Abstract
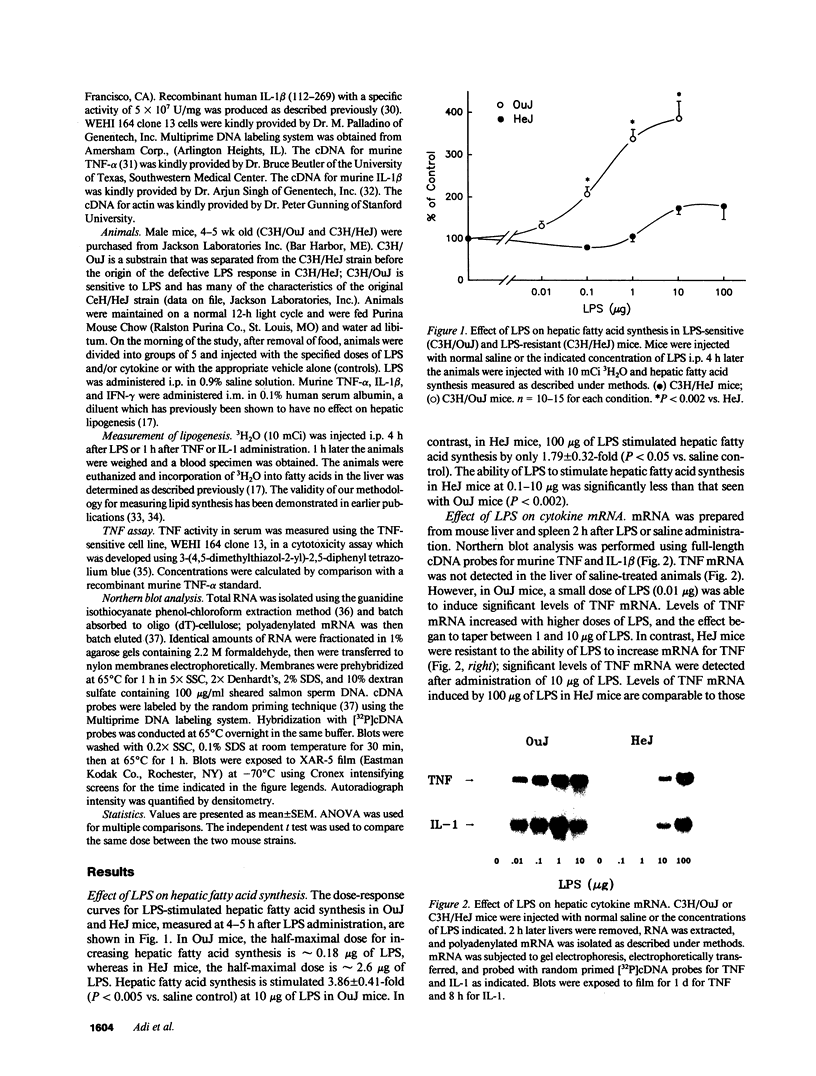
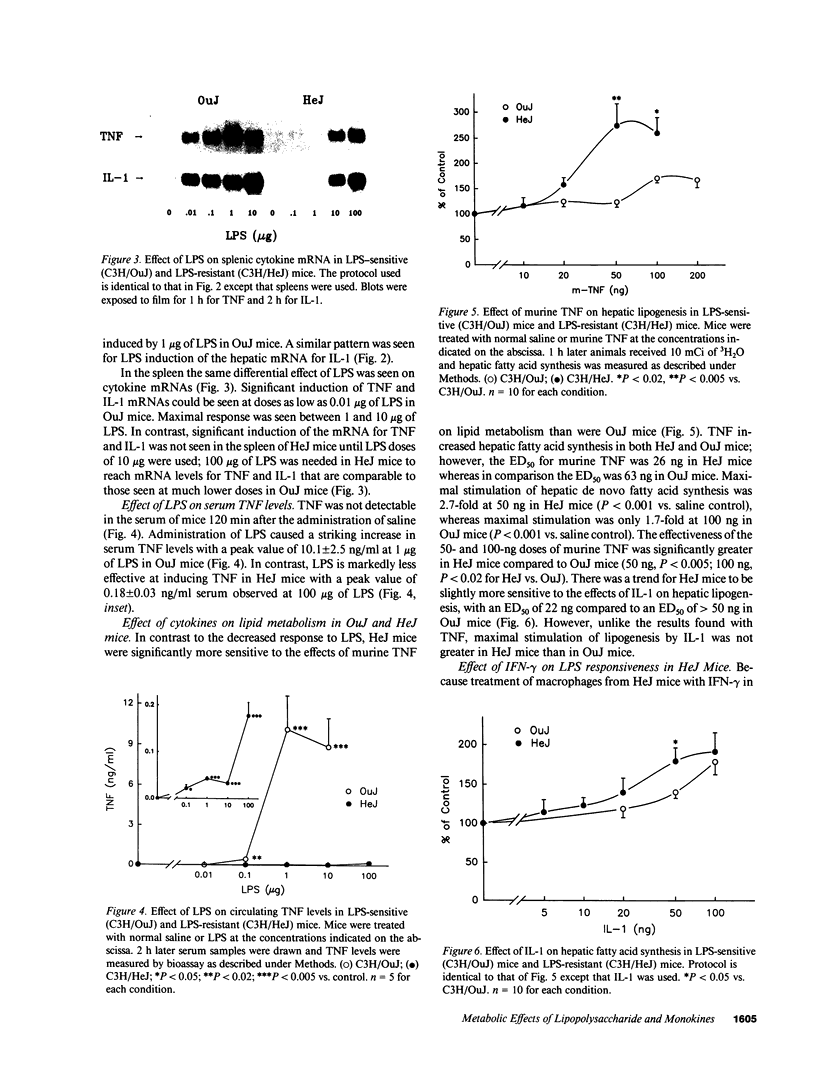
To examine the role of cytokines in mediating the lipogenic effects of endotoxin (LPS), we studied the effects of LPS and cytokines on hepatic fatty acid synthesis in LPS-sensitive C3H/OuJ mice and in LPS-resistant C3H/HeJ mice, whose macrophages are defective in the ability to produce tumor necrosis factor (TNF) and IL-1 in response to LPS. HeJ mice were 16-fold less sensitive than OuJ mice to the lipogenic effect of LPS. In OuJ mice, 10 micrograms of LPS caused a maximal increase in hepatic lipogenesis (3.86 +/- 0.41-fold), whereas in HeJ mice the maximal increase was only 1.79 +/- 0.32-fold after 100 micrograms of LPS. This lipogenic response paralleled the decreased ability of LPS to increase hepatic and splenic levels of mRNAs for TNF and IL-1 and serum levels of TNF in HeJ mice. In contrast, the maximal effect of TNF on lipogenesis was greater and the sensitivity to TNF was increased 2.4-fold in HeJ mice compared to OuJ mice. Administration of IFN-gamma before LPS in HeJ mice had no effect on IL-1 mRNA, but partially restored the LPS-induced increase in hepatic and splenic mRNA for TNF and serum TNF levels, which may account for the partial restoration of sensitivity to the lipogenic effect of LPS after IFN-gamma treatment. These results indicate that cytokines produced by mononuclear leukocytes mediate the lipogenic effects of LPS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apte R. N., Ascher O., Pluznik D. H. Genetic analysis of generation of serum interferon by bacterial lipopolysaccharide. J Immunol. 1977 Dec;119(6):1898–1902. [PubMed] [Google Scholar]
- Apte R. N., Pluznik D. H. Genetic control of lipopolysaccharide induced generation of serum colony stimulating factor and proliferation of splenic granulocyte/macrophage precursor cells. J Cell Physiol. 1976 Oct;89(2):313–323. doi: 10.1002/jcp.1040890214. [DOI] [PubMed] [Google Scholar]
- Beisel W. R. Metabolic response to infection. Annu Rev Med. 1975;26:9–20. doi: 10.1146/annurev.me.26.020175.000301. [DOI] [PubMed] [Google Scholar]
- Beutler B. A., Cerami A. Recombinant interleukin 1 suppresses lipoprotein lipase activity in 3T3-L1 cells. J Immunol. 1985 Dec;135(6):3969–3971. [PubMed] [Google Scholar]
- Beutler B., Krochin N., Milsark I. W., Luedke C., Cerami A. Control of cachectin (tumor necrosis factor) synthesis: mechanisms of endotoxin resistance. Science. 1986 May 23;232(4753):977–980. doi: 10.1126/science.3754653. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Beutler B., Tkacenko V., Milsark I., Krochin N., Cerami A. Effect of gamma interferon on cachectin expression by mononuclear phagocytes. Reversal of the lpsd (endotoxin resistance) phenotype. J Exp Med. 1986 Nov 1;164(5):1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caput D., Beutler B., Hartog K., Thayer R., Brown-Shimer S., Cerami A. Identification of a common nucleotide sequence in the 3'-untranslated region of mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chajek-Shaul T., Friedman G., Stein O., Shiloni E., Etienne J., Stein Y. Mechanism of the hypertriglyceridemia induced by tumor necrosis factor administration to rats. Biochim Biophys Acta. 1989 Feb 20;1001(3):316–324. doi: 10.1016/0005-2760(89)90116-1. [DOI] [PubMed] [Google Scholar]
