Abstract
Over the past 4 decades, much has been learned about the pathophysiology and treatment of osteoporosis, the prevention of fragility fractures, and the perioperative management of patients who have these debilitating injuries. However, the volume of published literature on this topic is staggering and far too voluminous for any clinician to review and synthesize by him or herself. This manuscript thoroughly summarizes the latest research on fragility fractures and provides the reader with valuable strategies to optimize the prevention and management of these devastating injuries. The information contained in this article will prove invaluable to any health care provider or health system administrator who is involved in the prevention and management of fragility hip fractures. As providers begin to gain a better understanding of the principles espoused in this article, it is our hope that they will be able to use this information to optimize the care they provide for elderly patients who are at risk of or who have osteoporotic fractures.
Keywords: geriatric medicine, geriatric trauma, metabolic bone disorders, nonoperative spine, osteoporosis, systems of care, upper extremity surgery, trauma surgery, foot and ankle surgery, fragility fractures
Scope of the Problem
Stephen Kates, MD
Fragility fractures represent an epidemic problem worldwide, as the population ages at a rate much greater than once predicted. In the United States, the aging of the population is a result of improved life expectancy coupled with the aging of the Baby -Boom generation (born 1946-1964). It is expected that these 77 million Baby Boomers will become senior citizens by 2026 (http://en.wikipedia.org/wiki/Post-World_War_II_baby_boom) and cause the fastest growing segment of the population to be the group more than 85 years old.4
Falls and fractures become much more prevalent with advancing age.5 Falls have been shown to precede most fractures. Hip fractures occur equally inside or outside the home, whereas other fragility fractures occur somewhat more commonly outside the home.6 Fractures occur throughout the year evenly with the exception of hip fractures that occur with a slightly higher likelihood in springtime.6 It has been shown that most patients who sustain a fracture and are more than 65 years old have weakened bone quality from osteoporosis or osteopenia, conditions that are largely untreated and silent until a fracture occurs, although osteoporosis is the most common disease of the bone.7 Osteoporosis is a metabolic bone disease characterized by low bone mass and microarchitectural deterioration of bone tissue that results in increased bone fragility and a consequent increase in fracture risk. Although bone mass is an important component of the disease, it is the combination of bone mass and bone quality that results in a bone’s overall strength and ability to resist fracture. Approximately 2.1 million osteoporotic fractures occur yearly in the United States8; in 2006, the rate of fragility fracture was listed as 1056 per 100 000 people.7 Most such fractures occur in those in the over-65 age-group.7 For most patients who experience such a fracture, this is their first osteoporotic fracture.9 The lack of treatment that commonly follows a serious osteoporotic fracture is worrisome: Reported rates of treatment after hip fracture are in the 10% to 20% range.8,10 Primary prevention of osteoporotic fractures is essential. Improvement in algorithms to identify patients at risk of fracture will be essential to improving the population’s health in the future.9
Hip Fractures
Stephen Kates, MD
The most serious fragility fractures occur in the hip; such fractures can lead to serious morbidity, are associated with a high mortality risk, and are the most expensive of all the fragility fractures.
Approximately 330 000 hip fractures occur yearly in the United States.13 The incidence of hip fractures seems to be decreasing over the past decade, but the prevalence of hip fracture is expected to increase to 550 000 by 2040, which may be a conservative estimate.5,14 In 2006, the hip fracture rate was listed as 78.7 per 10 000 people. The mortality rate is in the 20% to 24% range at 1 year; many patients will lose their independence after hip fracture.7,15 The in-hospital mortality rate between 1988 and 2007 was 4.9% for men and 2.6% for women.16 Older ages, male gender, and comorbid conditions are associated with a higher risk of in-hospital mortality.16 There has been a downward trend in in-hospital mortality since 1988 mostly attributed to lower risk of death in men.16 Inappropriate medication prescribing has been shown to be an independent predictor of long-term mortality in patients with hip fracture.17 Mortality after hip fracture is high not only in the first year after fracture but remains higher than baseline during the subsequent 5 years as well.15
The cost of caring for hip fractures was reported to be US$17 billion dollars in 1997, and it is estimated that it will grow to US$62 billion by 2040.18 This number may also represent a conservative estimate because the medical consumer price index consistently outpaces the general consumer price index. In 2007, the average cost for inpatient care of a hip fracture had increased approximately to US$42 000.13,19 Nearly all patients with hip fractures are admitted to the hospital for care, and most hip fractures are treated surgically. The average length of hospital stay for a hip fracture in 2007 was 6.4 days19,20; it is very troublesome that population-based studies have shown a decline in use of osteoporosis medication after hip fracture from 40% in 2002 to 20.5% in 2011.21 Patients on treatment prior to fracture are more likely to be treated after fracture.21 Even more troublesome is data showing that proton pump inhibitor use is associated with risk of hip fracture. Proton pump inhibitor medications are among the most commonly used drugs in the United States today.22
Admission to the hospital
Bernardo J. Reyes, MD and Simon C. Mears, MD, PhD
Typically, a patient with an acute hip fracture is unable to walk, is seen in the emergency department (ED), admitted to the hospital, and then the fracture is surgically repaired. Despite the seeming simplicity of this pathway, many roadblocks stand in the way of optimal care.
The first potential roadblock is the delay between injury and presentation to the ED, which can be extensive. As an example, a patient who lives alone may not be found for hours to days after injury. These unfortunate patients are often unable to move and become dehydrated or even develop rhabdomyolysis with renal failure. Decubitus ulceration from lying in one position on the floor may occur.
When initially seen by emergency medical service personnel, the patient typically complains of hip or groin pain. Patients with suspected hip fractures are usually transported to the ED by ambulance on a back board or stretcher; these devices are hard and can lead to additional pressure on the sacrum and thereby potentially to pressure ulcers.23 The hip fracture patient is at particular risk for pressure ulcers from the time of fracture to arrival at the ED, and indeed, throughout care.
The next potential roadblock is the ED itself. In the United States, ED overcrowding is epidemic, and the patient with a hip fracture is often lost within the system.24 A short length of stay (less than 4 hours) in ED is typically seen in a well-functioning system. Unfortunately, in a busy hospital, the length of time spent in the ED may be considerably longer.25 Lack of appropriate triage will lengthen the stay in the ED, especially for an elderly patient who does not appear to require acute care. In addition, the environment is frequently noisy, seemingly chaotic, and often confusing and frightening for the elderly patient and promotes the development of delirium.26
Tips to avoid delays in ED
Regularly monitor time in ED as a parameter of interest.
Limit and streamline tests in the ED (a short hip fracture order set).
Multidisciplinary approach to admit patient to floor quickly.
Work with hospital administration to remove roadblocks to quick admission.
Consider an early admission pathway for patients with hip fracture to improve care.
Critical steps in ED
Rapid X-ray when there is concern for hip fracture.
Avoidance of unnecessary advanced imaging (computed tomography [CT] scans and magnetic resonance imaging’s [MRI’s]).
Identify medical unstable patients who may require intensive care unit admission.
Early rehydration with isotonic crystalloid.
Pain control and consider regional nerve block.27
Essential laboratory work and electrocardiogram (ECG).
Rapid consultation with orthopedics and medical/hospitalist/geriatrician team.
Promote quick admission to hospital room.
The initial step in evaluation of the patient with a hip fracture is obtaining a problem-focused history and performing a physical examination. The clinician may need to obtain information from a family member, medical records, or a nursing home (most often via a call to the nursing supervisor) in addition to questioning the patient. During this time, collecting information to complete a comprehensive geriatric assessment might be appropriate if it does not delay surgery. With this information, key decisions can be made regarding goals of care, possible outcomes as well as forecast potential complications.
The nature of the fall must be determined to see whether there was a contributing event such as a stroke or syncope. Other potential causes for fracture should be sought, including a history suggestive of metastatic cancer. Acute medical problems such as myocardial infarction must be ruled out. An accurate list of home medications as well as obtaining the patient’s medical history is critical. Early assessment of the patient’s mental status is necessary. An abbreviated mini-mental examination will help determine whether the patient has memory loss. Examination of cognition should be completed only on patients without delirium and in which pain is well controlled in order to avoid inaccurate results. A social history that assesses the patient’s preinjury level of activity and independence is also important. As elderly patients might find decision making overwhelming, contacting the patient’s health care proxy and or family members is appropriate early in the evaluation process. In addition, advanced directives must be determined and documented prominently in the medical record. Depending on the institution, patient’s limited life support advanced directives might be suspended for the surgical intervention.
The physical examination should be initiated by the ED provider who should inspect for other injuries. Basic laboratory studies and an ECG should be ordered. A whole-body CT scan is not required for the patient with an isolated fragility fracture and should be avoided unless specifically indicated because of concern about more extensive injury or illness.28
The physical examination should focus on the injured extremity. Most often a patient with a hip fracture has groin pain and pain with hip motion. Fracture displacement causes the leg to be shortened and externally rotated (Figure 1). The hip should not be excessively moved on examination because it is painful and may increase bleeding. Conventional radiographs are the best method for diagnosing a hip fracture. They should be ordered as follows: anterior–posterior (AP) and tube lateral (cross-table) views of the involved hip and an AP view of the pelvis (Figure 2a, b). An AP view with gentle traction can be very helpful in determining the pattern of the fracture. If radiographs are negative despite hip pain, a MRI scan is the best way to confirm a hip fracture. If metastatic cancer is the cause of the fracture, additional conventional radiographs and advanced imaging studies will likely be needed to evaluate the entire femur, and consideration should be given to additional imaging to find the primary lesion, if not already known.
Figure 1.
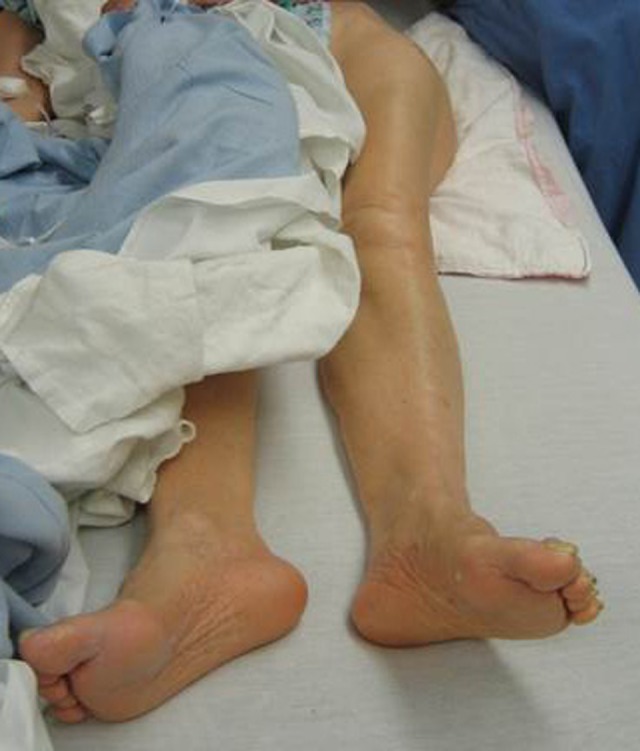
Clinical photograph of the lower extremities of a patient with a left hip fracture. The left side is shortened and externally rotated.
Figure 2.
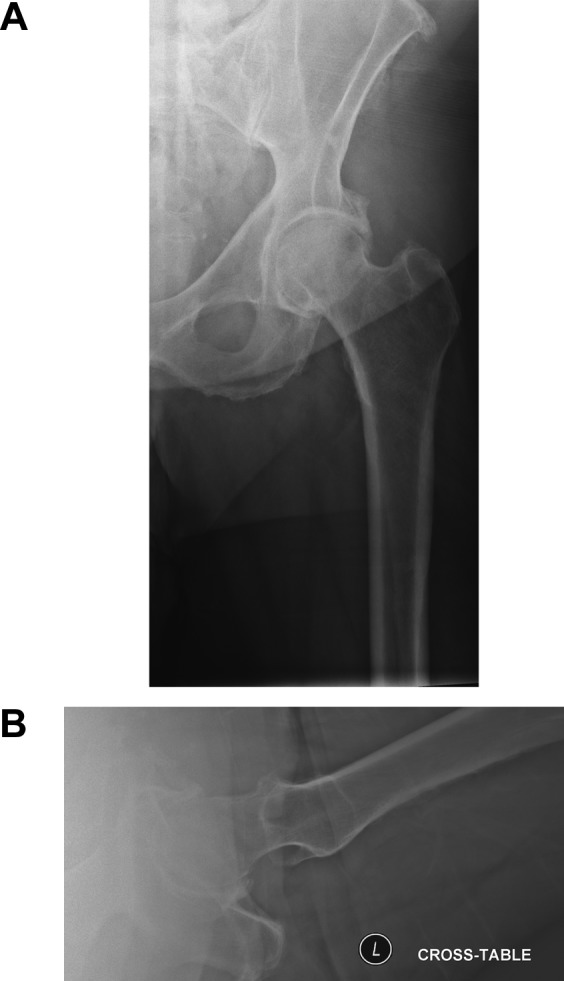
Standard radiographic views of the hip (A) anterior–posterior (AP) and (B) lateral views of the hip.
Pain Control
Pain management must be started in the ED as part of the initial orders given for emergency care. Proper pain management is humane and may reduce the likelihood of developing delirium.29 Pain control is best accomplished with small doses of narcotic medicine, for example, 1- to 2-mg doses of intravenous morphine (Merperidine should not be used in older adults) that can be titrated to achieve the desired effect. Other regimens include the use of oral narcotic medications such as oxycodone. In patients with renal or hepatic insufficiency, hydromorphone is the narcotic of choice. If available, a peripheral nerve block can help with pain relief.30 The use of traction is not helpful in terms of pain relief for patients with hip fractures and may contribute to pressure ulceration.27,31 In the ED, it is important to achieve effective pain control without excessive sedation.
Triage and Admission
At this point, the type of hospital admission is determined. The medical stability of the patient must be ascertained. Unstable patients may require critical care admission. Most patients should be admitted to an orthopedic surgeon or medical service, depending on the care model of the institution. Clear benefits exist to streamlining this process and admitting patients to a hospital floor as quickly as possible.32,33
Low-pressure mattresses should be used to avoid pressure sores, and nurses should be trained to recognize and prevent them. A full skin examination with particular focus on the heels and sacrum must be performed and documented during the admission process. It is important to document any pressure ulcer present on hospital admission.
To prevent skin inflammation and pain in female patients (or in males with incontinence or voiding difficulties), a Foley catheter is often placed while the patient is in the ED.
Screening for Urinary Tract Infections
The Infectious Diseases Society of America was unable to determine the clinical benefits of screening for and treatment of bacteriuria prior to a surgical procedure with prosthetic implantation, including orthopedic procedures. Urinalysis should be performed and urinary tract infections should be documented and treated if the patient is symptomatic. Although chronic urinary tract infections or colonization may not be symptomatic, urinary tract infections may increase the risk of superficial wound infections. Therefore, patients who are undergoing surgical procedures with implantation of hardware are often treated with antibiotics in the perioperative period.
Perioperative Hydration
In the ED, hydration of the patient should be started. Patients with hip fractures are almost always dehydrated. The physiologic stress response to surgery and trauma induces inflammation, catabolism, and fluid retention depleting even more the intravascular volume. Typically, isotonic saline is used for repletion of intravascular volume.
A Cochrane review has failed to identify the best crystalloid for preoperative hydration. The amount of intravenous hydration that a patient will need is based on clinical judgment. Based on the available studies a range between 2 and 5 L is a safe estimate. Some studies have questioned the accuracy of assessing euvolemia using urine output, vital signs, or oxygen tonometry. Other methods like central venous catheters also have been less accepted as reliable. In terms of the type of crystalloid to be used, isotonic (normal) saline could be started at 100 to 200 mL/h, and the fluid status should be carefully followed.34 Caution is needed to avoid volume overload because many seniors have cardiac disease, are predisposed to heart failure, and excess of chloride might cause hyper-chloremic metabolic acidosis.
