore evaluated the frequencies of APOBEC3-type YC mutations in F-MuLV DNA in a subset of FV-infected WT and mA1 KO mice using NGS.
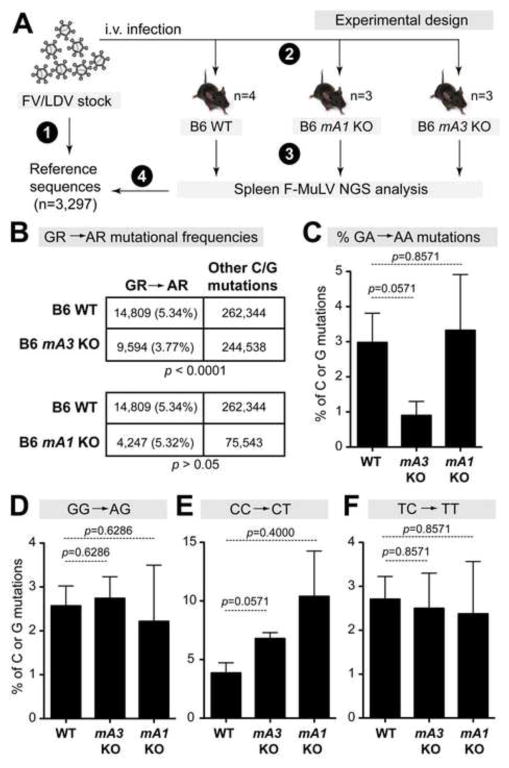
The FV inoculum stock is a quasispecies, raising the technical challenge of calculating mutation frequencies in infected mice relative to this stock. We previously addressed this issue by generating a set of 15 env sequences from the FV/LDV inoculum stock that served as reference sequences (Smith et al., 2011). Using traditional cloning and Sanger sequencing approaches, we found that the frequency of F-MuLV GA→AA and GG→AG mutations (or GR→AR, where R=purine) in FV/LDV infected mA3 KO mice were reduced relative to WT mice (Smith et al., 2011). However, 15 sequences may not completely capture the diversity of the retroviral quasispecies. We therefore characterized the FV/LDV inoculum stock using NGS methods, and obtained 169,014 sequence reads (mean length = 398 bp) from viral RNA. We note that in this dataset, each sequence was represented at least twice. To generate reference sequences, the data were collapsed into 3,297 unique sequences. We then amplified F-MuLV using the same Illumina primers from splenic DNA from FV/LDV-infected B6 WT (n=4), mA1 KO (n=3) and mA3 KO (n=3) mice. The retroviral quasispecies in these mice were compared to the inoculum reference sequences to evaluate mutational profiles at dinucleotide contexts associated with mA1 and mA3 activity (Fig. 2A). In effect, F-MuLV mutations in infected mice that were found in the reference sequences were considered as preexisting mutations and were excluded in subsequent analyses (see Materials and Methods).
Figure 2. APOBEC1 does not enhance retroviral C→T and G→A mutations in vivo.
(A) Experimental design. Next-generation sequencing (NGS) was utilized to profile the quasispecies diversity in the (1) FV inoculum, which was used to (2) infect WT, mA1 KO and mA3 KO mice. (3) NGS analysis were performed on 7 dpi splenocytes from infected mice, and (4) FV mutations were compared between infected mice and the inoculum. (B) Analysis of G→A mutations. C or G mutations were combined from all mice from each cohort, and the proportion of GA→AA and GG→AG (or GR→AR, where R=purine) mutations were evaluated by 2×2 contingency analysis. P values were computed using Fisher’s exact test. The percentage of C or G mutations that fit the (C) GA→AA; (D) GG→AG; (E) CC→CT; and (F) TC→TT dinucleotide contexts were computed for individual mice. Bars and error bars correspond to mean and SEM, respectively. Exact p values following a 2-tailed Mann-Whitney U test are shown.
On average, we obtained 24,098 F-MuLV sequence reads per mouse (range: 1,044 to 102,112). We initially combined all C or G mutations from each mouse strain and computed the percentage of GR→AR mutations. By Fisher’s exact test, mA3 KO, but not mA1 KO mice, had significantly lower GR→AR mutations than WT mice (Fig. 2B), confirming our previous results (Smith et al., 2011). We then calculated the frequencies of specific dinucleotide mutations on each FV-infected mouse. These analyses revealed that relative to WT mice, mA3 KO mice had 3.3-fold lower F-MuLV GA→AA mutations (Fig. 2C; statistical trend at p=0.057), whereas GG→AG mutations were similar between both strains of mice (Fig. 2D). These data suggested that mA3 preferentially mutated F-MuLV reverse transcripts in the TC instead of the CC context. As expected from the 2×2 contingency analyses in Fig. 2B, mA1 KO mice had similar percentages of GA→AA and GG→AG mutations relative to WT mice (Fig. 2C–D). Since mA1 could potentially edit retroviral RNA (Bishop et al., 2004b; Petit et al., 2009), we also evaluated the percentages of F-MuLV TC→TT and CC→CT mutations but found no differences in these dinucleotide mutation frequencies between mA1 KO versus WT mice (Fig. 2E–F). Thus, we found no evidence that mA1 significantly increased the mutational load of F-MuLV in vivo.