- Chedid L., Parant M., Damais C., Parant F., Juy D., Galelli A. Failure of endotoxin to increase nonspecific resistance to infection of lipopolysaccharide low-responder mice. Infect Immun. 1976 Mar;13(3):722–727. doi: 10.1128/iai.13.3.722-727.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Collart M. A., Belin D., Vassalli J. D., de Kossodo S., Vassalli P. Gamma interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986 Dec 1;164(6):2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Kelly N., Bernton E., Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989 Jun 1;169(6):2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czuprynski C. J., Brown J. F. Recombinant murine interleukin-1 alpha enhancement of nonspecific antibacterial resistance. Infect Immun. 1987 Sep;55(9):2061–2065. doi: 10.1128/iai.55.9.2061-2065.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darlington G. J., Wilson D. R., Lachman L. B. Monocyte-conditioned medium, interleukin-1, and tumor necrosis factor stimulate the acute phase response in human hepatoma cells in vitro. J Cell Biol. 1986 Sep;103(3):787–793. doi: 10.1083/jcb.103.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A., Cannon J. G., Mier J. W., Bernheim H. A., LoPreste G., Lynn D. L., Love R. N., Webb A. C., Auron P. E., Reuben R. C. Multiple biological activities of human recombinant interleukin 1. J Clin Invest. 1986 Jun;77(6):1734–1739. doi: 10.1172/JCI112495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinarello C. A. Interleukin-1 and interleukin-1 antagonism. Blood. 1991 Apr 15;77(8):1627–1652. [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987 Jul;80(1):184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Serio M. K., Adi S., Moser A. H., Grunfeld C. Tumor necrosis factor stimulates hepatic lipid synthesis and secretion. Endocrinology. 1989 May;124(5):2336–2342. doi: 10.1210/endo-124-5-2336. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Adi S., Staprans I., Neese R., Shigenaga J., Doerrler W., Moser A., Dinarello C. A., Grunfeld C. Effect of interleukin-1 on lipid metabolism in the rat. Similarities to and differences from tumor necrosis factor. Arterioscler Thromb. 1991 May-Jun;11(3):495–500. doi: 10.1161/01.atv.11.3.495. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Serio M. K., Moser A. H., Dinarello C. A., Grunfeld C. Multiple cytokines stimulate hepatic lipid synthesis in vivo. Endocrinology. 1989 Jul;125(1):267–274. doi: 10.1210/endo-125-1-267. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Soued M., Staprans I., Gavin L. A., Donahue M. E., Huang B. J., Moser A. H., Gulli R., Grunfeld C. Effect of tumor necrosis factor (TNF) on lipid metabolism in the diabetic rat. Evidence that inhibition of adipose tissue lipoprotein lipase activity is not required for TNF-induced hyperlipidemia. J Clin Invest. 1989 Apr;83(4):1116–1121. doi: 10.1172/JCI113991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., MacRae G., Lear S., Moser A. H., Zsigmond G., Siperstein M. D. De novo sterologenesis in the intact rat. Metabolism. 1983 Jan;32(1):75–81. doi: 10.1016/0026-0495(83)90160-9. [DOI] [PubMed] [Google Scholar]