The goal is to correctly diagnose the hip fracture, stabilize the patient medically for any acute needs, and admit the patient to the hospital. These goals must be accomplished quickly and in a thoughtful and caring manner.32,33
Preoperative Medical Assessment
Bernardo Reyes, MD and Simon C. Mears, MD, PhD
Preoperative medical assessment of the patient with hip fracture starts in the ED. The goal of the preoperative medical assessment is to make surgical repair as safe as possible in a timely manner. The ideal timing of fracture repair is within 24 hours after fracture.27,35 Early surgical repair improves results by decreasing initial pain, length of stay, and complications.35–37 There is also an association between early surgical repair and benefits in mid- and long-term outcomes.38
The preoperative medical assessment is meant to risk stratify the patient, improve reversible acute medical abnormalities, and prevent complications common in the geriatric patient.33 The use of an interdisciplinary team approach (including orthopedics, geriatrics or internal medicine/ hospitalist/ family medicine, anesthesiology, nursing, and therapists) to fracture care and the level of experience of the providers are very important factors in achieving the best outcomes.32,33 It is important that the anesthesia team be involved in this process to avoid delay in surgical intervention. The goals of the team must be to optimize the patient for early surgical repair. Coordination and cooperation among surgeons, anesthesiologists, and others is critical. This team approach should minimize unnecessary preoperative tests and consultations, which can add expense and cause delay.32,33 The goal of early surgery should always be kept in mind, and any test that is ordered should have a clear and immediate benefit to the patient. Evaluation or procedures that are not needed for a surgical decision should be avoided.
For patients arriving from a skilled nursing facility (SNF), an efficient method of transition to the inpatient hospital setting is essential. When the patient is transferred, a summary listing the patient’s most recent history and physical examination and medication list is needed. Attention to mental status including dementia and delirium is important. A confusion assessment method (CAM) and some form of mental status testing will help to determine this status. The short form mini-mental test39 and the mini-cog test40 are good examples of short tests to look for dementia. It is important to recognize cognitive problems because they can predict the development of delirium during the hospital stay.29
Standard laboratory tests, including a basic metabolic profile, complete blood count, prothrombin time (international normalized ratio [INR]), and partial thromboplastin time, should be obtained. If the electrolytes are abnormal, these abnormalities should be corrected.
Cardiopulmonary Evaluation
Preoperative cardiac evaluation should be tailored with the assumption that the patient requires early surgery. The aim of this evaluation is to diagnose and treat possible absolute contraindications for surgery. The American Heart Association (AHA) recognizes 4 major contraindications for surgery, namely, ongoing or recent acute coronary syndrome (within two weeks of surgery), decompensated heart failure, uncontrolled arrhythmia, and severe valvular disease.
The extent of the investigations to rule out any of these conditions should be proportional to the medical history and the history of present illness. For example, patients with prodromal symptoms such as chest pain, palpitations, or loss of consciousness are more likely to require a more comprehensive workup than a patient who had a fall due to extrinsic factors only. Based on current scientific guidelines, patients with an exercise capacity of 4 or more metabolic equivalents (METs) without symptoms should proceed to planned surgery.41 This determination is made by asking patients about their activity level. Patients with an activity level of 1 to 3 METs can dress themselves, walk around the house, or walk a block at 2 mph. At 4 METs, a patient can climb a flight of stairs, walk a block at 4 mph, run a short distance, or do heavy housework. At 10 METs, a patient can participate in strenuous sports.
The ECG should be reviewed to rule out abnormalities and compared with a previous tracing, if possible. New or acute changes should be followed with analysis of serum troponin level to rule out myocardial infarction.
Rate and rhythm should be assessed. The use of additional tests, such as echocardiograms or stress testing, should be used only in compelling circumstances—for example, for the patient with severe aortic stenosis or pulmonary hypertension, for whom the anesthesiologist may need the results of an echocardiogram to enable appropriate care during surgery. The routine use of echocardiogram is associated with delay or surgical repair of hip fractures and only on a focused group of patients has it demonstrated to change perioperative management.41
Anemia and Transfusions
The hemoglobin level should be checked to make sure that the patient does not need blood transfusion before repair of the hip fracture. Blood transfusion should be considered if the preoperative hemoglobin is below 8 g/dL because it likely represents a risk to a patient who will incur surgical blood loss, leading to an additional decrease in the hemoglobin level.27,42
Coagulopathies
The prothrombin time/INR should be checked because the patient may be on chronic anticoagulation therapy or have a condition affecting coagulation. The treatment of patients with a markedly elevated INR is controversial, with options ranging from watchful waiting to the use of oral vitamin K or fresh-frozen plasma.43 If the INR is less than 1.5, surgical intervention may proceed. The treatment of an elevated INR is complicated by the acute need for the patient with a hip fracture to undergo surgical fixation. The use of oral vitamin K (oral is the preferred route) may expedite this process. The fastest reversal is with the use of fresh-frozen plasma. The use of fresh-frozen plasma appears to be safe and significantly reduces time to surgical repair.44,45
For patient taking newer anticoagulants like dabigatran, rivaroxaban and apixaban, there is no reversal therapy therefore, based on these products’ package insert, a prudent time between the last dose of these medications and surgery is approximately 48 hours. This could be extended on patients with abnormal renal of hepatic function.
The need of bridge therapy for those patients taking Coumadin at admission is determined by the risk of a thrombotic event versus the risk of bleeding. The risk of thrombosis among high-risk patient is 0.9%, 1.2%, and 1.8% for patients with atrial fibrillation, mechanical valves, and history of recent deep venous thrombosis, respectively. The corresponding risk of major bleeding is 2.0%, 2.7%, and 1.9%, respectively, regardless of the use of bridging therapy.
Bridging therapy should be considered in patients with mitral mechanical valves, atrial fibrillation with a stroke risk prediction CHADS2 score of 4 or more, and venous thromboembolism (VTE) within the past 3 months of surgery.46,47
β-Blockers
The use of β blockers before surgery has been the objective of controversy. The AHA strongly recommends continuing β-blockers in patients undergoing surgery who are receiving β-blockers for the treatment of any cardiac condition. For those patients with high cardiac risk and who are naive to this group of medications, AHA recommends to start and titrate a short-acting β-blocker (ie, metoprolol tartrate) to achieve a heart rate between 80 and 60 beats/min. Due to findings of more recent studies, the routine administration of perioperative β-blockers, particularly in higher fixed-dose regimens begun on the day of surgery is not recommended.41 Additional beta blockade may decrease the risk of cardiac events but give a higher risk of hypotension, stroke, and death.48
Of note, the validity of the data that were used to write the current AHA guidelines has been questioned. Nonetheless, more recent large observational studies confirmed the benefits of perioperative β-blockers in patient undergoing orthopedic procedures when the patients have history of heart failure or an acute coronary syndrome within 2 years of their surgery. Therefore, although more evidence is needed, for now, starting these medications before surgery in selective populations seems to be beneficial.
Patients with Pulmonary Disease
Postoperative pulmonary complications can occur in up to 50% of patients with chronic pulmonary disease. Preoperative pulmonary evaluation (including pulmonary function tests) does not predict respiratory complications in nonelective surgery. Steroids and bronchodilators may be indicated, although the risk of producing arrhythmia or myocardial ischemia by beta agonists must be considered. Respiratory infections should be treated as soon as possible as they can affect outcomes significantly.
Regardless of any preexisting cardiopulmonary condition, chest radiographs are commonly ordered as a part of preoperative evaluation. Although it has been found that rarely changes management, patients who received preoperative chest radiographs have a lower rate of pulmonary complications.49
Optimize the patient for early fracture repair!
- Medical optimization
- The team works toward early surgical repair;
- hydrate the patient;
- recognize cognitive dysfunction (delirium and dementia);
- optimize electrolytes;
- correct coagulopathy;
- diagnose aortic stenosis, pulmonary hypertension, myocardial infarction;
- reconcile medications; and
- solidify advanced directives.
- Tests to avoid
- Echocardiogram (may be useful if severe aortic stenosis or severe pulmonary hypertension is suspected);
- cardiac stress test;
- pulmonary function test; and
- routine subspecialist consultation.
Anesthesia Management
Omar I. Ahmed, MD, Jean-Pierre P. Ouanes, DO and Frederick E. Sieber, MD
Currently, the anesthesiologist may select from a variety of techniques to enable the surgeon to perform hip fracture repair. These include spinal, epidural, or general anesthesia. Many studies have been performed to try to determine whether one technique is better than the other. No differences have been found between techniques in the current literature.50,51
However, there is much evidence to suggest that regional versus general anesthesia is associated with better outcomes in patients with hip fracture.27 Researchers reviewed data from 400 US hospitals to determine whether neuraxial anesthesia or general anesthesia had better outcomes following primary hip or knee replacements.52 They found that the neuraxial group had an 80% lower 30-day mortality rate and 30% to 50% lower risk of major complications including stroke, renal failure, pneumonia, or need for mechanical ventilation. Recently, a close examination of a retrospective cohort of patients with hip surgery specifically looked at regional versus general anesthesia with a primary outcome of inpatient mortality and secondary outcomes of cardiovascular and pulmonary complications. In this review of over 18 000 cases, patients who received regional anesthesia had a significant reduction, up to 29%, in pulmonary complications and mortality.53 Similarly, a meta-analysis of patients with hip fracture has shown that, compared with general anesthesia, regional anesthesia is associated with reduced incidence of deep vein thrombosis, decreased early mortality, but longer operative times.54
Spinal and epidural anesthesia has been shown to decrease intraoperative blood loss.55 A variety of mechanisms have been proposed to explain the beneficial effects of regional anesthesia on perioperative blood loss. The decreased blood loss is most likely the result of arterial and venous hypotension below the level of the neuraxial blockade. In a study of regional versus general anesthesia for total hip arthroplasty, patients were randomized to 1 of the following 3 anesthetics: (1) epidural anesthesia alone, (2) general anesthesia with spontaneous ventilation, or (3) general anesthesia with positive pressure mechanical ventilation.56 The beneficial effects of neuraxial anesthesia on blood loss may be lost with positive pressure ventilation unless induced hypotension is employed.
A recent review examined whether general or regional anesthesia is associated with a greater risk of postoperative delirium.57 Most studies examining elective surgery suggest no difference between regional and general anesthesia in terms of in postoperative delirium. In contrast to elective procedures, however, evidence suggests that type of anesthesia influences postoperative delirium after the urgent surgery of hip fracture repair. A Cochrane review compared outcome differences in patients with hip fracture who received regional and general anesthesia.58 Based on 5 randomized controlled trials, the number of patients who experienced a postoperative state of confusion (delirium) was 11 (9.4%) of 117 in the regional anesthesia group and 23 (19.2%) of 120 in the general anesthesia group (relative risk 0.50, 95% confidence interval 0.26-0.95; overall effect z = 2.12, P = .03). The authors concluded that with hip fracture surgery, regional anesthesia, compared with general anesthesia, is associated with a 2-fold reduced risk of acute postoperative confusion.
Controlling the level of sedation during regional anesthesia has been shown to prevent delirium in high-risk populations. A recent randomized double-blind trial examined the question of whether light or deep sedation could decrease the incidence of postoperative delirium.59 In elderly patients undergoing hip fracture repair with spinal anesthesia, patients were randomized to receive either light or deep sedation with propofol and then were followed postoperatively for delirium. The study showed that in this high-risk population, patients with light sedation had a 50% lower incidence of postoperative delirium than did those with deep sedation. The effect was associated with a mean reduction in almost 1 day of delirium for the light sedation group. This study points to the role of excessive sedation during the perioperative period as a risk factor for delirium in patients with hip fracture.
In considering neuraxial anesthesia, it is important to determine whether the patient is taking anticoagulants. Epidural and spinal hematomas are rare but devastating complications with spinal and epidural anesthesia. The reported incidence is less than 1 in 150 000.60 The leading risk factor for epidural hematoma is anticoagulation use. For guidelines concerning administration of spinal or epidural anesthesia in patients who are taking anticoagulants, we refer the reader to the American Society for Regional Anesthesia and Pain Medicine consensus statement on neuraxial anesthesia and anticoagulation.61
Peripheral nerve blocks may be attempted to provide surgical anesthesia and analgesia for lower extremity surgery.55 However, consistent blockade may prove challenging due to individual variations in nerve distributions and variable spread, especially in the case of the psoas compartment or 3:1 blocks. For hip fractures, both the lumbar plexus and the sciatic nerve distributions need to be covered. The lumbar plexus must be covered to include the lateral femoral cutaneous and femoral nerves. For surgeries and fractures at and below the knee, both the femoral and the sciatic nerve distributions need to be covered. In some patients, the obturator nerve may also contribute to sensory innervation of the medial knee.
Pain secondary to the fracture itself may make performing a regional technique challenging. However, appropriate preoperative sedation during the block can facilitate regional and neuraxial anesthesia. Older adults may have dementia or other neurological conditions. Such underlying problems will challenge anesthetic plans and may oftentimes lead practitioners to select general anesthesia over regional to manage the patient’s lack of cooperation.
In summary, debate continues as to the best anesthetic technique for hip fracture surgery. The current literature shows little difference between general and spinal techniques.51 Data quality is poor, and there may be differences in outcomes if the depth of sedation were controlled. Further study is required to determine whether one method is better than another. Regional techniques such as obturator or iliac fascial nerve block help with pain in the perioperative period.27
Anesthesia for Hip Fractures
Current literature shows no difference between general and spinal anesthesia for patients with hip fracture.
Literature is flawed as depth of sedation may be the key factor and this has not routinely been measured.
Additional regional techniques such as nerve blocks may help with pain control both while waiting for surgery and after surgery.
Surgery
Simon C. Mears, MD, PhD
The type of surgery needed to manage a hip fracture is determined by the fracture type (femoral neck, intertrochanteric or subtrochanteric; Figure 3) and the individual needs of the patient. Femoral neck fractures may be classified as stable or unstable, depending on the fracture pattern, displacement, and angulation. Stable femoral neck fractures are nondisplaced fractures or valgus-impacted fractures with no angulation on a lateral radiographic view. Some nondisplaced fractures may require MRI imaging for visualization.27
Figure 3.
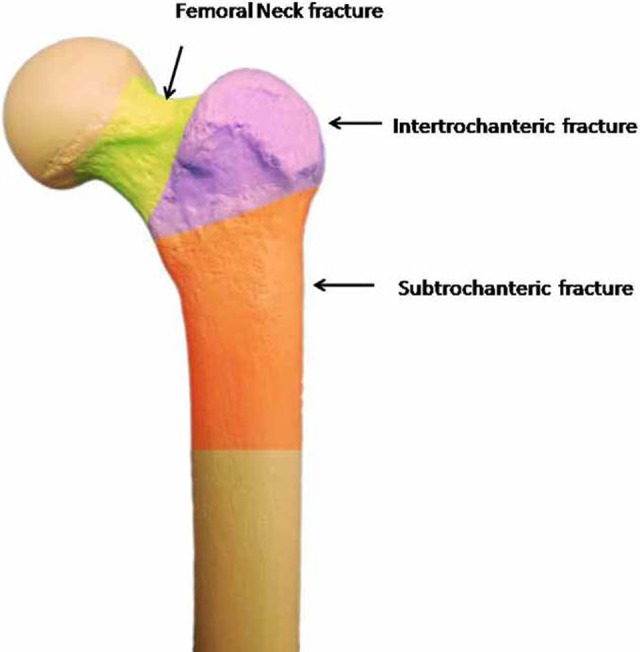
This image shows the 3 typical locations of hip fractures, namely, femoral neck, intertrochanteric, and subtrochanteric regions.