DISCUSSION
The identification of APOBEC3 sparked investigations on whether the evolutionarily related APOBEC1 enzyme could also act as a retrovirus restriction factor. This was supported by cell culture studies that demonstrated that APOBEC1 could inhibit retroviruses including HIV-1 (Bishop et al., 2004a; Bishop et al., 2004b) and F-MuLV (Petit et al., 2009). However, a key experiment to strengthen this conclusion, similar to studies done on APOBEC3, would be to compare retrovirus replication levels in WT versus APOBEC1-null mice. Here, we took advantage of a previously described mA1 KO mouse (Hirano et al., 1996) and evaluated its ability to support FV replication. Our results showed that mA1 deficiency did not augment acute FV replication. Moreover, under experimental conditions that unraveled a role for mA3 in retroviral evolution in vivo, we found no evidence for mA1 acting in a similar capacity. Even if our NGS approach (which analyzed several thousand-fold more sequence clones than a typical 3D-PCR) missed low levels of mA1-mediated viral RNA editing, our in vivo data suggest that these mutations had no impact on acute FV replication. Interestingly, our results with mA3 complement a recent report that the human APOBEC3 proteins could impact HIV-1 evolution in vivo (Kim et al., 2014).
The mechanism(s) underlying the discrepancy in the activity of APOBEC1 against FV in vitro (Petit et al., 2009) and in vivo (this study) remains unclear, but one possibility is that overexpression of deaminases may alter their normal localization and allow these proteins to deaminate non-physiological targets. For example, endogenous human APOBEC3A is cytoplasmic, but upon transfection, it can induce genotoxicity due to mislocalization in nuclear compartments (Land et al., 2013). Endogenous APOBEC1 interacts with APOBEC1-complementation factor (ACF), forming an ‘editosome’ that promotes its association with apoB mRNA (Wedekind et al., 2003). We speculate that APOBEC1 overexpression may offset the stoichiometric balance between APOBEC1 and ACF, allowing non-ACF-complexed APOBEC1 to edit additional targets (Sowden et al., 1996; Yang et al., 2000). In fact, transgenic expression of APOBEC1 in mouse livers resulted in hepatocellular carcinoma, presumably due to aberrant RNA editing of additional host genes (Yamanaka et al., 1995).
The lack of antiretroviral activity of mA1 in vivo raises the question of why this gene is expressed in immune compartments. One possibility is that APOBEC1 may have remnant AID-like activity. However, APOBEC1 could not compensate for AID when transduced into AID-deficient B cells (Eto et al., 2003). A second possibility is that mA1 may have activity against endogenous retrotransposons (Ikeda et al., 2011). However, given that we did not detect an impact of mA1 on exogenous retrovirus infection in vivo, it would now be critical to confirm if mA1 indeed inhibits endogenous retrotransposons in vivo. A third possibility is that mA1 may have activity against other viruses, such as herpesviruses (Gee et al., 2011). However, we note that even though the APOBEC3 proteins were shown to edit herpesviruses in vitro (Suspene et al., 2011), mA3-deficiency did not augment murine gammaherpesvirus-68 replication in vivo (Minkah et al., 2014). Interestingly, differential RNAseq studies identified intestinal and hepatic tissue genes other than apoB that were modified by mA1, but unlike apoB, these mutations were exclusively found in the 3′ untranslated region of target RNAs and did not alter gene coding capacity (Blanc et al., 2014; Rosenberg et al., 2011). A similar RNASeq approach may have to be performed to unravel a potential function of mA1 in immune compartments.
In a previous report (Li et al., 2013), we showed that Ribonuclease L, a potent antiviral factor against several RNA viruses, can significantly inhibit F-MuLV in vitro. However, Ribonuclease L deficiency had no impact on FV replication in vivo. Thus, our findings on mA1 KO mice provide a second example of a disconnect between the in vitro and in vivo activities of retrovirus restriction factors. These results could have practical implications in prioritizing retrovirus restriction factors for therapeutic targeting against HIV-1. At the time of this writing, over 30 HIV-1 restriction factors have been identified through in vitro screens (Abdel-Mohsen et al., 2014; Schoggins et al., 2011), but only a handful of these factors have bona fide counterpart viral antagonists. Since our results demonstrate that some retrovirus restriction factors that function potently in vitro may turn out to be unimportant in vivo, it may be premature to develop antiviral strategies based on some restriction factors until their in vivo importance is confirmed. As highlighted by this work and others, mouse knockout models may help delineate candidate retrovirus restriction factors for in-depth basic and translational studies (Rehwinkel, 2014).
CONCLUSIONS
Cell culture studies demonstrated that APOBEC1 can significantly inhibit retroviruses and promote retroviral C→T and G→A mutations. However, the physiological relevance of these findings remain unclear. To complement these in vitro studies, we evaluated if APOBEC1 deficiency in mice would enhance acute retroviral replication and decrease deaminase mutations following infection with pathogenic murine Friend retrovirus (FV) complex. The data indicate that APOBEC1 did not restrict acute FV replication or promote retroviral C→T and G→A DNA mutations. Our findings question both the generality of APOBEC1 as a potent innate retrovirus restriction factor and its role as a significant driver of retroviral evolution in vivo.