- Feingold K. R., Wiley M. H., MacRae G., Moser A. H., Lear S. R., Siperstein M. D. The effect of diabetes mellitus on sterol synthesis in the intact rat. Diabetes. 1982 May;31(5 Pt 1):388–395. doi: 10.2337/diab.31.5.388. [DOI] [PubMed] [Google Scholar]
- Fiser R. H., Denniston J. C., Beisel W. R. Infection with Diplococcus pneumoniae and Salmonella typhimurium in monkeys: changes in plasma lipids and lipoproteins. J Infect Dis. 1972 Jan;125(1):54–60. doi: 10.1093/infdis/125.1.54. [DOI] [PubMed] [Google Scholar]
- Fong Y. M., Marano M. A., Moldawer L. L., Wei H., Calvano S. E., Kenney J. S., Allison A. C., Cerami A., Shires G. T., Lowry S. F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Invest. 1990 Jun;85(6):1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Kaye D., O'Leary W. M. Serum lipids in infection. N Engl J Med. 1969 Nov 13;281(20):1081–1086. doi: 10.1056/NEJM196911132812001. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Glaister D., Chen E., Goeddel D. V., Pennica D. Two interleukin 1 genes in the mouse: cloning and expression of the cDNA for murine interleukin 1 beta. J Immunol. 1986 Dec 1;137(11):3644–3648. [PubMed] [Google Scholar]
- Grunfeld C., Adi S., Soued M., Moser A., Fiers W., Feingold K. R. Search for mediators of the lipogenic effects of tumor necrosis factor: potential role for interleukin 6. Cancer Res. 1990 Jul 15;50(14):4233–4238. [PubMed] [Google Scholar]
- Grunfeld C., Gulli R., Moser A. H., Gavin L. A., Feingold K. R. Effect of tumor necrosis factor administration in vivo on lipoprotein lipase activity in various tissues of the rat. J Lipid Res. 1989 Apr;30(4):579–585. [PubMed] [Google Scholar]
- Guckian J. C. Role of metabolism in pathogenesis of bacteremia due to Diplococcus pneumoniae in rabbits. J Infect Dis. 1973 Jan;127(1):1–8. doi: 10.1093/infdis/127.1.1. [DOI] [PubMed] [Google Scholar]
- Hagberg L., Hull R., Hull S., McGhee J. R., Michalek S. M., Svanborg Edén C. Difference in susceptibility to gram-negative urinary tract infection between C3H/HeJ and C3H/HeN mice. Infect Immun. 1984 Dec;46(3):839–844. doi: 10.1128/iai.46.3.839-844.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajduk S. L., Moore D. R., Vasudevacharya J., Siqueira H., Torri A. F., Tytler E. M., Esko J. D. Lysis of Trypanosoma brucei by a toxic subspecies of human high density lipoprotein. J Biol Chem. 1989 Mar 25;264(9):5210–5217. [PubMed] [Google Scholar]
- Harris H. W., Grunfeld C., Feingold K. R., Rapp J. H. Human very low density lipoproteins and chylomicrons can protect against endotoxin-induced death in mice. J Clin Invest. 1990 Sep;86(3):696–702. doi: 10.1172/JCI114765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan M. M., Vogel S. N. Production of tumor necrosis factor by rIFN-gamma-primed C3H/HeJ (Lpsd) macrophages requires the presence of lipid A-associated proteins. J Immunol. 1988 Dec 15;141(12):4196–4202. [PubMed] [Google Scholar]