Femoral Neck Fractures
Nondisplaced femoral neck fractures are treated with surgery because there is a 20% chance of displacement with nonoperative treatment.62 This risk increases to 79% when the patient is more than 70 years old.63 Surgery typically involves fixation with 2 to 3 cannulated screws (most typically, 3), with the patient on a fracture table. The use of washers seems to improve fixation in osteoporotic bone. The position of screws is important: They should be spread apart and placed next to the cortex of the femoral neck inferiorly, superiorly, and posteriorly. An inverted triangle pattern has been shown to lead to significantly less nonunions than a triangle pattern of screw insertion.64 The bottom screw must be above the level of the lesser trochanter to prevent a stress riser in the subtrochanteric areas that can result in subtrochanteric fracture.65 The screw threads should not cross the fracture line and should be placed as deeply into the head as possible without head penetration. The results of screw fixation for stable fractures are satisfactory with revision rates approximating 10%; the more stable the fracture, the better the results.66,67 Some limbs may later develop shortening, osteonecrosis, nonunion, or screw cutout. The degree of posterior tilt does not seem to affect the results of screw fixation. In a review of 382 patients with either Garden I or Garden II fractures, the rate of revision was 19% at 5 years, with no difference between fracture types.68 Hemiarthoplasty may also be an option for nondisplaced fractures. No studies have directly compared screw fixation versus hemiarthroplasty for nondisplaced fractures. The satisfaction of patients with displaced fractures with hemiarthroplasty is higher and the revision rate lower than patients with nondisplaced fractures treated with screw fixation.67
If the fracture is unstable, the choice of treatment is based on an algorithm that uses information about the patient and the surgeon.69 The basic choices are reduction and internal fixation, hemiarthroplasty, or total hip arthroplasty: Open reduction and internal fixation (ORIF) should be reserved for very young patients. Hemiarthroplasty is an excellent choice for the older or medically infirm patient with a relatively normal acetabulum, and total hip arthroplasty has been shown to give the best outcomes for the active elderly patient.70 The choice of surgery should also be tempered by the surgeon’s skill. For instance, those less familiar with total hip replacement will achieve better results with hemiarthroplasty. The goal of surgery should be to achieve the best result with the fewest reoperations in the timeliest manner.
Internal fixation has a higher rate of reoperation and lower patient satisfaction than hemiarthroplasty for displaced fractures. This has been shown true a long-term follow-up. The rate of reoperation for internal fixation is about 23%.71 Internal fixation has also been shown to be inferior to hemiarthroplasty for patients with severe cognitive dysfunction.72 Internal fixation is more expensive than hemiarthroplasty when the cost of reoperation is considered.73
For arthroplasty procedures, there is debate about which type of femoral prosthesis should be used. Although uncemented stems are used most commonly in the United States, the role of the cemented stem in very elderly patients (more than 85 years old) with hip fracture should not be forgotten and may be superior to uncemented stems.74 Excellent long-term results with cemented stems should give assurance that a well-placed stem will last the length of the patient’s life.74,75 The cemented stem has the advantage of a lower fracture rate (both insertional and later peri-prosthetic fractures) and easier use in the patient with advanced osteoporosis and the stovepipe or Dorr type C anatomy of the femur.75 Several randomized and long-term studies have shown significantly lower periprosthetic fracture rates with the use of cemented stems for hemiarthroplasty.27,76–78 Cemented stems do have the potential disadvantage of acute intraoperative hypotension at the time of cement insertion. When larger numbers of patients (11 116 cases with hemiarthroplasty) were examined in the Norwegian Hip Fracture Register, the rate of intraoperative death was higher for the use of cemented stems (26 of 8639 patients) compared to uncemented stems (1 of 2477 patients), although the rate of fracture and implant failure was higher for the uncemented stems (97% 5-year survival of cemented stems vs 91% for uncemented stems).79 Uncemented stems can be used in osteoporotic bones, but their placement is difficult, especially for the surgeon who performs hip replacements infrequently, such as may be the case when an on-call surgeon performs the hip fracture procedure. If an uncemented stem is selected, many designs have been shown to be effective in Dorr type C bones, including those with proximally coated, rectangular, or fully coated designs. Uncemented stems have a higher risk of intraoperative fracture.27,75 The experience of the surgeon in using the stem most familiar to them is the most important factor for success.
If a hemiarthroplasty is selected, a uni- or bipolar type of head may be used.27 In the past, a unipolar head was associated with poor femoral fixation, which leads to poor results. With the use of a well-fixed stem, there seems to be no advantage to the use of a bipolar construct in terms of range of motion or pain level.80 It is possible that later acetabular erosion is more common with the unipolar head.81 The hemiarthroplasty does leave the patient susceptible to wear of the articular cartilage or pain in the hip secondary to mismatch of the size of the selected head and the native acetabulum. This potential disadvantage has led to the use of total hip arthroplasty for patients who are active or physiologically young. Several randomized controlled trials have shown that, in such patients, total hip arthroplasty has proven superior for pain relief and functional outcomes.70,75,82,83 Patient recorded outcomes are best with total hip arthroplasty when compared to hemiarthroplasty or internal fixation.84
The better functional outcomes of total hip replacement do not come without potential cost. The rate of dislocation after total hip replacement is higher than after hemiarthroplasty. The dislocation for total hip replacement after fracture has been shown to be higher than after care for osteoarthritis. It is unclear whether this is due to anatomical differences such as a laxer capsule in patients with fracture or whether this is due to the skill level of nonarthroplasty surgeons performing a more technically challenging procedure. It is thought that the use of an anterolateral approach and larger bearing surfaces will help to reduce dislocation rates.
Intertrochanteric Fractures
Intertrochanteric fractures have been classified by several systems,85 but they are more practically termed stable or unstable (Figure 4). Stable fractures typically have 2 or 3 parts with intact medial and lateral buttresses and should be treated with sliding hip screw fixation. The lateral buttress allows for a firm end point to the sliding of the screw.86 The sliding hip screw works by having a firmly anchored screw in the femoral head. The screw slides in the barrel of the side plate, allowing for compression of the neck of the femur against the greater trochanter. Over time and with weight bearing, the screw may slide, further compressing the fracture. The key factor in the success of the hip screw is the placement of the screw within the femoral head. The screw should be as deep as possible and centered with the head. The importance of the position has been quantified by the tip-apex distance, that is, the distance between the tip of the screw and the apex of the femoral head on the posterior–anterior and lateral views. When this distance is <25 mm and the chance of success and healing is excellent. If the tip-apex distance is >25 mm and the rate of failure is increased.87
Figure 4.
The AO/OTA classification of the extra-capsular proximal femur fractures (intertrochanteric-subtrochanteric region). According to this classification system, the femur is labeled bone 3, and the proximal femur segment is labeled 1. The “A” types are extracapsular fractures. Types A1.1 to A2.1 are generally considered to be stable patterns. Types A2.2 to 3.3 are usually considered unstable fractures.
Unstable fractures are characterized by comminution, a reverse obliquity fracture line, or extension into the shaft of the femur. In these cases, the lateral buttress is not intact and will not provide an end point to sliding, so a sliding hip screw has a higher rate of failure in these fracture patterns.88 The unstable fracture is best treated with an intramedullary nail because it provides the buttress for the proximal fragment.27 A fixed angle device, such as an angled blade plate, may also be considered.
There are 3 important technical points concerning the insertion of an intramedullary nail. First, the fracture must be reduced before nail insertion and open reduction performed if necessary. Second, the proximal part of the nail must be medialized during insertion to prevent additional iatrogenic fracture. Third, the nail must be held still in the femoral canal during hip screw insertion so that the screw does not migrate proximally, a step that is critical in assuring assure a low tip-apex distance.
A short or a long intramedullary nail may be used. Although the long nail may protect more of the femoral shaft, the bone can be at risk of fracture distally around the end of the nail above the knee. The nail may also cause an intraoperative fracture at the anterior cortex of the distal femur because of a mismatch between the anterior bow of the nail and that of the femur. Care must be taken during nail insertion to avoid fracture. Good evidence does not exist for the choice of a short versus long nail for unstable intertrochanteric fractures.89
The goal of hip fracture surgery is to permit the patient to bear weight as tolerated after surgery.90 Elderly patients usually cannot limit their weight bearing or follow mobility restrictions. Allowing patients to bear weight will help with mobilization and recovery and is recommended when stable surgical repair has been achieved.91 The surgeon should choose a procedure that will allow full weight bearing immediately postoperatively.
Treatment of Femoral Neck fractures
Nondisplaced: screw fixation
Displaced low activity level: hemiarthroplasty
High activity level: total hip arthroplasty
Treatment of Intertrochanteric fractures
Stable fractures with intact lateral wall: sliding hip screw and side plate
Unstable fractures: intramedullary hip screw
Surgical Complications of Hip Fractures
Simon C. Mears, MD, PhD
An important goal of hip fracture repair is to minimize reoperation—“single shot surgery”. This goal should guide surgical decision making. Despite sound decision making and meticulous surgical technique, complications can occur which require further surgery. A second hip fracture surgery is more likely to be associated with an adverse event because the patient is further debilitated than during their initial fracture. Results of reoperation are not as good when compared to primary repair.92 Patients requiring a second surgery are often those with the most medical comorbidities and with the poorest bone quality. Surgical complications differ between those associated with arthroplasty and those associated with ORIF.
Arthroplasty-Related Complications
Infection
Infection is the most feared complication of arthroplasty. Rates of infection after arthroplasty range between 0.2% to 0.8%.93,94 Infection risks are higher in smokers, morbid obesity, uncontrolled diabetes, poor dentition, or open wounds or other sites of infection. Due to the urgent nature of hip fracture surgery, most of these risk factors cannot be altered prior to fracture repair. In contrast, elective arthroplasty for an arthritic condition can be postponed until patient-specific factors can be modified or resolved. Rates of infection after hemiarthroplasty for hip fracture have been reported at 1.3% in the Scandinavian database.95 Infections can occur immediately after the procedure or later. The patient with a wound that does not heal or continues to drain after hip replacement is likely to have infectious process. Workup for infection should include an initial Erythrocyte Sedimentation rate (ESR) and C-reactive protein (CRP) test. If either is elevated, a hip aspiration should be performed.96
Any wound that continues to drain should suggest infection. Aggressive surgical treatment of this is required, with washout of the joint and exchange of any possible bearing surfaces. Cultures should be taken prior to antibiotic administration, to help guide antibiotic treatment. If washout fails or if the infection is diagnosed after several weeks, strong consideration should be given to removal of the implants with 1-stage or 2-stage treatment and subsequent reimplantation. Implant removal can be a very difficult decision to make in a patient with frail hip fracture. The causative organism should be sought and sensitivities should guide treatment.
Loosening
Loosening is a late complication of arthroplasty. Any painful arthroplasty should be evaluated radiographically. Radiographic signs of loosening include lines around the prosthesis. A loose implant should be assessed for infection with ESR/CRP testing and aspiration of the joint. If this is workup is negative, the implant may be aseptically loose. Revision surgery is required with removal of the loose implant.
Fracture
Periprosthetic fracture may occur during component insertion or in the early or late postoperative period. Uncemented prostheses have a significantly higher rate of periprosthetic fracture than cemented prostheses.76–78 Intraoperative fractures, if noticed, can be treated with cerclage wires or cables. Treatment of postoperative fractures depends on whether the implant is loose or stable. The Vancouver classification is widely used to help guide treatment.97 Loose implants require revision and stabilization of the fracture.98 Stable implants require fixation of the fracture. Modified plates that allow for screw fixation around implants have been developed which are helpful for periprosthetic fracture fixation.
Dislocation
Dislocation is a known risk of arthroplasty. The risk is higher with total hip arthroplasty when compared to hemiarthroplasty. The risk of dislocation is higher if components are malpositioned. Typically, this is retroversion of the stem or cup. Surgical approach also can affect dislocation risk.27 Posterior approaches have a higher dislocation risk of hemiarthroplasty when compared to anterolateral approaches.95 In total hip arthroplasty, increasing the head size decreases dislocation risk. Risk of dislocation after total hip arthroplasty for hip fracture is thought to be higher than after total hip arthroplasty for osteoarthritis. It is unclear whether this is due to surgeon skill or anatomical differences. Some theorize that the hip capsule is tighter in patients with osteoarthritis and that the looser capsule or more normal capsule in a hip fracture patient allows for higher dislocation risk. Additional issues include retained fragments of bone in the acetabulum and improper head size for bipolar /monopolar replacement. A good “suction fit” between head and acetabulum is needed with hemiarthroplasty. Clearly, attention to component position is very important in arthroplasty after hip fracture. Dislocation of prostheses is generally treated with an initial closed reduction. If this is not possible open reduction must be performed. Strong consideration should be given to using larger head size or a constrained liner in patients with total hip dislocation. During an open reduction or revision surgery, component position must be very carefully checked and revised if indicated.
Wear
It is possible for there to be wear issues after both hemiarthroplasty and total hip arthroplasty. Hemiarthroplasty may lead to cartilage wear and acetabular erosion. Bipolar implants may also develop wear of the plastic liner after many years. Total hip replacement may develop polyethylene liner wear. Wear should be assessed radiographically at 5- and 10-year intervals after surgery. Significant polyethylene wear may cause osteolysis and in such cases revision should be performed.
Pain
Severe pain is thought to persist in about 6% of patients after hip replacement.99 Pain may occur for several reasons after arthroplasty. Hemiarthroplasty can be painful do to a mismatched head size to the acetabulum. It is possible for the acetabular cartilage to wear resulting in arthritic pain. Stiffness of the stem within the femur may cause proximal thigh pain. This is more common with uncemented fully coated prostheses. Soft tissue pain may occur around the trochanter or psoas tendon or posterior superior iliac spineregion. This is generally treated with physical therapy and injections.
Limp
Limp may occur do to damage to the abductor mechanism. Risk of limp is higher using an anterolateral approach compared with the posterior approach for arthroplasty. This may lead to a Trendelenburg-type gait. This will usually resolve in approximately 1 year.
Leg length discrepancy
Arthroplasty can lead to a leg length inequality. Careful trialing and templating can help reduce the risk of leg length differences. Treatment should be with a shoe lift on the contralateral side.
Open Reduction and Internal Fixation-Related Complications
Infection
Continuedredness or drainage after ORIF should suggest infection (Figure 5). Aggressive washout and debridement should be performed if drainage is not improving. Cultures should help guide the use of long-term intravenous antibiotics. Stable fracture fixation is left in place until healing occurs. Infection must be suppressed during this period. Unstable implants should be revised.
Figure 5.