MATERIALS AND METHODS
Mice
Wild-type B6 and BALB/c mice were purchased from The Jackson Laboratory. B6 mA3 KO mice (generously provided by Dr. Warner Greene, Gladstone Institutes) were derived from a gene-trap 129P2 mouse embryonic stem cell line (BayGenomics) (Santiago et al., 2008) and backcrossed into B6 for 9 generations. The derivation of mA1 KO mice, backcrossed for 10 generations into B6, were described previously (Hirano et al., 1996). Mice were handled in accordance with the recommendations of the NIH Guide for the Care and Use of Laboratory Animals and approved by the UCD IACUC committee [permit number B-89709(10)1E].
FV infections
Initial FV and FV/LDV stocks were provided by Dr. Kim Hasenkrug (Rocky Mountain Laboratories, NIAID). These stocks were amplified in BALB/c mice and SFFV titers were quantified as previously described (Santiago et al., 2008). Mice were infected intravenously through the retro-orbital route with 10,000 SFFU of FV. At 7 dpi, bone marrows, spleens and plasma samples were obtained for analyses.
Plasma viral load and infectious titer quantification
F-MuLV RNA copies were determined by qPCR as previously described (Santiago et al., 2011). Briefly, RNA was extracted from 5 μl of plasma and added to 1× One-Step Taqman Reverse-Transcriptase-PCR reaction mixture (Applied Biosystems) containing primers designed in F-MuLV env as previously described. T7-transcribed RNA standards (Ambion) were used to calculate F-MuLV copy numbers. For infectious titer determination, 5 μl of plasma was incubated into Mus dunni cells in a 48-well plate, and after 2 days, DNA from the cells were extracted using the DNAeasy kit (Qiagen). F-MuLV DNA copies were determined using qRT-PCR as with the viral load estimations, normalizing to 100 ng of input DNA.
Flow cytometry
Bone marrow and splenocytes were initially stained for the F-MuLV glyco-gag protein using the mAb 34 antibody for 1 h, then co-stained with: Ter119-FITC (clone TER-119), CD3-Alexa700 (17A2), CD11b-PE (M1/70) (BD Biosciences); CD11c-PE-Cy7 (eBioscience); CD19-PerCP-Cy5.5 (6D5) (BioLegend) and anti-mouse IgG1-APC (Columbia Biosciences) for 30 min. An LSR-II flow cytometer (BD Biosciences) was used to capture up to 250,000 events per sample, and Flowjo software (Treestar) was used to analyze the data. Glyco-gag+ cells were gated based on biological controls using uninfected splenocytes.
PCR of F-MuLV DNA for Illumina sequencing
Genomic DNA was extracted from FV-infecrted mouse splenocytes using the DNAEasy blood and tissue kit (Qiagen). Amplicons were produced using Phusion Hi-fidelity DNA Polymerase (New England Biolabs) and 10 μM of forward and reverse primers. To increase diversity in the sample, a four-nucleotide random sequence (NNNN) was added 5′ of the 6 bp index (or barcode) sequence. The primers were purchased from Eurofins MWG Operon. The sequences (5′-3′) are shown below:
Forward: AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT NNNN INDEX1 GCCCTGTGCAACACTACCCT
Reverse: CAAGCAGAAGACGGCATACGAGATGTGACTGGAGTTCAGACGTGTGCTCTTCCGATCT NNNN INDEX2 TCGTCGATTCTGCAGTACAACC
Amplification was performed in a BioRad S1000 thermocycler using the following conditions: 98°C for 30 s; 35 cycles of 98°C for 10 s, 60°C for 15 s, and 72°C for 18 s; and one final extension step of 72°C for 7 mins. Amplicons were gel-extracted using the QIAquick gel extraction kit (Qiagen), and subsequently quantified by Quant-IT PicoGreen dsDNA assay kit (Invitrogen) and mixed at equimolar amounts for paired-end 2×250 bp sequencing on the Illumina MiSeq.
Quality control of NGS data
The quality score (Q score) of 20 and minimum length of 100bp were set to prefilter and remove the low quality sequences from both forward and reverse reads using custom Perl scripts. The program PANDAseq (Masella et al., 2012) was used to assemble contigs from paired-end reads. Subsequently, sequencing primers and gene-specific primer sequences were removed from both ends of the contigs. The total numbers of contigs assembled was counted and sequences that occurred only once were excluded from further analysis.
Assembly of F-MuLV reference sequences
Total RNA was extracted from the FV inoculum using the RNAEasy kit (Qiagen), and cDNA was synthesized using the QuantiTect reverse transcription kit (Qiagen). The same PCR procedure as described above in ‘PCR of F-MuLV DNA for Illumina sequencing’ was used to amplify the FV cDNA in triplicate. Illumina MiSeq sequencing results were quality filtered as described above. Subsequently, all filtered sequences were collapsed into unique sequences while keeping track of the count number of each unique sequence. A total of 3,297 unique sequences (average length 398 bp) were used to build an FV quasispecies reference database. It should be noted that each unique sequence was represented at least twice in the original dataset.