- Huemer H. P., Menzel H. J., Potratz D., Brake B., Falke D., Utermann G., Dierich M. P. Herpes simplex virus binds to human serum lipoprotein. Intervirology. 1988;29(2):68–76. doi: 10.1159/000150031. [DOI] [PubMed] [Google Scholar]
- Kaufmann R. L., Matson C. F., Beisel W. R. Hypertriglyceridemia produced by endotoxin: role of impaired triglyceride disposal mechanisms. J Infect Dis. 1976 May;133(5):548–555. doi: 10.1093/infdis/133.5.548. [DOI] [PubMed] [Google Scholar]
- Keay S., Grossberg S. E. Interferon inhibits the conversion of 3T3-L1 mouse fibroblasts into adipocytes. Proc Natl Acad Sci U S A. 1980 Jul;77(7):4099–4103. doi: 10.1073/pnas.77.7.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler V., Sappino A. P., Grau G. E., Piguet P. F., Vassalli P. The inducing role of tumor necrosis factor in the development of bactericidal granulomas during BCG infection. Cell. 1989 Mar 10;56(5):731–740. doi: 10.1016/0092-8674(89)90676-4. [DOI] [PubMed] [Google Scholar]
- Lees R. S., Fiser R. H., Jr, Beisel W. R., Bartelloni P. J. Effects of an experimental viral infection on plasma lipid and lipoprotein metabolism. Metabolism. 1972 Sep;21(9):825–833. doi: 10.1016/0026-0495(72)90005-4. [DOI] [PubMed] [Google Scholar]
- Leong J. C., Kane J. P., Oleszko O., Levy J. A. Antigen-specific nonimmunoglobulin factor that neutralizes xenotropic virus is associated with mouse serum lipoproteins. Proc Natl Acad Sci U S A. 1977 Jan;74(1):276–280. doi: 10.1073/pnas.74.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane A., Minagawa T., Kato K. Endogenous tumor necrosis factor (cachectin) is essential to host resistance against Listeria monocytogenes infection. Infect Immun. 1988 Oct;56(10):2563–2569. doi: 10.1128/iai.56.10.2563-2569.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Rosenstreich D. L., Scher I., Campbell G. H., MacDermott R. P., Formal S. B. Genetic control of susceptibility to Salmonella typhimurium in mice: role of the LPS gene. J Immunol. 1980 Jan;124(1):20–24. [PubMed] [Google Scholar]
- Patton J. S., Shepard H. M., Wilking H., Lewis G., Aggarwal B. B., Eessalu T. E., Gavin L. A., Grunfeld C. Interferons and tumor necrosis factors have similar catabolic effects on 3T3 L1 cells. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8313–8317. doi: 10.1073/pnas.83.21.8313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter D. H., Dinarello C. A., Punsal P. I., Colten H. R. Cachectin/tumor necrosis factor regulates hepatic acute-phase gene expression. J Clin Invest. 1986 Nov;78(5):1349–1354. doi: 10.1172/JCI112721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifkin M. R. Identification of the trypanocidal factor in normal human serum: high density lipoprotein. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3450–3454. doi: 10.1073/pnas.75.7.3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robson H. G., Vas S. I. Resistance of inbred mice to Salmonella typhimurium. J Infect Dis. 1972 Oct;126(4):378–386. doi: 10.1093/infdis/126.4.378. [DOI] [PubMed] [Google Scholar]