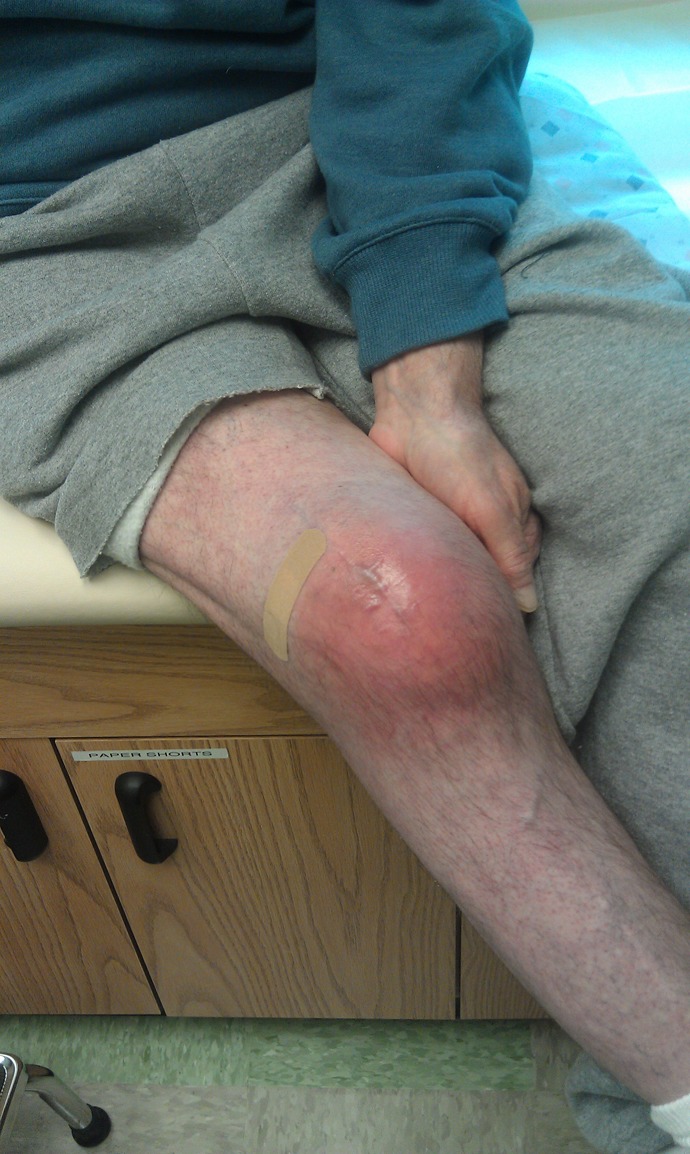
Wound infection after patella fracture surgery.
Nonunion/fixation failure
If fracture healing does not occur, eventually the fixation device will fail. With hip fracture fixation, the bone around the implant may also fail. This is especially true if positioning of the implants is poor. Most commonly this is due to superior positioning of a hip screw. In this case, the implant does not get purchase in the best possible bone (Figure 6). Poor positioning may lead to cut out of devices through the femoral head. This may occur with screws, sliding hip screws, or intramedullary hip screws. If cut out of a device occurs, the metal screw protrudes into the acetabulum which usually causes severe pain. Treatment entails conversion to an arthroplasty.100 If the fracture does not heal, treatment may vary depending upon the exact fracture pattern. Femoral neck nonunion is treated with conversion to arthroplasty. Intertrochanteric or subtrochanteric nonunion is a more difficult problem. This may be either treated with conversion to a complex arthroplasty or with consideration for refixation. Selection of treatment options will depend on bone quality and the exact nonunion pattern.
Figure 6.
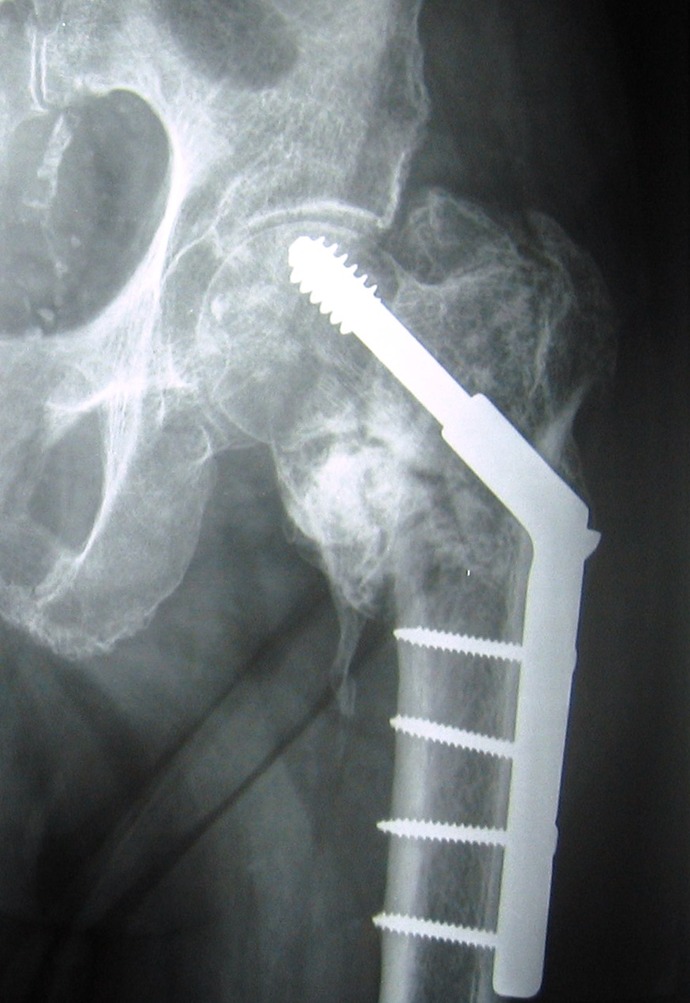
A nonhealing intertochanteric hip fracture with cutout of a sliding hip screw.
Shortening/leg length discrepancy
Hip fracture typically leads to shortening of the limb. This gives the patient a leg length discrepancy and leads to weakness of the hip musculature due to shortening of the lever arm around the hip. Initial treatment should be with a shoe lift. If the leg length discrepancy is bothersome enough to the patient and they’re healthy enough, the only way to get further length is to convert the repair to a hip arthroplasty. This may be a partial or total hip replacement depending on the activity level of the patient and the state of the acetabular cartilage.
Osteonecrosis/osteoarthritis
Osteonecrosis is a risk after repair of femoral neck fracture and less commonly after intertrochanteric fractures. Onset of osteonecrosis is delayed and occurs after 1 to 2 years. The head of the femur collapses leading to arthritis. Treatment is with conversion to hip replacement. Osteoarthritis may also be preexisting or worsen after fracture repair. Treatment is also with conversion to arthroplasty if the pain is severe enough.
Conversion to Arthroplasty
Conversion after screw fixation is relatively straightforward.101 The screws must be removed which creates a weak area in the lateral femur. Care must be taken not to split the bone and not to insert the stem outside of the femur. Conversion after a sliding hip screw is more complicated. The device must be removed. The hip should be dislocated first prior to implant removal. Dislocation puts a lot of force on the bone and puts the bone at risk of fracture after implant removal. Conversion after sliding hip screw can be more difficult due to heterotopic bone formation and trochanteric malpositioning. Conversion after intramedullary nail fixation is more difficult than after sliding hip screw. Removal of the nail can be difficult. The nail may be overgrown with heterotopic bone and damage to the abductors occurs with extraction. Conversion after nailing puts the patient at higher risk of greater trochanter fracture, abductor deficiency, limp, and dislocation.102
Stem selection can be based on surgeon preference but should have some sort of distal fixation for conversion after Sliding hip screw (SHS) or Intramedullary nail (IM) nail devices. The stem can be cemented or uncemented. Longer stemmed, calcar replacing, or modular implants may be required (Figure 7). The surgeon may select either a hemiarthroplasty or a total arthroplasty depending on the state of the acetabular cartilage and the activity level of the patient.
Surgical complications after hip fracture repair often result in further surgery to correct the problem.
Surgeons should try to avoid further surgery in hip fracture patients.
Most commonly, failure leads to revision surgery or conversion of a repair to a hip arthroplasty.
Figure 7.
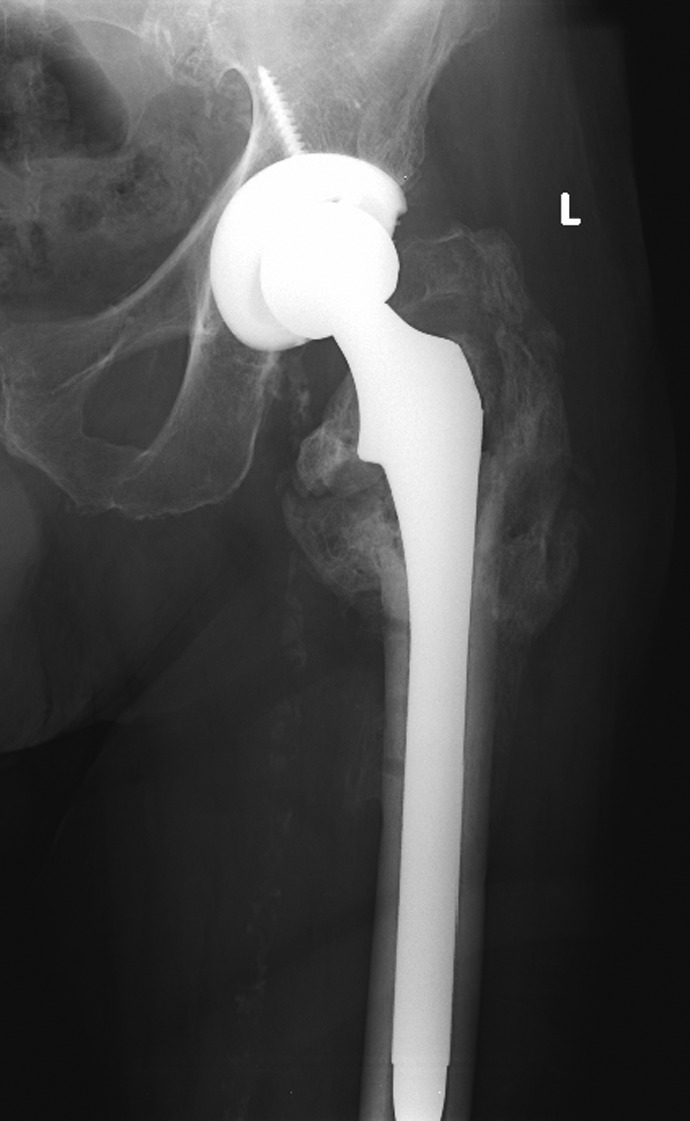
A long stemmed calcar replacing stem used to treat failed fixation in an intertrochanteric hip fracture.
Pain Management
Omar I. Ahmed, MD, Jean-Pierre P. Ouanes, DO and Frederick E. Sieber, MD
Assessment of postoperative pain in the elderly patients can be challenging for several reasons. There appears to be both an age-related increase in pain threshold and a tendency for older adults to underreport pain.103,104 Cognitive impairment can also make pain assessment and treatment difficult. In general, pain-intensity scales may be used for assessment. Numerical rating scales and verbal descriptor scales have been used successfully in cognitively intact elderly patients, whereas visual analog scales may lead to frequent nonscorable responses with the elderly patients.104 In patients with mild to moderate dementia, the 0 to 10 pain assessment tool and the verbal descriptor scale have been found to have adequate but not perfect reliability and validity.105 In patients with advanced dementia, pain assessment may be performed with one of several pain assessment tools available for seniors with dementia.105
If used intraoperative, peripheral nerve blockade can be continued into the postoperative setting with the use of continuous catheters. Local anesthetic delivered through these catheters target the appropriate nerves either in a continuous infusion or in patient-controlled modality. A recent systemic review of 83 studies looked at various pain management techniques for hip fractures in older patients.106 Overall, peripheral nerve blockade was seen as an effective way to reduce acute pain in this population while reducing the incidence of delirium. The use of peripheral nerve blockade reduces use of opioid and systemic analgesic interventions. Peripheral nerve blocks may be used both in the operating room for postoperative pain but also in the ED for fracture pain. In the ED, nerve blocks have been shown to significantly lower pain from the hip fracture.107,108 Implementation of nerve blocks in the ED can be difficult and either requires availability of a trained anesthesiologist or training of ED physicians in block techniques. An ultrasoundmachine in the ED is also helpful. Protocols to allow for this on a routine basis are necessary, and organizational roadblocks are common in organizing this service for patients.
When selecting opioids for pain management, there is no difference in cognitive outcome when comparing fentanyl, morphine, and hydromorphone109; meperidine is the only opioid that has been definitively associated with delirium, and it should be avoided.110 With regard to the mode of opioid administration, there is no difference in cognitive outcome between intravenous and epidural administration.109 To summarize the relationship between postoperative delirium and pain management with opioids in patients with hip fracture, the strongest evidence supports avoiding meperidine, and there is only weak evidence that the mode of administration is an important factor.
Intravenous patient-controlled analgesia (IV PCA) is a commonly used delivery method of systemic opioids in the postoperative setting. Because of its ability to take into account the wide variability between patients, IV PCA has been proven to be associated with better patient outcomes and satisfaction when compared to traditional nurse-administered bolus regimens.111,112 In the elderly population, IV PCA has been used successfully but with special considerations related to comorbidities, polypharmacy, decreased pain perception, declined physiologic reserves, and changes in pharmacokinetics.103,113,114 These factors warrant slow titration of opioids even in the PCA setting. Furthermore, patients with baseline dementia or cognitive dysfunction are generally poor candidates for IV PCA. Elderly patients are also at greater risk of developing respiratory depression, therefore a background or basal infusion of opioid is generally not recommended.
The push for multimodal analgesia is of great importance in the elderly population. Given the likely comorbidities and increased sensitivities to opioids, the usage of multiple approaches to treating pain should be utilized. Opioids themselves may induce delirium, and elderly patients may have increased cerebral sensitivity to them.115 Use of regional analgesia alongside nonopioid pharmacologic interventions, such as acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs), these pain treatment modalities act synergistically to reduce pain and spare the usage of opioids.116 Care should be taken however with the use of multiple pain medicines in the elderly patient. Some may promote delirium, and practitioners should be aware of the Beer list created by the American Geriatrics Society.117 For instance, scopolamine patches are a common adjunct used to prevent postoperative nausea in multimodal pathways for hip and knee replacement. In elderly patients, these patches put the patients at higher risk of delirium. Nonsteroidal anti-inflammatory drugs may cause acute kidney injury in those with renal insufficiency. Multimodal methods should be built into order sets so that poor medicine choices can be avoided. Effective control of acute pain and the reduction in chronic pain rely on a strong multimodal analgesic plan in the perioperative and postoperative periods.116,118 Early surgery is likely one of the best ways to decrease pain in the patient with hip fracture. After surgery, pain levels are relatively low with multimodal pain control.119
In summary, pain control for patient with hip fracture starts in the ED. Multimodal techniques are best and should be integrated into the clinical care pathway.27 Development of a service to provide peripheral nerve blocks in the ED may help to decrease pain while patients are waiting for hip fracture repair.
Keys to Pain Management in the Patient With Hip Fracture
Good control of postoperative pain reduces delirium and improves a patient’s ability to participate in rehabilitation.
Peripheral nerve blocks may help pain before and after surgery.
Multimodal techniques are helpful to decrease narcotic requirement.
Care is required to avoid medicines that may promote delirium in the elderly patients.
Early surgery is a good pain management strategy.
Wound Care and Infection Prevention
Stephen L. Kates, MD, and Amy Kates, MS, RN-BC
Wound infection is a serious complication that is best avoided. Prevention of infection has been studied for over 150 years. There are many factors involved in prevention of infection and they are reviewed below by type. It should be remembered that it is every health care provider’s obligation to try to prevent infection in their patients.