Analysis of dinucleotide mutations in NGS data
The MiSeq sequencing results of each FV infected mouse sample were scanned and aligned with the FV reference database using USEARCH (Edgar, 2010). Custom Perl scripts were used to compare each sequence to the aligned reference sequence in order to determine the mutation frequency and to identify mutations as AID-type (RC) or APOBEC3-type (YC) based on their dinucleotide context. To quantify the percentage of AID and APOBEC3-type mutations, we utilized mutations in C or G bases as a denominator, since these are the mutations that could be directly mapped as an AID or APOBEC3-type deamination.
Statistical analysis
Data were analyzed using a 2-tailed unpaired Student’s t test. If the data exhibited a skewed distribution based on Kolmogorov-Smirnov normality test, 2-tailed unpaired Mann-Whitney U test was utilized. 2×2 contingency tables were analyzed using Fisher’s exact test. All statistical tests were performed using GraphPad Prism 5.0.
Highlights.
The role of APOBEC1 in retrovirus infection was tested in APOBEC1-null mice
Acute retrovirus replication was similar in wild-type and APOBEC1 KO mice
Deep-sequencing unraveled a role for APOBEC3 in retrovirus evolution in vivo
In contrast to APOBEC3, APOBEC1 did not impact retroviral evolution
Retrovirus restriction factors shown as potent in vitro may not be relevant in vivo
Acknowledgments
We thank Warner Greene for the mA3 KO mice and Kim Hasenkrug for FV reagents and protocols. This work was supported by the National Institutes of Health (NIH) Grant R01 AI090795 (to M.L.S.) and the University of Colorado Department of Medicine Early Career Scholar Program (to M.L.S.). N.O.D. was supported by grants (HL-38180, DK-56260 and DK-52574, murine core). M.S.H. was supported by the Tim Gill Foundation Endowment to the UCD ID Division and S.X.L. was supported by the Molecular Pathogenesis of Infectious Disease T32 AI052066 and the Colorado Clinical and Translational Sciences Institute TL1 TR000155.
Footnotes
AUTHORS’ CONTRIBUTIONS
M.L.S., B.S.B. and K.G. designed the research; B.S.B. performed the mouse infections, virological quantifications and flow cytometry in Figure 1 and Table 1; K.G. performed the NGS experiments and developed computational tools to analyze NGS data in Figure 2; N.O.D. provided critical reagents and expertise; M.S.H., S.X.L. and K.J.H. assisted in quantifying virus infection levels; K.G., B.S.B. and M.L.S. analyzed data; M.L.S., N.O.D., B.S.B. and K.G. wrote the paper; all authors read and approved the manuscript; and M.L.S. supervised the work.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdel-Mohsen M, Deng X, Liegler T, Guatelli JC, Salama MS, Ghanem Hel D, Rauch A, Ledergerber B, Deeks SG, Gunthard HF, Wong JK, Pillai SK. Effects of alpha interferon treatment on intrinsic anti-HIV-1 immunity in vivo. J Virol. 2014;88:763–767. doi: 10.1128/JVI.02687-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beale RC, Petersen-Mahrt SK, Watt IN, Harris RS, Rada C, Neuberger MS. Comparison of the differential context-dependence of DNA deamination by APOBEC enzymes: correlation with mutation spectra in vivo. J Mol Biol. 2004;337:585–596. doi: 10.1016/j.jmb.2004.01.046. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Holmes RK, Sheehy AM, Davidson NO, Cho SJ, Malim MH. Cytidine deamination of retroviral DNA by diverse APOBEC proteins. Curr Biol. 2004a;14:1392–1396. doi: 10.1016/j.cub.2004.06.057. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Holmes RK, Sheehy AM, Malim MH. APOBEC-mediated editing of viral RNA. Science. 2004b;305:645. doi: 10.1126/science.1100658. [DOI] [PubMed] [Google Scholar]
- Bishop KN, Verma M, Kim EY, Wolinsky SM, Malim MH. APOBEC3G inhibits elongation of HIV-1 reverse transcripts. PLoS Pathog. 2008;4:e1000231. doi: 10.1371/journal.ppat.1000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V, Davidson NO. APOBEC-1-mediated RNA editing. Wiley Interdiscip Rev Syst Biol Med. 2010;2:594–602. doi: 10.1002/wsbm.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V, Park E, Schaefer S, Miller M, Lin Y, Kennedy S, Billing AM, Ben Hamidane H, Graumann J, Mortazavi A, Nadeau JH, Davidson NO. Genome-wide identification and functional analysis of Apobec-1 mediated C-to-U RNA editing in mouse small intestine and liver. Genome Biol. 2014;15:R79. doi: 10.1186/gb-2014-15-6-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanc V, Xie Y, Luo J, Kennedy S, Davidson NO. Intestine-specific expression of Apobec-1 rescues apolipoprotein B RNA editing and alters chylomicron production in Apobec1 −/− mice. J Lipid Res. 2012;53:2643–2655. doi: 10.1194/jlr.M030494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL. Expression of the familial hypercholesterolemia gene in heterozygotes: mechanism for a dominant disorder in man. Science. 1974;185:61–63. doi: 10.1126/science.185.4145.61. [DOI] [PubMed] [Google Scholar]