- Rosenstreich D. L., Vogel S. N., Jacques A. R., Wahl L. M., Oppenheim J. J. Macrophage sensitivity to endotoxin: genetic control by a single codominant gene. J Immunol. 1978 Nov;121(5):1664–1670. [PubMed] [Google Scholar]
- Schindler R., Clark B. D., Dinarello C. A. Dissociation between interleukin-1 beta mRNA and protein synthesis in human peripheral blood mononuclear cells. J Biol Chem. 1990 Jun 25;265(18):10232–10237. [PubMed] [Google Scholar]
- Seganti L., Grassi M., Mastromarino P., Panà A., Superti F., Orsi N. Activity of human serum lipoproteins on the infectivity of rhabdoviruses. Microbiologica. 1983 Apr;6(2):91–99. [PubMed] [Google Scholar]
- Shortridge K. F., Ho W. K., Oya A., Kobayashi M. Studies on the inhibitory activities of human serum lipoproteins for Japanese encephalitis virus. Southeast Asian J Trop Med Public Health. 1975 Dec;6(4):461–466. [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of host responses to endotoxin. Infect Immun. 1972 Jan;5(1):107–113. doi: 10.1128/iai.5.1.107-113.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultzer B. M. Genetic control of leucocyte responses to endotoxin. Nature. 1968 Sep 21;219(5160):1253–1254. doi: 10.1038/2191253a0. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Ulevitch R. J., Johnston A. R., Weinstein D. B. New function for high density lipoproteins. Their participation in intravascular reactions of bacterial lipopolysaccharides. J Clin Invest. 1979 Nov;64(5):1516–1524. doi: 10.1172/JCI109610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel S. N., Moore R. N., Sipe J. D., Rosenstreich D. L. BCG-induced enhancement of endotoxin sensitivity in C3H/HeJ mice. I. In vivo studies. J Immunol. 1980 Apr;124(4):2004–2009. [PubMed] [Google Scholar]
- Wakabayashi G., Gelfand J. A., Burke J. F., Thompson R. C., Dinarello C. A. A specific receptor antagonist for interleukin 1 prevents Escherichia coli-induced shock in rabbits. FASEB J. 1991 Mar 1;5(3):338–343. doi: 10.1096/fasebj.5.3.1825816. [DOI] [PubMed] [Google Scholar]
- Watson J., Riblet R. Genetic control of responses to bacterial lipopolysaccharides in mice. I. Evidence for a single gene that influences mitogenic and immunogenic respones to lipopolysaccharides. J Exp Med. 1974 Nov 1;140(5):1147–1161. doi: 10.1084/jem.140.5.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson J., Riblet R., Taylor B. A. The response of recombinant inbred strains of mice to bacterial lipopolysaccharides. J Immunol. 1977 Jun;118(6):2088–2093. [PubMed] [Google Scholar]
- Wolfe R. R., Shaw J. H., Durkot M. J. Effect of sepsis on VLDL kinetics: responses in basal state and during glucose infusion. Am J Physiol. 1985 Jun;248(6 Pt 1):E732–E740. doi: 10.1152/ajpendo.1985.248.6.E732. [DOI] [PubMed] [Google Scholar]
- von Jeney N., Günther E., Jann K. Mitogenic stimulation of murine spleen cells: relation to susceptibility to Salmonella infection. Infect Immun. 1977 Jan;15(1):26–33. doi: 10.1128/iai.15.1.26-33.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]