Host Factors are factors intrinsic to the patient. Some of these factors are disease states and others are related to patient behaviors. The host factors include Existing foci of infection elsewhere in the body have been shown to contribute to development of a surgical site infection, presumably through a hematogenous route in many cases. These include issues such as dental, gastrointestinal, urinary, and pulmonary infections and other bony infection foci.120,121 Control of other sites is recommended prior to elective and semielective surgery.121–124
Diabetes is a disease that is reported to be increasing in frequency. Diabetes control is often assessed by the glycosylated hemoglobin level (HbA1C). This is a modifiable risk factor in the perioperative period by medically assisting the diabetic patients to improve their glucose control for elective surgeries. When the surgery is urgent, glucose control should be done by protocol to keep the serum glucose level between 100 and 180 mg/dL.125,126 Nutrition is another modifiable risk factor for the development of infection. Patients may be malnourished as evidenced by history, examination, and laboratory findings of low serum albumin level <3.5g/dL and serum transferrin < 200 mg/dL and total lymphocyte counts < 1200 cells/mm3.127 Morbid obesity is an independent risk factor for infection.124 Particularly for elective surgery, nutritional interventions may be useful.123 Skin condition is sometimes a risk factor that can be modified prior to surgery. In many cases, the skin condition is not optimal for surgery. Infections, blisters, abrasions, and skin tears may cause delay in definitive care and require use of other treatments such as spanning external fixation until the skin condition has improved. Chronic skin conditions such as yeast infections and psoriasis can also be managed medically prior to surgery to reduce the risk of developing a surgical site infection. Some patients are chronic carriers of methicillin-resistant Staphylococcus aureus (MRSA), particularly in their nares. Decolonization of the nares has been shown to be helpful in reducing infections.121,126,128,129
Smoking is a risk factor for wound infection as well as delayed bone and wound healing.121 Smoking cessation should be encouraged. Immune system diseases such as HIV infection also increase the risks of surgical site infections130 as do autoimmune diseases such as Rheumatoid Arthritis.123 These can be managed medically but cannot be eliminated as risk factors. Steroid use is a risk factor for infection as well. There is some controversy as to the level of effect this medication has on infection rates. Disease modifying anti-rheumatic drugs such as antitumor necrosis factor and anti-interleukin 1 biologic antagonists in particular also increase a patient’s risk of surgical site infection or delayed wound healing.131 These medications should be discontinued prior to surgery and not restarted until the wound has fully healed.131 Patients from a lower socioeconomic status, patients with anemia, and patients with comorbidities (ASA score ≥3) also have an elevated risk of infection.121 Preoperative transfusion with allogeneic blood has been suspected to increase the risk of surgical site infection but the evidence for this is weak.
As hip fracture surgery is urgent and delay worsens results, many host factors cannot be improved as much as the practioner would like. In these cases, the risks of delay have to be weighed versus the risk of the correctable host factor.
Trauma Situations
Trauma situations offer many challenges when considering surgical site infections. The situation is urgent or emergent and there is less time to properly prepare the patient for surgery. The patient may present to the hospital with open, contaminated wounds, abrasions, blisters, and other sites of injury (Figure 8). These are several special situations that should be considered when prevention of infection is analyzed. The open wound may be contaminated with foreign material. When there is foreign material or an implant present, the bacterial load required to cause infection is markedly lower (100 organisms/gm of tissue).132 Formal debridement of the wound is an essential element in the care of open wounds. Likewise, abraded or blistered skin will increase the risk of developing a surgical site infection. Often, it is best to allow such skin to heal prior to performing a surgical approach through or adjacent to it. The burden of comorbidity carried by the patient also contributes heavily to outcomes. The patient with many comorbidities will be more likely to develop an infection with surgery.133 Additional features that carry a worse prognosis are deep wound contamination, necrotic tissue in the wound and delays in treatment.134
Figure 8.
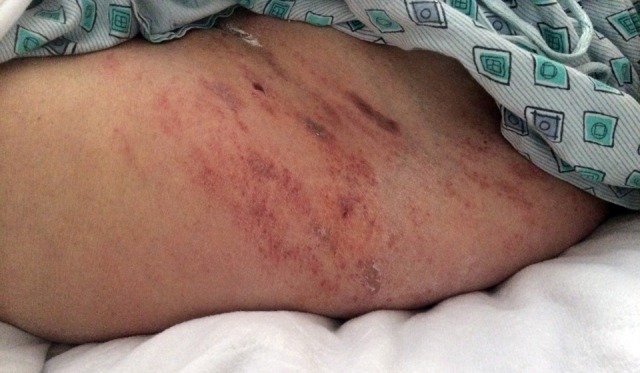
Abraded skin at the site of a hip fracture.
Preoperative Factors
In 2004, 63% of hospital-acquired infections were caused by MRSA.135 There are many preoperative factors that can be modified to help reduce the risk of surgical site infection. These include preoperative medical optimization of health issues, treatment of active infections elsewhere, fluid resuscitation, and rewarming, all essential in the patient with trauma.
Preoperatively, it is essential that correct preoperative prophylactic antibiotics be chosen for the surgery to be performed.126 Essentially, all cases in which prostheses are inserted, or open reduction and fixation of a fracture is performed, will need a first-generation cephalosporin (2 g) or vancomycin (1 g) for patients with penicillin allergiy. The antibiotic should be completely infused prior to incision.136 Redosing should be done if the surgery is greater than 3 to 4 hours of duration136 or for blood loss >2 L. A total duration of <24 hours is recommended.137 Hair removal should not be done with a razor as this increases the risk of infection.121 If needed, a surgical clipper offers the safest method for hair removal.121
Operating Room Factors
It is generally felt that many surgical site infections are initiated in the operating room. There are many possible factors to consider. These will be divided into “operating room,” “surgical team,” “surgeons,” and “facility factors”. Not all of the factors have evidence related to orthopedic surgery but have been accepted as important in infection prevention. Some cannot ethically be studied such as the use of rubber gloves or gowns.
Many infections in surgical sites come from the patient’s own flora.121 Thus, preparation of skin is vitally important in prevention.138 The skin should be initially cleansed of any gross contamination with an antibacterial soap. The actual preparation has been shown to be superior when chlorhexidene gluconate (CHG) with alcohol is used compared with iodoform-based antiseptics.139 Iodine with alcohol has also been shown to have very low infection rates.137,140 Occlusive iodine-impregnated drapes help to reduce surgical site infection141,142; however, this has not been conclusively demonstrated in orthopedic surgery.143 Many surgeons traditionally change scalpel blades after the skin incision. There is no evidence to show this has reduced infection rates.144 The irrigation fluid used during the irrigation process and the mode of delivery remain controversial topics. The low pressure irrigation systems cause less muscle damage but are somewhat less effective in removal of contamination.145 Despite this, low pressure irrigation seems a best practice for infection reduction in open fractures. Irrigation containing castile soap, benzalkonium chloride, bacitracin and other antibiotics has been studied. The detergents seem most effective in reducing contamination but must be washed out with saline to reduce risks of wound dehiscence.146,147 Operating room traffic has been shown to increase infection rates in several studies148–150 and thus should be minimized.
Hemostasis is important to reduce hematoma formation which can predispose to infection.121,123 The use of drains has not been shown to reduce surgical site infections after fracture fixation or arthroplasty.151–153 The wound should be closed in a manner that allows healing to proceed primarily.137 Bandages should be occlusive for at least the first 48 hours to improve healing and reduce infection.154,155 During the perioperative period, the patient’s body temperature should be maintained between 36°C and 37.5°C.156 Reductions in core temperature of 1.9°C have been shown to triple the incidence of wound infections with colon surgery.157
The surgical team concept is important in many ways to the success of an operation. The team itself may also contribute to the rate of infections experienced by the patient.126 Team members should all wear appropriate impervious gowns and protective gear. Minimized talking and movement helps to reduce infection.158 The team members should all be competent at their roles126 and can communicate well together to improve safety.159 To minimize the infection risk, all members of the team should cleanse their hands/forearms with CHG solutions for at least 2 minutes.137 Gloves used should be inspected for damage regularly and should be powder free.140 Double gloves are recommended for orthopedic surgeons. Changing the outer gloves at least hourly is advised for surgeons. The team should observe each other and external personnel such as observers for breaks in the sterile field.158 The team should minimize their own traffic and not take breaks during surgery if possible.158
The surgeons contribute to the infection prevention effort in many ways. The surgeon should foster a culture of safety in the team and promote it. The surgeon’s level of experience and skill contribute to duration of surgery, particularly for the routine or frequently performed procedures. Duration of surgery contributes to development of surgical site infections—shorter is better.122,160,161 Clean scrub attire and head covers should be worn at all times in surgery.158
The Facility itself may contribute to reduced infections. Ultraclean air is recommended for operating rooms with frequent (15/hour) air exchanges.121,137 Laminar airflow is controversial in efficacy. The environmental surfaces in the operating room should be kept clean after each surgery.121 Instruments should be sterilized in the sterile processing area for a full cycle of sterilization.121,158 Flash sterilization should be avoided and is not as good as full sterilization.121,158 The facility should supply an adequate number of clean scrub clothes for the surgical team to wear and change as required.158 Construction in the operating room area is a particular risk for contamination of the room environment and introducing unwanted contamination or leaking fluids. Appropriate measures must be taken to avoid this contamination.158 During warmer season, insects may enter the operating room area and appropriate efforts to eliminate them must be undertaken.
Postoperative Period
The postoperative period is important as well. Wound care should include an occlusive dressing that remains in place for at least 24 to 48 hours or longer. Prophylactic antibiotics should be used for less than 24 hours.126 All personnel who have contact with a surgical wound should be gloved, preferably with sterile gloves.121 There is evidence that the physicians should wash their hands before and after examining wounds. Dressing changes with antibiotic ointments lessen surgical site infections.137 Other issues include avoiding allogeneic blood transfusions which is controversial but transfusion seems to increase the likelihood of infection.123 Finally, postoperative glucose control helps the patients lessen their risk of infection.125 This is most effectively done with a standard glucose control protocol. Anticoagulation should be carefully dosed and monitored to avoid hematoma formation. It is important to avoid postoperative falls in the hospital that can cause wound dehiscence. The distance between patient beds and hospital occupancy seems to contribute to infection in some studies.162,163
Postdischarge management should also include careful management of anticoagulation. The wound itself presents a controversial issue. There is not adequate evidence of best practices for bandaging. Monitoring for signs of infection should include observation by the patient and family members. Sutures or staples should not be removed until the incision has healed fully to prevent dehiscence.
Staff education is an important element of any prevention program—ideally covering many of the issues listed earlier. Finally, patient and family education is essential to allow the patient to partner with the surgeon to achieve the best outcomes.
Deep wound infection involving the implant is uncommon but devastating in its impact: Approximately half of such patients die and few survivors regain mobility.12
The entire team must recognize the importance of infection prevention throughout the hospitalization of the patient with fragility fracture.
Infection prevention includes maximizing host factors as well as meticulous intraoperative and postoperative care.
Fluid and Blood Management
Stephen L. Kates, MD
In the postoperative period, careful fluid management is essential for a good outcome. It may prove difficult to determine whether the patient is normovolemic, dehydrated, or fluid overloaded. Maintaining the patient’s urine output without diuretics at a rate of 30 to 35 mL/h or 250 mL/8 hours is usually an acceptable indication of normovolemia. The experienced medical consultant following the patient regularly is usually in the best position to provide advice on this issue. It is generally best to use an isotonic saline solution to assure volume adequacy while monitoring serum electrolyte laboratory values for hypokalemia, hyponatremia, or bicarbonate changes. It has been shown that properly hydrated patients have better survival rates.164 Those patients admitted with an elevated serum blood urea nitrogen have been shown to be at increased risk of mortality and require extra attention to fluid management.165
Increasing evidence suggests that allogeneic blood transfusions may be harmful to patients and may contribute to infections.166 Increasingly, many clinical practice guidelines are recommending restrictive policies should be used regarding red blood cell transfusions. There is good evidence as to the appropriate hemoglobin level ≥8 g/dL is appropriate for the elderly patient with cardiac comorbidities after hip fracture based on the NIH sponsored “FOCUS” trial “Safety and Effectiveness of Two Blood Transfusion Strategies in Surgical Patients With Cardiovascular Disease.”42 Another study has shown that there was no reduction in incidence or severity of delirium in individuals after hip fracture surgery when hemoglobin levels were less than 10 g/dL.167
Hydrate patients to achieve urine output of 30 to 35 mL/h using isotonic saline
Based on current best evidence, the red cell transfusion threshold should be hemoglobin <8 g/dL.42
Pressure Sore Prevention
Stephen L. Kates, MD, and Amy Kates, MS, RN-BC
Pressure sores have a very negative impact on the recovery of the elderly patient with a fracture. They are often painful and interfere markedly with the patient’s rehabilitation efforts. Pressure sores take months to heal and often become infected, which may result in wound infection, readmission to hospital, additional surgery, or death. Regions to be checked include the buttocks, hips, heels, and elbows at least daily for the development of redness or blister, which indicate a beginning pressure sore. The most commonly used prediction tools are the Norton and Braden scales. The Braden scale assesses risk level based on a point system for sensory perception, moisture level, activity level, mobility, nutrition, friction, and shear using scores from one to three or four.168 The maximum total score is 23; a score of 18 or less indicates high risk. The Norton scale uses a 1 to 4 scoring system and rating patients in each of 5 subscales, namely, physical condition, mental condition, activity, mobility, and incontinence. A score of less than 14 indicates a high risk of pressure ulcer development. The Norton scale generally identifies more patients at high risk than the Braden scale.169 A recent study has shown that handgrip strength accurately predicts development of a pressure sore in the hospital and at 30 days.170
A pressure sore can be staged171,172 by determining whether it has partial or full thickness skin loss or by grading it on a 1 to 4 Braden scale: stage 1, non-blanching erythema of the skin; stage 2, partial-thickness skin loss, such as a blister or shallow ulcer; stage 3, a deep ulcer not penetrating the fascia and with no undermining; and stage 4, extensive soft-tissue loss with exposure of tendon, muscle, or bone and undermining of the skin.
Treatment of the pressure sore is based on stage and involves relief of pressure and shearing stresses on the skin, debridement of any necrotic tissues, and dressing changes. Rarely, surgical coverage with a muscle flap is required.
Avoiding the pressure sore is the best approach.27 Early surgery has been shown to reduce the risk of pressure ulcer.173,174 Frequent repositioning of bedbound patients with hip fracture was not shown in a recent study to reduce the risk of development of pressure ulcer.175 Early mobilization following surgery seems to be a useful approach to avoidance of pressure ulcer. Pressure-reducing mattresses and surfaces have not been shown to reduce development of pressure ulcers in a recent study.176 Avoidance of pressure sources such as avoidance of compression stockings in bed or braces is also a useful technique. Avoiding or minimizing delirium (see earlier discussion) will reduce the likelihood of developing a pressure sore.171 Nutritional status seems to have an important role in development of pressure sore and should be attended to during the hospital stay.
All patients with a hip fracture should be assessed and cared for with a view to minimizing development of a pressure ulcer.27
Thromboprophylaxis
Stephen L. Kates, MD
The development of a perioperative thrombosis is a common event in the elderly patient with a fracture. One study has shown a higher risk of developing VTE in patients with intertrochanteric and subtrochanteric fractures when compared with femoral neck fractures.177
It has become a standard of care in most hospitals in the United States to use a prophylactic strategy for hospitalized patients with a lower extremity fracture.27,43,178 However, currently there is no one accepted standard of prophylaxis and controversy exists. For example, mechanical means include sequential pneumatic compression devices and foot pumps placed on the legs; these devices are somewhat effective in the reduction in thrombosis.179,180 Compression stockings are of limited benefit and must be carefully applied and removed to prevent skin injury. To avoid the development of a pressure sore, such stockings should not be left on the elderly patient with a fracture while in bed. Mechanical devices may also serve to tether the patient to the bed and thus increase the risk of falls and delirium. Early surgery and early mobilization have been shown to reduce the likelihood of thrombosis and should be instituted whenever possible.43
Pharmacologic Prophylaxis
Pharmacologic means commonly used to prevent VTE include unfractionated heparin, low-molecular weight heparin, warfarin, and factor XA inhibitors.
Heparin and low-molecular weight heparins
Heparins significantly reduce the risk of venous thrombosis and embolism, but they also increase the incidence of bleeding into the wound and at other sites. Considerations for use of the low-molecular weight heparins include its high cost and the need to inject the medication subcutaneously. Weekly platelet counts are required to check for the development of heparin-induced thrombocytopenia. Low-molecular weight heparins such as dalteparin and enoxaparin have been shown to be very effective as prophylaxis of VTE after hip fractures.43 Unfractionated heparin is typically used as a twice daily subcutaneous injection and is inexpensive. It is also effective as a prophylactic agent, particularly in the inpatient setting where the twice daily administration is less problematic. It also carries the risk of heparin-induced thrombocytopenia.