- Chesebro B, Britt W, Evans L, Wehrly K, Nishio J, Cloyd M. Characterization of monoclonal antibodies reactive with murine leukemia viruses: use in analysis of strains of friend MCF and Friend ecotropic murine leukemia virus. Virology. 1983;127:134–148. doi: 10.1016/0042-6822(83)90378-1. [DOI] [PubMed] [Google Scholar]
- Conticello SG, Thomas CJ, Petersen-Mahrt SK, Neuberger MS. Evolution of the AID/APOBEC family of polynucleotide (deoxy)cytidine deaminases. Mol Biol Evol. 2005;22:367–377. doi: 10.1093/molbev/msi026. [DOI] [PubMed] [Google Scholar]
- Duggal NK, Emerman M. Evolutionary conflicts between viruses and restriction factors shape immunity. Nat Rev Immunol. 2012;12:687–695. doi: 10.1038/nri3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Eto T, Kinoshita K, Yoshikawa K, Muramatsu M, Honjo T. RNA-editing cytidine deaminase Apobec-1 is unable to induce somatic hypermutation in mammalian cells. Proc Natl Acad Sci U S A. 2003;100:12895–12898. doi: 10.1073/pnas.2135587100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee P, Ando Y, Kitayama H, Yamamoto SP, Kanemura Y, Ebina H, Kawaguchi Y, Koyanagi Y. APOBEC1-mediated editing and attenuation of herpes simplex virus 1 DNA indicate that neurons have an antiviral role during herpes simplex encephalitis. J Virol. 2011;85:9726–9736. doi: 10.1128/JVI.05288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein JL, Kita T, Brown MS. Defective lipoprotein receptors and atherosclerosis. Lessons from an animal counterpart of familial hypercholesterolemia. NEJM. 1983;309:288–296. doi: 10.1056/NEJM198308043090507. [DOI] [PubMed] [Google Scholar]
- Gonzalez MC, Suspene R, Henry M, Guetard D, Wain-Hobson S, Vartanian JP. Human APOBEC1 cytidine deaminase edits HBV DNA. Retrovirology. 2009;6:96. doi: 10.1186/1742-4690-6-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halemano K, Guo K, Heilman KJ, Barrett BS, Smith DS, Hasenkrug KJ, Santiago ML. Immunoglobulin somatic hypermutation by APOBEC3/Rfv3 during retroviral infection. Proc Natl Acad Sci U S A. 2014;111:7759–7764. doi: 10.1073/pnas.1403361111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper MS, Barrett BS, Smith DS, Li SX, Gibbert K, Dittmer U, Hasenkrug KJ, Santiago ML. IFN-alpha treatment inhibits acute Friend retrovirus replication primarily through the antiviral effector molecule Apobec3. J Immunol. 2013;190:1583–1590. doi: 10.4049/jimmunol.1202920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris RS, Petersen-Mahrt SK, Neuberger MS. RNA editing enzyme APOBEC1 and some of its homologs can act as DNA mutators. Mol Cell. 2002;10:1247–1253. doi: 10.1016/s1097-2765(02)00742-6. [DOI] [PubMed] [Google Scholar]
- Hasenkrug KJ, Chesebro B. Immunity to retroviral infection: the Friend virus model. Proc Natl Acad Sci U S A. 1997;94:7811–7816. doi: 10.1073/pnas.94.15.7811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He JY, Cheng HJ, Wang YF, Zhu YT, Li GQ. Development of a real-time quantitative reverse transcriptase PCR assay for detection of the Friend leukemia virus load in murine plasma. J Virol Methods. 2008;147:345–350. doi: 10.1016/j.jviromet.2007.10.009. [DOI] [PubMed] [Google Scholar]
- Hirano K, Young SG, Farese RV, Jr, Ng J, Sande E, Warburton C, Powell-Braxton LM, Davidson NO. Targeted disruption of the mouse apobec-1 gene abolishes apolipoprotein B mRNA editing and eliminates apolipoprotein B48. J Biol Chem. 1996;271:9887–9890. doi: 10.1074/jbc.271.17.9887. [DOI] [PubMed] [Google Scholar]