Warfarin
Warfarin inhibits the production of vitamin-K-dependent coagulation factors in the liver. It has a long half-life, and dosing is often troublesome in the elderly patients. Effects of the dose are not seen until 48 hours after the dose is taken orally. Although warfarin is inexpensive and easy for the patient to take, it requires frequent, often inconvenient, and in the aggregate expensive laboratory testing (INR) to monitor and adjust dosage. It may cause bleeding complications, particularly if the INR values are greater than 3. The effects of warfarin are reversible with the administration of vitamin K orally or parenterally.
Factor XA inhibitors
This newer class of medications inhibits activated factor X and thereby anticoagulates the patient. Fondaparinux sodium is very effective for thromboprophylaxis, but it also can result in bleeding complications.181 It is currently available in a subcutaneous form and is costly. Fondaparinux is recommended as the best primary choice for VTE prophylaxis by the National Institute for Health and Clinical Excellence in the United Kingdom.182 A recent manuscript has demonstrated efficacy of rivaroxaban, an orally administered factor Xa inhibitor to be efficacious in management of lower extremity fractures. It is not approved for this use in the United States at this time for VTE prophylaxis in patients with hip or lower extremity fracture.183 Factor Xa inhibitors are not reversible with medication.
Aspirin
The American Academy of Orthopaedic Surgeons (AAOS) and American College of Chest Physicians have both produced evidence-based guidelines on VTE prophylaxis in patients with major joint replacement and hip fracture. They are in concordance on the use of aspirin. Aspirin is not an appropriate sole option for prevention of VTE after hip fracture184 but can be considered as an option if part of a multimodal approach to prevention including other means such as mechanical compression devices.185 Aspirin has not been found to have a lesser risk of bleeding complications when compared with other pharmacologic options.185
Summary
Pharmacologic prophylaxis for VTE should be undertaken postoperatively for all patients with a hip fracture. Because the available evidence is mostly based on consensus statements from various organizations, the choice of therapy is the clinician’s preference. Fondaparinux or low-molecular weight heparin for 28 to 35 days after surgery seems to be the best evidence-based recommendation at this time.43,186,187 Warfarin is an alternative reasonable choice for therapy and is often used in patients who were taking warfarin prior to fracture.
All patients with a major lower extremity fracture should receive prophylactic anticoagulation for pharmacologic postoperative prophylaxis—unless strongly contraindicated.27
Nutrition
Stephen L. Kates, MD, and Amy Kates, MS RN-BC
Nutrition is an essential part of care of the elderly patient with a fracture.27 Proper nutrition allows for uneventful wound healing and, ultimately, better recovery. The patient who is unable to eat postoperatively has a very poor prognosis. Malnutrition is a part of the geriatric syndrome known as “frailty.”188 Malnutrition was found in 48% of patients with hip fracture in a recent study.189 A serum albumin level less than 3 g/dL has been associated with poor outcomes after hip fracture.190
Screening for malnutrition has been studied. Screening tools while quick and easy do not perform as well as a complete nutritional assessment in correctly diagnosing malnutrition.189 Routine nutritional assessment should be standard in management of older patients with fragility fracture.189,191
Generally, patients with fragility fracture should be fed orally. Nasogastric feeding is uncomfortable, likely a precipitant of delirium, is associated with aspiration pneumonia, and should be avoided. Parenteral feeding should also be avoided if at all possible, as it has a risk of sepsis, metabolic abnormalities, and delirium.
The diet should consist of small portions with high-caloric content. Foods should be easily chewable because many elderly patients have impaired dentition. Nutritional supplementation consisting of liquid oral supplements between or with meals may be useful for decreasing complications, improving rehabilitation, reducing pressure sores, and improving muscle strength. Some high-caloric drinks or shakes may not be well tolerated by the elderly patients, and assistance from a dietician is often very useful. A recent study of a multidisciplinary nutritional approach to patients with hip fracture showed improved nutritional intake and better outcomes for the patients.192 Another recent study showed the Mini Nutritional Assessment to be predictive of gait status and mortality at 6 months after hip fracture.193 Cost-effectiveness of nutritional interventions has been studied as well and found to be effective at 3 months when the result was weight gain.194 However, the authors noted no improvement in quality-adjusted life-years.194
Proper nutrition of the patient with a fragility fracture is an essential element for a successful recovery.
Rehabilitation
Stephen L. Kates, MD, and Simon C. Mears, MD, PhD
The goal of rehabilitation after fracture is to restore the patient to the preinjury activity status. This is a difficult goal to achieve, as many patients lose functional status and independence after hip fracture.
In most cases, rehabilitation should begin immediately after surgery. The patient should be mobilized to stand and then walk with a walker as soon as possible after surgery but always within 24 hours. The preinjury functional status is the therapeutic target and should be the basis of planning of the rehabilitation program. In the United States, patients are typically transitioned to an acute rehabilitation center or a subacute nursing facility depending on their ability to perform 3 hours of rehabilitation per day. In some limited cases, patients with very high functional status may be discharged home with home services. Overall, long-term differences in outcomes between these different approaches have not been seen.195 The transition period is one of particular vulnerability for the hip fracture patient.196 Meticulous attention to detail in the discharge process is very important to decrease complications and readmissions.
Weight bearing as tolerated should be recommended for patients with hip fractures. In addition, most elderly patients cannot comply with limited weight bearing restrictions. Appropriate pain control will allow the patient to participate effectively in his or her rehabilitation. There is some evidence that scheduled dosing of pain medicine may improve results of rehabilitation.197 In many cases, however, delirium and dementia interfere with rehabilitation. Delirium should be prevented to allow rehabilitation to progress. Dementia frequently gives care providers problems in the rehabilitation process and slows rehabilitation.198,199 Rehabilitation programs have been shown to be effective in the patient with dementia although the best approach is currently unknown.200
The length and intensity of rehabilitation after hip fracture is a topic of great importance. Studies have shown that long periods of rehabilitation improve function.201,202 A recent randomized trial examined an extended home program using a physical therapist. Improved results were seen using this approach.203 The cost of long-term approaches is of huge importance in the current heath care environment. Currently, there is no consensus on the best method for the rehabilitation of the patient with a fragility fracture, and this area requires additional study.204
All patients with hip fractures should be weight bearing as tolerated after surgery.
The best type of rehabilitation program is unknown although extended rehabilitation improves function over time after hip fracture repair.
Models of Care in Current Use in the United States
Stephen L. Kates, MD
There are several different models of care in current use in the United States, and there is some evidence to suggest that improvements in the system of care will improve patient outcomes and costs of care.11,32,33,205 When considering how to care for a patient with a fragility fracture, there are several models of care to be considered, each of which represents a different system or approach to the delivery of care. The common models in use in the United States are traditional care, closed panel-health maintenance organization (HMO), and comanaged (Rochester model).
Traditional Care
In this model of care, the patient with a fragility fracture enters through the ED and is evaluated. This evaluation is often delayed because elderly patients tend to suffer quietly and are perceived as low acuity problems to assess. The diagnosis may be quite apparent to the nurse triaging the elderly patient, but they are frequently placed in the hallway or back of the ED. When a decision is being made to admit the patient to the hospital, there is frequently a dispute that occurs between the medical and the surgical physicians as to who should accept the patient onto their hospital service. This type of “turf war” is unfortunately common, and the patient becomes the victim in such a case. Such a situation must be avoided.
Nonetheless, once admitted, the patient must be seen by a surgeon and often by a medical physician for “clearance” for surgery. Many medical physicians feel uncomfortable with this role of giving “clearance” for surgery, and they request specialty consultations and additional testing before surgery is approved. The result is a delay in surgical intervention that can be especially detrimental for an elderly patient.
When the patient has been cleared for surgery, the anesthesiologist becomes involved. An unclear clearance note or a perceived lack of diagnostic testing may result in surgery being delayed or canceled.
In most cases, postoperative care is dependent on the surgeon. The comorbid conditions may present substantial challenges medically in the postoperative period. Often, patients are restricted to “nonweight bearing” status by the surgeon, which interferes with their ability to participate in rehabilitation and typically relegates the elderly patient to a bed-to-chair activity status.
Discharge to a SNF is common, and the patient may or may not recover from the injury. In most cases, there is no treatment prescribed for osteoporosis upon discharge nor is there a referral made for treatment of the osteoporosis.
Closed Panel HMO
The patient is admitted to a designated facility for care or transferred there if originally admitted to a nonparticipating hospital. The patient is usually admitted to the hospitalist and assessed medically. Surgery is typically mandated within 24 hours of admission. Postoperative care is provided primarily by the hospitalist, with the orthopedic surgeon as the consultant. At the 72-hour point, the stable patient is transferred to inpatient rehabilitation, which is also operated by the HMO. This procedure results in a very short length of stay and very orderly care. Follow-up care is arranged by the closed-panel HMO and may not be with the operating surgeon.
This model of care has resulted in a very successful rate of post-fracture osteoporosis management. Kaiser Permanente’s “Healthy Bones Program” is one such system that has published successful outcomes.206
Comanaged Care (A Care Model Used at the University of Rochester and Other Institutions)
In this model of care, an emphasis is placed on the rapid admission of the patient through the ED or as a direct admission to the floor from other facilities. A fast-track approach is undertaken in the ED, with rapid admission after assessment of medical stability. The patient is admitted by agreement to the orthopedic surgery service. The patient is seen by the orthopedic surgeon, and then a consultation is obtained from the geriatric medicine/hospitalist service. The emphasis of this consultation is to ensure medical optimization for early surgery. A detailed assessment of the comorbid conditions and medications is also obtained. The patient is risk stratified for the appropriate operative risk level. Additional consultations and diagnostic testing are rarely obtained.
Early surgery, typically in less than 24 hours, is provided for all optimized patients. The risk stratification and comprehensive assessment is reassuring to the anesthesia physician, and thus cancelation of surgery is a rare event.
Postoperatively, all patients are comanaged by medicine and surgical services, and care is by standard protocol. Patients are advised to bear weight as tolerated so they may participate effectively in their rehabilitation. The stable patient is discharged on the third hospital day. This model of care has been shown to result in reduced length of stay, reduced complication rates, and lower costs than that of usual care.11,32,33,207 Another group of authors found that this model of care reduced hospital stay and time to surgery at their center.208 One recent manuscript demonstrated that most clinical outcome measures improved significantly with implementation of this care model.209 One unintended consequence was an increase in in-hospital mortality during the implementation phase of the program.209 Yet another program showed that implementation of such a program reduced complications, reduced length of stay, and resulted in restoration of prefracture place of residence.210 A recent systematic review and meta-analysis of orthogeriatric care models concluded that collaboration between orthopedic surgeons and geriatricians could improve mortality after hip fracture.211 Routine scheduled geriatric consultation was recommended for care of older patients with fracture.211
Summary
The system or model of care used has a profound impact on the quality of care and outcomes for the patient with a fragility fracture. Standardizing care will provide better care to such patients. Attention to details and avoidance of adverse events should be important goals when instituting such a system. Physician leadership and collaborative interdisciplinary care are fundamental concepts in such a system. Improvements in quality will directly result in improvement in costs of care. An organized and standardized system of care for the patient with a fragility fracture will afford a better outcome for that patient and be of benefit to the health care system.27
Costs of Care
Stephen L. Kates, MD
Health care costs in the United States have assumed a serious front-page role in the public’s awareness. Costs have become a hotly debated topic in political, economic, and business forums. Several public groups have run educational events on ways to reduce health care costs and improve health care economics. Hip fracture is the third most costly diagnosis in American medicine.212 Despite this problem, there has been little progress on reducing health care costs, although the pace of growth seems to have slowed somewhat in 2013. Typically, costs of care are inversely proportional to quality of care.213,214
Why is hip fracture care so costly? In 2007, average hospital charges were ∼US$42 000 for hip fracture based on the Agency for Healthcare Research and Quality (AHRQ)/Health Care Utilization Project (HCUP) data.19 The few analyses that have been performed on costs show that most of the expense is incurred on the first few days after hip fracture.215,216 However, it should be noted that costs continue to accrue during the postacute care phase of recovery after hip fracture. High charges are incurred from skilled nursing facilities, home care services as well as lesser charges for durable medical equipment, prescription drug charges, and physician charges. Adding to this problem is that 14.5% of patients with hip fracture are readmitted to a hospital facility within 30 days of discharge.217 The readmission charges are frequently similar to the costs of initial hospitalization (Kates et al., submitted for publication, GOS).
It is clear that our present system of care for the patients with hip fracture (and fragility fractures in general) is expensive and is not providing acceptable value of care (high mortality, large number of readmissions, and frequent poor outcomes). It is unlikely that specific surgical improvements will have any effect on improving the situation. Additionally, simply changing the payment model will be unlikely to have significant impact on improving the value of care. A complete retooling of the present system of care for fragility fractures is needed to achieve double-digit impact on outcomes and costs.215,216 Lean business methods have been proposed as a method for improving health care in general.218,219 A recent publication details this process for geriatric fracture program development.213 Even with lean business methods, some of the fundamental problems of coordination of care, misaligned incentives, and lack of an integrated system of care will all have adverse impacts on costs of care. The misaligned incentives in particular work against significant cost improvements. Each provider in the present system is incentivized to maximize profitability rather than reduce costs of care. This will, no doubt, be a subject of great debate and intense effort over the ensuing decade.
Some methods to improve costs of care for fragility fractures include utilization of the lean business model when designing the system of care.213 Additionally, integrating the system of acute care with the system of postacute rehabilitation care and outpatient care as a “bundle of care” may offer the appropriate incentives to reduce costs and improve quality. Some methods of inpatient cost reduction are already well described.213,215,216 A comprehensive geriatric fracture program system requires somewhere between 70 and 100 patients per year to be cost effective to the hospital in the present payment model.214,220 Table 1 describes some suggestions for cost improvement.
Table 1.
Methods to Reduce Costs of Care for Fragility Fracture Patients.
| Area for attention | Method | Expected outcome |
|---|---|---|
| Time to surgery | System changes—prioritize geriatric fracture cases, operating room should be available for these cases | Reduced length of stay and costs, improved outcomes, improved patient satisfaction |
| Iatrogenic errors with fragility fracture cases | Implement standardized order sets | Reduced length of stay, reduced readmissions, reduced costs, reduced errors |
| Implant costs | Negotiated lower costs with vendor, use single vendor | Reduced errors in operating room, reduced storage for implants, reduced costs |
| Prescription drugs | Use standard drug regimens in standard order sets, design protocols with help of pharmacists | Reduced errors in prescribing; drastically reduced costs |
| Delirium | Improved pain management by protocol, allow patient to keep glasses and hearing aids, avoid tethers | Reduced length of stay, improve participation and rehabilitation, reduced pressure sores, improved satisfaction, reduced costs |
| Standardized medical consultation | Use of a standardized consultation form | Reduced time to surgery, reduced likelihood of cancelation of surgery, reduced length of stay, and reduced medical errors |
| Medical complications during hospital stay | Medical comanagement by design on a daily basis. A designated team of consultants seems to work best | Improved recognition of problems, reduction in complications, and improvement in satisfaction |
| Osteoporosis management | Standard protocols for diagnostic testing and treatment postfracture, implement a fracture liaison service | Reduced refracture rate and improved patient outcomes. Will save money for a system in the long run |
| Pressure sore avoidance | Improved nutrition and reduce time to surgery | Reduced readmission and patient safety issues |
| Eliminate unnecessary testing – (advanced imaging and echocardiography) | Standard order sets and protocols | Reduced costs, reduced length of stay, improved patient safety by eliminating unnecessary testing |
| Errors in surgical decision-making | Standardized surgical protocol—surgical poster as a “Point of service tool” | Use evidence-based best practices to fix fragility fracture correctly |
| Medication errors | Medication reconciliation done by medical consultant—many drugs can be stopped | Reduced medication costs, drug–drug interactions and errors |
| Discharge problems | Standard social work consultation on admission with discharge planning beginning on admission | Reduced length of stay and improved patient and family satisfaction; reduced length of stay |
| Readmission within 30 days | Correct any new problem that occurs during hospitalization prior to discharge, short time to surgery, proper bowel regimen, better handoff to receiving facility | Fewer readmissions reduced mortality rate |
| Blood transfusions | Implement evidence-based guidelines—transfusion threshold Hb < 8 | Reduced costs, reduced infections |
Cost of Care
Average costs per hospital episode are US$42 000.
Current system leads to maximized charges.
Bundled care may provide better incentives to improve care and decrease costs.
Standardization and lean management principles offer opportunities to decrease cost in hip fracture care.
Data Collection, Quality Assurance, and Research
Steven Olson, MD
The goal of this text is to enable the clinician to use the best available evidence to guide clinical care of the geriatric patient with low-energy fractures. Reaching this goal is dependent upon the availability of robust outcome data in this patient population. The area of outcomes assessment has also grown rapidly in the past decade. Increasingly, research into outcomes is segregating into 2 major areas, namely, clinical outcomes and quality improvement.221,222 The area of outcomes research in clinical medicine assesses the ability of treatment interventions to improve the health of patients diagnosed with specific clinical pathology. Most clinicians are familiar with this type of research. Research into clinical outcomes seeks to understand what diagnostic or treatment modality leads to improvement in patients outcomes,222 whereas quality improvement research examines the processes and defect rates in the delivery of health care. This type of research has been traditionally the focus of hospital administrators and is relatively new to many clinicians. Research in quality improvement seeks to understand how well treatment is delivered to the patient.221,223 Newer concepts such as cost-effectiveness and value span both of these lines of research. Value is defined simply as the benefit/cost of a treatment.224 The benefit is often a clinical outcome measure. Whereas the denominator requires critical evaluation of the resources we use to deliver health care through techniques like cost-effectiveness analysis. Robert Kaplan and Michael Porter of Harvard Business School have highlighted the need for improvement in measuring costs before when can expect to effectively control them225.
The role of data in research
Clinical data may also be gathered to gain new, generalizable knowledge for research. Data gathered for purposes of answering a research question will need to be carefully collected and should be of the highest quality. Obtaining research data requires expertise in data collection and database management, which are often best done by a dedicated data manager or research associate. An operational definition for each data point is central to maintaining reliability of data. Because database integrity is of the utmost importance, integrity and validity checks need to be performed routinely.
There are many important areas of fragility fracture care that need active ongoing research. Some aspects of clinical practice are studied because of a short-term outcome. An example a short-term outcome is pain control in the perioperative period for patients with hip fracture. Strong clinical evidence indicates that regional analgesia techniques improve preoperative pain control.226,227 The use of regional techniques adds an additional expense in the initial care of the patient. Research into the effect of regional analgesia techniques in overall opioid medication use, incidence of delirium, or length of stay is needed. An improvement in any of these parameters would provide a means to add the resources required to provide this treatment in the initial care as well as identifying which patients benefit from these techniques. Additional needed research can be identified throughout the continuum of care delivery for patients with fragility fractures.
Research examining the geriatric patients with hip fracture will require a range of different clinical outcome parameters. These will include basic information such as patient demographics (age, gender, pre-injury living situation, etc) and fracture outcomes (union rates, time to union, hip range of motion, etc). Outcomes scores are sorted into the types of outcomes that are measured.222 Joint specific measures, such as the Harris Hip Score, will measure joint function parameters. Condition-specific outcome measures may include the Musculoskeletal Function Assessment (MFA) or Short Musculoskeletal Function Assessment (SMFA) or Western Ontario McMaster osteoarthritis index (WOMAC) and will measure musculoskeletal function. Quality-of-life measures will assess the overall function of a patient using a SF-36, or EuroQol as a measures. Some of these measures such as the EuroQol allow the investigator to calculate a quality of life-year measure that is useful for cost-effectiveness analysis.222 Often all 3 of these types of outcomes may be measured. In addition, in the geriatric population with hip fracture, a measure of mental status function may be necessary to validate these other measures.222
The Role of Data in the Development of a Fragility Fracture Program
Fragility fracture programs typically have 2 distinct components;, a clinical pathway for management of acute orthopedic injuries and related medical comorbidities and a clinical pathway to ensure that osteoporosis treatment is started and appropriate follow-up treatment is arranged after discharge. These programs typically involve at least 3 or more medical specialties, namely, Orthopaedic Surgery, Hospital Medicine, Anesthesiology, Geriatrics, Endocrinology, or Emergency Medicine.
Performance and outcome data are critical in gaining and subsequently maintaining administrative support for special orthopedic care programs. It is helpful for the clinician to have an understanding of how hospital administrators perceive value created by an efficient orthopedic surgery practice.228 When starting a program, it is important to establish a working partnership with the hospital or health system. Hospitals in the United States that receive funds from Centers for Medicare & Medicaid Services (CMS) actively track a number of data points for reporting to a variety of organizations.229 These typically include assessment of in-hospital mortality, length of stay, reoperation rate, and readmission rate. Other important data points to consider include time to operation, percentage of patients discharged on osteoporosis treatment, and so on. This information should be compared with available benchmarks from national, international, or other regional medical centers providing care to such patients. Organizations such as University Hospital Consortium or American Association of Medical Colleges provide bench mark data for a variety of medical conditions.
The comparison of the health center’s current practice to benchmark data or peer-reviewed published best practices can highlight opportunities for improvement. Data collection should address the areas of opportunity along with major national bench mark data points. Assessment of the yearly volume of admissions, types of procedures, costs, and reimbursement allows for financial planning and sets the stage for developing a realistic business plan. Documentation of changes over time provides evidence of program efficacy and sustainability. Data are also important for compliance and billing purposes.
The Role of Data in Quality Assurance
Medical quality of care can be improved by evaluation of individual cases, individual incidents, and trends. Regular morbidity and mortality review is important to identify clinical issues and to reinforce best practices. Review of the cost of care by treating surgeon or medical provider can help identify differences in practices, implant use as well as complications. Program managers can use an individual provider’s clinical outcome data to respond to incidents or to help educate and guide a provider whose performance falls below expectation; data can also be used to recognize and acknowledge individuals whose performance exceeds expectation. Most surgeons are very competitive by nature—making appropriate comparison data available within a physician group can drive change in an individual physicians practice.
Study of care delivery data allows the physician and hospital leaders to identify opportunities for improvement in the day-to-day delivery of care. Data collected on process measures (such as time spent in the ED, pain assessment and management, completion of falls prevention education, time to start of physical therapy, intensity of medical comanagement, and time to indwelling urinary catheter removal) can be tracked and compared with benchmarks. Data provide the foundation for process improvement.
Potential Performance Measures
The Institute of Medicine (IOM) published its landmark document “To Err Is Human: Building A Safer Health System” in 2000.230 This work addressed the issue of medical errors. The IOM defined medical errors as the failure of a planned action to be completed as intended or the use of a wrong plan to achieve an aim. The IOM outlined 4 goals. Goals 2 and 3 require the development and use of performance measures.
In the United States, The National Quality Forum (NQF) was developed to provide a forum to develop and implement quality measurement, data collection, and reporting standards throughout the health care community.231 Key among the aims of this group are establishing measurement priorities focused on national aims for quality improvement and endorsing quality measures and standardized methods for measurement and reporting. This group has membership that spans the public and private sector to include patients, providers, nursing and allied health, employers, insurers, and industrial producers and suppliers in health care.
The NQF promotes quality in medicine by evaluating and endorsing standardized performance measurements. The AHRQ and CMS have recognized the NQF as a legitimate means of vetting heath care priorities and developing validated measures of health care delivery. In effect the NQF has positioned itself to provide a “stamp of approval” for quality measures developed by health providers and hospital systems across the United States. Endorsements are re-evaluated every 3 years.231 There is a complex 9-step Consensus Development Process used for endorsing each quality initiative. Four primary criteria are utilized in the endorsement process:
Does a quality measure report high-impact priority issues with strong evidence that improvement will provide a distinct benefit to patients?
Is a quality measure scientifically acceptable—will measurements be reliable and valid?
Is a quality measure useable and relevant, able to be interpreted by all involved parties?
Is a quality measure feasible, able to be tracked using available resources?
The AHRQ maintains a clearing house of recognized performance measures for a variety of medical conditions from the US and international community. As of January 2014 this site listed 25 performance measures for patients with hip fractures created in the United States, Canada, the United Kingdom, and Australia.232 These measures cover areas including mortality, osteoporosis treatment, VTE prophylaxis, readmission rates, and secondary fracture rates. Through the use of registries and other large data sets, we can identify other aspects of care that will be the basis of additional performance measures.
National Databases or Registries
The AHRQ gives several reasons to consider establishing a registry.233 Registries are particularly suitable for situations where experimental research is not feasible or practical, such as:
natural history studies where the goal is to observe clinical practice and patient experience but not to introduce any intervention;
measures of clinical effectiveness, especially as related to compliance, where the purpose is to learn about how that affects outcomes, if at all, rather than to observe the effects of products used according to a study protocol;
studies of heterogeneous patient populations, since unlike randomized trials, registries generally have much broader inclusion criteria and fewer exclusion criteria. These characteristics lead to studies with greater generalizability (external validity); and
follow-up for delayed or long-term benefits or harm, since registries can extend over much longer periods than most clinical trials (because of their generally lower costs to run and lesser burden on participants).
Several countries have developed national databases concerning fragility fractures, but such an entity is not available in the United States at this time. One robust source of information is The United Kingdom’s Web-based National Hip Fracture Database.234 This is a collaborative project led by the British Orthopaedic Association and the British Geriatrics Society. The core data set includes elements of case mix, process, and outcomes. They collect data points that address matters of 6 key areas of review for every patient including234:
to orthopedic ward in 4 hours;
surgery in 48 hours;
development of pressure ulcers;
preoperative medical assessment;
bone health assessment and treatment at discharge; and
specialist falls assessment
Additional data points that are collected address these items.
age, gender, postal code;
source of admission;
walking ability;
site and type of fracture;
ASA grade;
date and time of surgery;
type of operation;
reoperation at 30 days;
bone protection medication;
date and time of discharge from acute ward;
date and time of final discharge from trust; and
residential status; walking ability and bone protection therapy at 30 days, 120 days, and 1 year
The hospitals in the United Kingdom are compelled to participate in the national registry. The availability of this large, national database will enable important clinical questions, such as surgical timing, anesthetic choices, implant issues, and postfracture osteoporosis care to be answered in the future.
In the United States, registries have been successfully constructed on a more limited basis. For example, the Kaiser Permanente Healthy Bones Program has permitted high-quality fracture follow-up care of osteoporosis by use of a computer registry. The availability of this registry has permitted clinicians to improve patient safety, quality of care, and cost-effectiveness.206,235 Research using this registry has focused on early secondary prevention and has reduced the incidence of subsequent fractures in the Kaiser Health system.236 The establishment of a national hip fracture database in the United States would be desirable to improve the quality of care for our aging population and to provide the data to allow physicians to be engaged in management of active delivery of care. It is increasingly clear that outcomes data should be used to drive decision making for fragility fracture care at the hospital level and national level.
In summary, outcomes information is critical to the success of fragility fracture programs. Tracking of data allows for quality assurance both locally and nationally through the use of registries. By following outcomes data, fracture programs can prove their value to hospital systems and society.
Outcomes measures to follow in a hip fracture program include:
short-term outcomes;
long-term outcomes;
joint function; and
musculoskeletal function or quality of life.
Role of the Mid-Level Practitioner in the Geriatric Fracture Center
Carie E. Bradt, PA-C
Mid-level practitioners include physician assistants and nurse practitioners. A physician assistant can provide a broad range of health care services under the supervision of a physician. Nurse practitioners may or may not work under the supervision of a physician. The exact definition of these delegations is state dependent. The work of the mid-level practitioner can reduce the clinical and nonclinical tasks for the physician. The decrease in resident work hours has led to many of the duties of the orthopedic resident staff now being performed by the mid-level practitioner in most academic centers. Community hospitals often employ mid-level practitioners to perform the daily inpatient medical care of patients. Duties include obtaining complete medical history and physical examination, ordering and interpreting laboratory test and X-rays, prescribing medications, daily rounds, admitting and discharging patients, and coordination of other medical consult services. These responsibilities allow the mid-level practitioner to serve as an integral part of the geriatric fracture care team and to function as a central contact point of care for the geriatric fracture patient.
The mission of the geriatric fracture center mid-level practitioner is to facilitate geriatric fracture patient care. The consistency provided by the mid-level practitioner to navigate the geriatric patient with fracture through the complex medical system can improve the quality of patient care, increase patient and family satisfaction, decrease adverse events, and reduce length of stay and costs.237,238 The patient enters the geriatric fracture care system either through the ED or as a direct admission from another facility.214 The mid-level practitioner performs the orthopedic evaluation of the patient with a complete history and physical examination.214 Admitting laboratory tests, radiographs, and notification of consult services are expedited. Essential information such as identifying family members, health care proxy, current complete medication lists, pharmacy information, and medical history from either their current care facility or their primary care physician can be obtained without delaying the admission process. The initial admission process is a time-consuming process in medically complex patients and is expedited by the physician assistant. The mid-level practitioner can serve as a navigator for the patient and the patient’s family.213 A navigator is able to educate the patient and family members and provide reasonable expectations and reassurance during the fracture care process. In some institutions, the mid-level practitioner can obtain surgical consent from the patient or family member.
The mid-level practitioner is ideally positioned to serve as the central contact person for coordination of care during the hospital stay of the patient with fracture. Geriatricians, orthopedic surgeons, the patient, and their families can rely on the physician assistant to carry out orders and adequately prepare the patient with fracture for surgery.
After surgery, the mid-level practitioner can play a key role in postoperative management and daily rounding. Upon return to the floor, intraoperative and postoperative concerns of the orthopedic surgeon or anesthesia team can be communicated to the physician assistant for follow-up. The mid-level practitioner can be readily available on the floor for more frequent rounding on the patient with fracture if medically necessary as well as monitor and react to laboratory results, medication changes, fluid management, and pain management. The mid-level practitioner can alert the surgeon or medical team if a complication should arise. The mid-level practitioner can provide sign-out to the overnight coverage team as needed.
The geriatric patient with fracture requires care coordination. Specialized nursing staff should understand the increased needs of the geriatric patient.213 Nurses directly communicate with the physician assistant about issues such as delirium or pain management. Older patient with fracture have increased difficulty executing activities of daily living. The mid-level practitioner can help to assure that there is sufficient support for these needs.
Decreased length of stay can directly influence postoperative outcomes such as postoperative delirium and hospital-acquired infection.237 It is important to return the geriatric patient with fracture to their preoperative environment as quickly and safely as possible. To accomplish this, it takes constant management and coordination of care from physical therapy, occupational therapy, social work, nursing staff, patient’s family and caregivers, and the medical team that will be caring for the patient after discharge from the hospital.238 Most of the geriatric patient with fracture will be discharged to a SNF or less frequently to home. Either discharge plan requires much communication regarding the patient’s capabilities and restrictions, and these are best assessed and determined by physical therapists and occupational therapists. The mid-level practitioner communicates with the social worker to increase the awareness of those limitations as determined by physical therapy and occupational therapy as well as family concerns. The mid-level practitioner can provide necessary preparation for discharge of the geriatric patient with fracture. Aftercare appointments with medical providers, consultants, the orthopedic surgeon, and osteoporosis clinic should all be coordinated prior to patient discharge. The mid-level practitioner can provide a comprehensive discharge summary for the nursing facility or home care agency that will assume patient care beyond the hospital.239
Beyond direct patient care and coordination of care, the mid-level practitioner can participate in monitoring and assessing program performance data. A quality management dashboard serves as an integral method to improve patient care and outcomes. Constant involvement in the direct operations of a geriatric fracture center places the physician assistant in a valuable position to evaluate these measures, develop, and implement performance improvement initiatives. The mid-level practitioner can help to implement new strategies and work to consistently improve current initiatives.