- Hoatlin ME, Kozak SL, Lilly F, Chakraborti A, Kozak CA, Kabat D. Activation of erythropoietin receptors by Friend viral gp55 and by erythropoietin and down-modulation by the murine Fv-2r resistance gene. Proc Natl Acad Sci U S A. 1990;87:9985–9989. doi: 10.1073/pnas.87.24.9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultquist JF, Lengyel JA, Refsland EW, LaRue RS, Lackey L, Brown WL, Harris RS. Human and rhesus APOBEC3D, APOBEC3F, APOBEC3G, and APOBEC3H demonstrate a conserved capacity to restrict Vif-deficient HIV-1. J Virol. 2011;85:11220–11234. doi: 10.1128/JVI.05238-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Abd El Galil KH, Tokunaga K, Maeda K, Sata T, Sakaguchi N, Heidmann T, Koito A. Intrinsic restriction activity by apolipoprotein B mRNA editing enzyme APOBEC1 against the mobility of autonomous retrotransposons. Nucleic Acids Res. 2011;39:5538–5554. doi: 10.1093/nar/gkr124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda T, Ohsugi T, Kimura T, Matsushita S, Maeda Y, Harada S, Koito A. The antiretroviral potency of APOBEC1 deaminase from small animal species. Nucleic Acids Res. 2008;36:6859–6871. doi: 10.1093/nar/gkn802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi S, Herz J, Maeda N, Goldstein JL, Brown MS. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci U S A. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jern P, Stoye JP, Coffin JM. Role of APOBEC3 in genetic diversity among endogenous murine leukemia viruses. PLoS Genet. 2007;3:2014–2022. doi: 10.1371/journal.pgen.0030183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PH, Mehta HV, Okeoma CM. A novel role for APOBEC3: Susceptibility to sexual transmission of murine acquired immunodeficiency virus (mAIDS) is aggravated in APOBEC3 deficient mice. Retrovirology. 2012;9:50. doi: 10.1186/1742-4690-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EY, Lorenzo-Redondo R, Little SJ, Chung YS, Phalora PK, Maljkovic Berry I, Archer J, Penugonda S, Fischer W, Richman DD, Bhattacharya T, Malim MH, Wolinsky SM. Human APOBEC3 Induced Mutation of Human Immunodeficiency Virus Type-1 Contributes to Adaptation and Evolution in Natural Infection. PLoS Pathog. 2014;10:e1004281. doi: 10.1371/journal.ppat.1004281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoepfel SA, Di Giallonardo F, Daumer M, Thielen A, Metzner KJ. In-depth analysis of G-to-A hypermutation rate in HIV-1 env DNA induced by endogenous APOBEC3 proteins using massively parallel sequencing. J Virol Methods. 2011;171:329–328. doi: 10.1016/j.jviromet.2010.11.016. [DOI] [PubMed] [Google Scholar]
- Kolokithas A, Rosenke K, Malik F, Hendrick D, Swanson L, Santiago ML, Portis JL, Hasenkrug KJ, Evans LH. The glycosylated Gag protein of a murine leukemia virus inhibits the antiretroviral function of APOBEC3. J Virol. 2010;84:10933–10936. doi: 10.1128/JVI.01023-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krisko JF, Martinez-Torres F, Foster JL, Garcia JV. HIV restriction by APOBEC3 in humanized mice. PLoS Pathog. 2013;9:e1003242. doi: 10.1371/journal.ppat.1003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land AM, Law EK, Carpenter MA, Lackey L, Brown WL, Harris RS. Endogenous APOBEC3A DNA cytosine deaminase is cytoplasmic and nongenotoxic. J Biol Chem. 2013;288:17253–17260. doi: 10.1074/jbc.M113.458661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois MA, Beale RC, Conticello SG, Neuberger MS. Mutational comparison of the single-domained APOBEC3C and double-domained APOBEC3F/G anti-retroviral cytidine deaminases provides insight into their DNA target site specificities. Nucleic Acids Res. 2005;33:1913–1923. doi: 10.1093/nar/gki343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois MA, Kemmerich K, Rada C, Neuberger MS. The AKV murine leukemia virus is restricted and hypermutated by mouse APOBEC3. J Virol. 2009;83:11550–11559. doi: 10.1128/JVI.01430-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SX, Barrett BS, Harper MS, Heilman KJ, Halemano K, Steele AK, Guo K, Silverman RH, Santiago ML. Ribonuclease L is not critical for innate restriction and adaptive immunity against Friend retrovirus infection. Virology. 2013;443:134–142. doi: 10.1016/j.virol.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly F, Steeves RA. B-tropic Friend virus: a host-range pseudotype of spleen focus-forming virus (SFFV) Virology. 1973;55:363–370. doi: 10.1016/0042-6822(73)90176-1. [DOI] [PubMed] [Google Scholar]
- Low A, Okeoma CM, Lovsin N, de las Heras M, Taylor TH, Peterlin BM, Ross SR, Fan H. Enhanced replication and pathogenesis of Moloney murine leukemia virus in mice defective in the murine APOBEC3 gene. Virology. 2009;385:455–463. doi: 10.1016/j.virol.2008.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMillan AL, Kohli RM, Ross SR. APOBEC3 inhibition of mouse mammary tumor virus infection: the role of cytidine deamination versus inhibition of reverse transcription. J Virol. 2013;87:4808–4817. doi: 10.1128/JVI.00112-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim MH. APOBEC proteins and intrinsic resistance to HIV-1 infection. Philos Trans R Soc Lond B Biol Sci. 2009;364:675–687. doi: 10.1098/rstb.2008.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangeat B, Turelli P, Caron G, Friedli M, Perrin L, Trono D. Broad antiretroviral defence by human APOBEC3G through lethal editing of nascent reverse transcripts. Nature. 2003;424:99–103. doi: 10.1038/nature01709. [DOI] [PubMed] [Google Scholar]
- Marques R, Antunes I, Eksmond U, Stoye J, Hasenkrug K, Kassiotis G. B lymphocyte activation by coinfection prevents immune control of Friend virus infection. J Immunol. 2008;181:3432–3440. doi: 10.4049/jimmunol.181.5.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masella AP, Bartram AK, Truszkowski JM, Brown DG, Neufeld JD. PANDAseq: paired-end assembler for illumina sequences. BMC Bioinformatics. 2012;13:31. doi: 10.1186/1471-2105-13-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkah N, Chavez K, Shah P, Maccarthy T, Chen H, Landau N, Krug LT. Host restriction of murine gammaherpesvirus 68 replication by human APOBEC3 cytidine deaminases but not murine APOBEC3. Virology. 2014;454–455:215–226. doi: 10.1016/j.virol.2014.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Navaratnam N, Morrison JR, Bhattacharya S, Patel D, Funahashi T, Giannoni F, Teng BB, Davidson NO, Scott J. The p27 catalytic subunit of the apolipoprotein B mRNA editing enzyme is a cytidine deaminase. J Biol Chem. 1993;268:20709–20712. [PubMed] [Google Scholar]
- Nishigaki K, Hanson C, Jelacic T, Thompson D, Ruscetti S. Friend spleen focus-forming virus transforms rodent fibroblasts in cooperation with a short form of the receptor tyrosine kinase Stk. P Proc Natl Acad Sci U S A. 2005;102:15488–15493. doi: 10.1073/pnas.0506570102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okeoma CM, Lovsin N, Peterlin BM, Ross SR. APOBEC3 inhibits mouse mammary tumour virus replication in vivo. Nature. 2007;445:927–930. doi: 10.1038/nature05540. [DOI] [PubMed] [Google Scholar]
- Persons DA, Paulson RF, Loyd MR, Herley MT, Bodner SM, Bernstein A, Correll PH, Ney PA. Fv2 encodes a truncated form of the Stk receptor tyrosine kinase. Nat Genet. 1999;23:159–165. doi: 10.1038/13787. [DOI] [PubMed] [Google Scholar]
- Petersen-Mahrt SK, Neuberger MS. In vitro deamination of cytosine to uracil in single-stranded DNA by apolipoprotein B editing complex catalytic subunit 1 (APOBEC1) J Biol Chem. 2003;278:19583–19586. doi: 10.1074/jbc.C300114200. [DOI] [PubMed] [Google Scholar]
- Petit V, Guetard D, Renard M, Keriel A, Sitbon M, Wain-Hobson S, Vartanian JP. Murine APOBEC1 is a powerful mutator of retroviral and cellular RNA in vitro and in vivo. J Mol Biol. 2009;385:65–78. doi: 10.1016/j.jmb.2008.10.043. [DOI] [PubMed] [Google Scholar]
- Powell-Braxton L, Veniant M, Latvala RD, Hirano KI, Won WB, Ross J, Dybdal N, Zlot CH, Young SG, Davidson NO. A mouse model of human familial hypercholesterolemia: markedly elevated low density lipoprotein cholesterol levels and severe atherosclerosis on a low-fat chow diet. Nature Med. 1998;4:934–938. doi: 10.1038/nm0898-934. [DOI] [PubMed] [Google Scholar]
- Rehwinkel J. Mouse knockout models for HIV-1 restriction factors. Cell Mol Life Sci. 2014 doi: 10.1007/s00018-014-1646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renard M, Henry M, Guetard D, Vartanian JP, Wain-Hobson S. APOBEC1 and APOBEC3 cytidine deaminases as restriction factors for hepadnaviral genomes in non-humans in vivo. J Mol Biol. 2010;400:323–334. doi: 10.1016/j.jmb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, Catalan N, Forveille M, Dufourcq-Labelouse R, Gennery A, Tezcan I, Ersoy F, Kayserili H, Ugazio AG, Brousse N, Muramatsu M, Notarangelo LD, Kinoshita K, Honjo T, Fischer A, Durandy A. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Robertson SJ, Ammann CG, Messer RJ, Carmody AB, Myers L, Dittmer U, Nair S, Gerlach N, Evans LH, Cafruny WA, Hasenkrug KJ. Suppression of acute anti-friend virus CD8+ T-cell responses by coinfection with lactate dehydrogenase-elevating virus. J Virol. 2008;82:408–418. doi: 10.1128/JVI.01413-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohlmann A, Gotthardt M, Hammer RE, Herz J. Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J Clin Invest. 1998;101:689–695. doi: 10.1172/JCI1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg BR, Hamilton CE, Mwangi MM, Dewell S, Papavasiliou FN. Transcriptome-wide sequencing reveals numerous APOBEC1 mRNA-editing targets in transcript 3′ UTRs. Nature Struct Mol Biol. 2011;18:230–236. doi: 10.1038/nsmb.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Greene WC. The role of the Apobec3 family of cytidine deaminases in innate immunity, G-to-A hypermutation and evolution of retroviruses. In: Domingo E, Parrish CR, Holland JJ, editors. Origin and Evolution of Viruses. Academic Press; London, UK: 2008. pp. 183–206. [Google Scholar]
- Santiago ML, Montano M, Benitez R, Messer RJ, Yonemoto W, Chesebro B, Hasenkrug KJ, Greene WC. Apobec3 encodes Rfv3, a gene influencing neutralizing antibody control of retrovirus infection. Science. 2008;321:1343–1346. doi: 10.1126/science.1161121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago ML, Smith DS, Barrett BS, Montano M, Benitez RL, Pelanda R, Hasenkrug KJ, Greene WC. Persistent Friend virus replication and disease in Apobec3-deficient mice expressing functional B-cell-activating factor receptor. J Virol. 2011;85:189–199. doi: 10.1128/JVI.01838-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt K, Guo K, Algaier M, Ruiz A, Cheng F, Qiu J, Wissing S, Santiago ML, Stephens EB. Differential virus restriction patterns of rhesus macaque and human APOBEC3A: implications for lentivirus evolution. Virology. 2011;419:24–42. doi: 10.1016/j.virol.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoggins JW, Wilson SJ, Panis M, Murphy MY, Jones CT, Bieniasz P, Rice CM. A diverse range of gene products are effectors of the type I interferon antiviral response. Nature. 2011;472:481–485. doi: 10.1038/nature09907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shay T, Kang J. Immunological Genome Project and systems immunology. Trends Immunol. 2013;34:602–609. doi: 10.1016/j.it.2013.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DS, Guo K, Barrett BS, Heilman KJ, Evans LH, Hasenkrug KJ, Greene WC, Santiago ML. Noninfectious retrovirus particles drive the APOBEC3/Rfv3 dependent neutralizing antibody response. PLoS Pathog. 2011;7:e1002284. doi: 10.1371/journal.ppat.1002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowden M, Hamm JK, Smith HC. Overexpression of APOBEC-1 results in mooring sequence-dependent promiscuous RNA editing. J Biol Chem. 1996;271:3011–3017. doi: 10.1074/jbc.271.6.3011. [DOI] [PubMed] [Google Scholar]
- Suspene R, Aynaud MM, Koch S, Pasdeloup D, Labetoulle M, Gaertner B, Vartanian JP, Meyerhans A, Wain-Hobson S. Genetic editing of herpes simplex virus 1 and Epstein-Barr herpesvirus genomes by human APOBEC3 cytidine deaminases in culture and in vivo. J Virol. 2011;85:7594–7602. doi: 10.1128/JVI.00290-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda E, Tsuji-Kawahara S, Sakamoto M, Langlois MA, Neuberger MS, Rada C, Miyazawa M. Mouse APOBEC3 restricts friend leukemia virus infection and pathogenesis in vivo. J Virol. 2008;82:10998–11008. doi: 10.1128/JVI.01311-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng B, Burant CF, Davidson NO. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993;60:1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- Tsuji-Kawahara S, Chikaishi T, Takeda E, Kato M, Kinoshita S, Kajiwara E, Takamura S, Miyazawa M. Persistence of viremia and production of neutralizing antibodies differentially regulated by polymorphic APOBEC3 and BAFF-R loci in friend virus-infected mice. J Virol. 2010;84:6082–6095. doi: 10.1128/JVI.02516-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vartanian JP, Guetard D, Henry M, Wain-Hobson S. Evidence for editing of human papillomavirus DNA by APOBEC3 in benign and precancerous lesions. Science. 2008;320:230–233. doi: 10.1126/science.1153201. [DOI] [PubMed] [Google Scholar]
- Wedekind JE, Dance GS, Sowden MP, Smith HC. Messenger RNA editing in mammals: new members of the APOBEC family seeking roles in the family business. Trends Genet. 2003;19:207–216. doi: 10.1016/S0168-9525(03)00054-4. [DOI] [PubMed] [Google Scholar]
- Yamanaka S, Balestra ME, Ferrell LD, Fan J, Arnold KS, Taylor S, Taylor JM, Innerarity TL. Apolipoprotein B mRNA-editing protein induces hepatocellular carcinoma and dysplasia in transgenic animals. Proc Natl Acad Sci U S A. 1995;92:8483–8487. doi: 10.1073/pnas.92.18.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Sowden MP, Smith HC. Induction of cytidine to uridine editing on cytoplasmic apolipoprotein B mRNA by overexpressing APOBEC-1. J Biol Chem. 2000;275:22663–22669. doi: 10.1074/jbc.M910406199. [DOI] [PubMed] [Google Scholar]
- Yu Q, Konig R, Pillai S, Chiles K, Kearney M, Palmer S, Richman D, Coffin JM, Landau NR. Single-strand specificity of APOBEC3G accounts for minus-strand deamination of the HIV genome. Nature Struct Mol Biol. 2004;11:435–442. doi: 10.1038/nsmb758. [DOI] [PubMed] [Google Scholar]