The mid-level practitioner is uniquely qualified to be the hub of complex wheel that is the geriatric fracture center program.
Having a consistent medical provider that can bring all parts of this complex process together as well as provide direct patient care can prove to be invaluable in both tangible and nontangible aspects of today’s medical environment.
A well-coordinated, interactive program driven by a capable mid-level practitioner can increase patient and family satisfaction, promote high-quality outcomes, decrease length of stay, decrease complication rates, and ultimately provide high-quality, low-cost care that is uniquely tailored to a complex, ever-growing patient population.237,238
Role of Nurses, Social Workers, Medical Assistants, and Therapists
Jill Bass, MSPT, MBA, Anna Olson, MOT, OTR, MBA, CLT, Nancy Temple, RN, MSN, CCM, CCDS, and Carol Crowell, RN, MSN, NEA-BC
Care of the patient with a fragility fracture is best accomplished collaboratively, utilizing an interdisciplinary approach. Trust is established with the understanding that every discipline puts the patient as its center of focus. With open, honest, and respectful communication, trust grows and the patient benefits. Each discipline is not an entity unto itself but part of the whole care continuum for the patient. Many modes of communication exist between disciplines. Use of the electronic health record allows just-in-time documentation and gives the clinicians access to necessary information at any point during the patient’s stay. While much focus is placed on the physician portion of care, in fact most of the work is done by medical professionals including nursing, care coordination specialists and therapists. The intent of this chapter is to make clear the role of these professionals and how incorporation of a team thinking approach can benefit the care of fragility fracture patients.
Nursing
Facilitation of communication
Communication, nurse to nurse, nurse to physician, and nurse to family are essential areas to the care of the elderly patient. Some specific tips or best practices will improve communication. The primary nurse for a patient should routinely round with the physician to hear information given to the patient and family. This helps build a rapport between the physicians and the nurses and allows for any clarification in the plan of care or discussions on changes in protocol that need to occur between the patient, nurse, and/or physician. Orthopedic mid-level practitioners provide another resource for the nursing staff especially throughout the working day. At change of shift, nurses should do a bedside report between the on-coming and off-going shifts. This report includes the patient and, with the patient’s permission, may include the patient’s primary support person and/or family members. The family is an important part of the care team. Questions or concerns from the patient or family, especially regarding discharge plans, can be addressed at that time. Using these techniques provides guaranteed times throughout the day to make sure the patient, family nurses, and doctors are all on the same page. This is critical for patients with fragility fracture who may have delirium or dementia.
Another technique to enhance communication is the use of “rapid rounds.” Rapid rounds are conducted daily on the orthopedic unit, driven by the bedside nurse and attended by the charge nurse, social worker, care coordinator and therapist. The objective of rapid rounding is to “plan for the day, plan for the stay.”240 In other words, it establishes what needs to be accomplished today to move the patient closer to discharge goals. The bedside nurse provides a brief (less than 1 minute) description of the patient’s current level of functioning, family support, and goals, plus any barriers to the discharge plan.
Other forums are essential to improve quality of care between disciplines. An Orthopaedic Quality Team consists of all disciplines, including physicians and service lines that touch the orthopedic patient. The quality team meets monthly to review length of stay, costs, and quality outcomes. This forum allows for open discussion of any barriers encountered and gives the team and physicians input into the care provided by each discipline from preoperative to postacute care. This process allows for a continuous cycle of improvement to the care of the patient with a fragility fracture. Another important mode of communication is monthly nursing leadership rounding on the nursing staff. This allows leadership to hear issues the staff may be experiencing, such as the need for more equipment or patient care issues.
It is important that leadership sets expectations of the orthopedic nursing unit at the time of hire. Orientation to the orthopedic unit should be with a preceptor that includes verbal education with handouts. After orientation, nurses and patient care techs (PCTs) should pass an orthopedic competency skills checklist including joint and spine anatomy and physiology, hip and knee precautions, weight bearing, transfers, bed mobility, and equipment. It is crucial that nurses and techs have some basic knowledge of the orthopedic patient. These concepts are taught in collaboration with our physical and occupational therapy partners and orthopedic mid-level practitioners in a full-day course on care of orthopedic patients. Also integral to their training is bowel, bladder, and pain management. We provide annual skills fair to maintain knowledge and competency.
Areas of focus for nursing staff
Special education is important in particular areas of risk of patients with fragility fracture. Pressure ulcers due to injury, immobility, poor nutrition, and length of time to surgery are of particular concern. Specific education is available through online National Database of Nursing Quality Indicators (NDNQI), review course for assessment of pressure ulcers. NDNQI is a national nursing quality measurement program setup by the American Nursing Association. It allows members to benchmark and compare nursing quality measures (staffing, falls, healthcare acquired pressure ulcers, catheter associated urinary tract infections, etc) against national, regional, and state norms for similar type magnet hospitals. Comparative data are very helpful to develop facility goals and targets. These targets standardize knowledge on the pressure ulcer staging process. Monthly, facility-wide skin assessments are completed by a trained group of staff. Data collected from these assessments should be placed in hospital reporting software as well as reported to NDNQI. The data can then be trended by the facility and each department to assess goal attainment.
Prevention of falls requires a team effort that includes a patient’s family and all hospital employees. As best practice, it is an expectation that all patients are ambulated using a gait belt. Integration of a fall risk into standard nursing practice is very important. An assessment of fall risk using a tool such as the Johns Hopkins assessment tool should be made every shift.241 Patients with high risk of falls must be signaled to all providers as high risk. One method is the use of yellow armbands and the use of door frame signage, so all staff and departments that touch the patient are aware of the patient’s risk of falls. The use of booties with traction treads, and bed alarms may also prevent falls. We discuss fall interventions with family, so they can assist in keeping the patient safe. Every orthopedic hospital room should have a “Please call-Don’t fall” sign to remind patients and families.
Patients with fragility fracture are at very high risk of delirium. Nursing staff must be educated on what delirium is and how to recognize the condition. Every patient should be assessed each shift using the CAM. Positive assessments are reported to the physician to initiate a delirium order set. Education on delirium is very important and an important tool is the Hospital Elder Life Program (HELP) program developed by Dr Sharon Inouye (http://www.org/public/public-main.php).242 Education of nurses in delirium and delirium assessment as well as appropriate pain control techniques in patients who are elderly or who have dementia are critical for the improvement of care in the patient with fragility fracture. Other programs that may help with delirium include the standardization of the PCT role in caring for the elderly patients, a Hearing/Vision Program, which provides patients with assistance in the daily use of glasses, hearing aids, and magnifying lenses to keep them aware of their surroundings; a Mealtime Assistance Program, which provides help and companionship during meals; and a Sleep Enhancement Program, a program of warm beverages, hand massage and relaxation techniques to induce sleep. These are evidence-based practices that may help delay or prevent delirium in the hospitalized patient.
Resources for nursing education
Several excellent resources are available online to help with improvements in nursing care for patients with fragility fracture. The Nurses Improving Care of Healthsystem Elders (NICHE) program was started at the New York University School of Nursing and now has over 500 members throughout the United States. The NICHE program includes an interactive 24/7 e-Learning center, Project management support/mentoring for NICHE-based hospital initiatives as well as a Geriatric Institutional Assessment Profile tool. The International Collaboration of Orthopaedic Nursing has put forth an excellent 2-part document with a toolbox to help nurses care for patients with fragility fracture. This document stresses the importance of Pain, Delirium, Pressure Ulcers, Fluid Balance/Nutrition and Constipation/Catheter Associated Urinary Tract Infection.243,244
Therapy
Therapists treating patients with fragility fracture should make a concerted effort to build relationships with the treating physicians. Initial communication should address expected protocols, preferences, and expectations. Protocols can include special procedures, precautions, and approaches as well as direction to therapists who can expect to be consulted. Establishing preferred communication methods and discharge expectations could prevent treatment delays and increase consistency in patient communication. Specific recommendations are weight bearing status, the presence of hip precautions, wound care, and timing of mobilization. Through intentional communication with the treating physicians, therapists can establish rapport and an open line of communication that is essential within an interdisciplinary team approach to patient care.
Physical and occupational therapy services should be evidence-based and move beyond historical protocols to meet new environmental demands. Patients should be treated on an individualized basis, and frequency of treatment can be adjusted to facilitate improved outcomes.27 Treatment frequency can be increased to assist patients with more skilled needs. By adjusting treatment frequency and intensity, physical and occupational therapies can decrease the length of stay and positively impact discharge options.
As with all patient populations, therapists must individualize intervention approaches to meet the needs of the patient and family. Many patients with fragility fractures will have cognitive deficits. These deficits can be related to dementia, delirium within the hospital setting, and/or pain medication. Education and intervention must be provided at a level to facilitate their comprehension and participation. One-step instructions are ideal when providing cues during treatment. Repetition of education and new functional approaches can serve as an effective method for improving long-term recall, motor memory, and consistent incorporation of new learning into daily functional activities. Additionally, patients with fragility fractures often have family and other caregivers at the bedside. These individuals will be caring for the patient after discharge from the acute hospital setting and should be included in all education and training. Interventions with the patient and family should include functional mobility, adapted activities of daily living, adaptive equipment use, transfer training, fall prevention, and home safety/modification. Techniques and education across the multidisciplinary team should be consistent to maximize new learning and prevent unnecessary confusion for the patient and family.
The early mobilization of orthopedic patients following surgery is critical for a patient’s functional independence and prevention of postoperative complications. Mobilization on the day of surgery requires a team approach and begins with clear expectations from the physicians. Communication of expectations to therapists, nursing staff, and patients establishes a cohesive treatment environment. Therapists play a key role in early mobilization in 2 ways: one, physical therapists often will be the first discipline mobilizing the patient on the day of surgery. Two, physical and occupational therapists can provide training to all nursing staff on the safe mobilization of orthopedic patients. This training is essential for mobilization on the day of surgery and throughout a patient’s hospital stay.
Training other disciplines, such as nursing, to mobilize orthopedic patients can be an effective method for increasing patient mobilization. Educational modules should include several components. Physician protocols, preferences, and expectations should be shared. Precautions for different surgical interventions impacting mobilization should be explained. Transfer training should address bed mobility, transfers, gait belt use, proper body mechanics and safe use of adaptive equipment. Skill competencies addressing varying levels of assistance needs will increase staff comfort in mobilizing patients throughout their hospital course. A series of classes is an effective way to provide comprehensive knowledge and skills training. Classes can be divided by location of surgical intervention or injury (spine, lower extremity, upper extremity, etc). Following education of specific protocols and precautions, transfer and mobilization techniques can be paired with the surgical intervention or injury location to provide context for skills competencies. During the spine class, bed mobility and log rolling can be addressed. A lower extremity class is an appropriate setting to address 2-person transfers and modifications to adaptive equipment, such as the height of walkers and bedside commodes. An upper extremity class can incorporate 1-person or minimal assistance transfers to a chair or to the bathroom. Long-term success of a multidisciplinary approach to patient mobilization will require ongoing training as new employees are hired as well as at annual competency fairs.
Care Coordination and Care Transitions
The coordination of care for the hospitalized orthopaedic patient with fragility fracture begins on the day of admission. Optimally, social workers and or nurse care coordinators (SW/CC) will receive an order for consultation triggered from the admission order set, initiated in the electronic health record (EHR). Patients may also be identified for SW/CC intervention through nurse screens in the EHR and rapid rounding. Once identified for evaluation and assessment, the SW/CC should meet with the patient and family to determine the patient’s goals for discharge. Based on therapy evaluations and recommendations, physician orders and patient/family goals and preferences, a referral is made to the appropriate level of postacute provider. As almost all patients with fragility fracture will require assistance postdischarge, this coordination should be standardized.
In the United States, based on the patient’s diagnosis and level of functioning, the patient may qualify for either an acute rehabilitation placement or a placement in a SNF. The CMS requires a 3-day medically necessary inpatient stay including a minimum of 3 midnights prior to transfer to a SNF. Time spent in the ED or in observation status prior to the inpatient admission does not count toward the inpatient hospital stay (Medicare Benefit Policy Manual Chapter 8-Coverage of Extended Care, http://www.cms.gov/Regulations-and-Jcn/Guidance/Guidance/Manuals/Downloads/bp102c08.pdf).245 Con versations between the attending physician, case managers, therapists, patient, and family should produce a discharge care plan that is in agreement with patient/family goals, appropriate for the patient’s level of functioning, and compliant with Medicare benefits. For an SNF stay, the patient is required to participate in 1 to 3 hours of therapy per day. Per CMS, SNF care is covered if the patient requires skilled nursing services or skilled rehabilitative services on a daily basis, and the services delivered are reasonable and necessary for the treatment of the illness or injury (Medicare Benefit Policy Manual Chapter 8-Coverage of Extended Care, http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c08.pdf).
Certain diagnoses will qualify a patient for an acute inpatient rehabilitation placement. Contrary to an SNF placement referral, the patient does not require a 3 midnight inpatient stay in order to qualify for an acute rehabilitation placement. The CMS has determined 13 medical conditions that automatically qualify for an acute rehabilitation stay. A full listing of the 13 conditions can be found on the CMS Web site, but of particular relevance to the population with fragility fracture is fracture of the femur. In the acute rehabilitation setting, the patient is required to participate in 3 hours of therapy per day and will generally stay 10 to 14 days. The patients will participate in physical, occupational, and speech therapy in combination to achieve the required 3 hours of therapy per day.
Care Coordination Medicare Requirement and Expectations
Skilled nursing facility
Requires a medically necessary inpatient stay spanning 3 midnights.
Physical, occupational, and speech therapy of 1 to 3 hours required per day while in facility.
Acute rehabilitation facility
Does not require a 3-day inpatient stay.
Requires 1 of 13 particular diagnoses (includes fractures of femur).
Physical, occupational, and speech therapy for 3 hours required per day while in facility.
As most patients will need to go to either an SNF or acute rehabilation facility, optimal care should facilitate communication between the hospital, the facility, and the surgeon. One method of coordinating care across the continuum is to organize a team of social workers, care coordinators, therapists, and hospital leaders (including a lead surgeon and medical doctor). The team should then seek out postacute care providers (skilled nursing facilities, long-term acute care and acute rehabilitation hospitals) to form a partnership with the goal of improved communication and outcomes between the acute care and postacute care settings. The team should tour the postacute care facilities and invite the facilities to participate in training of physician-recommended protocols for therapies. Training can be conducted by the therapy department and offered either on-site or to the participating facility. Following the meeting and training, a postacute care partnership meeting should be setup and scheduled quarterly to support improved outcomes and communication. Multidisciplinary communication between RNs, physicians, social workers, care coordinators, therapists, and postacute care providers shared with the patient and family promotes coordinated care from admission through discharge, with a safe transition to the postacute care setting.
Summary
Nursing, therapy, and care coordination plays a very important role in the care of the patient with fragility fracture. These groups must work hand in hand with the physician leadership and administration to provide optimal care. Specific education based on the needs of these elderly patients must be facilitated. Interdisciplinary care is extremely important to provide an excellent continuum of care.
Nursing Pearls
Nurses should round with physicians.
Rapid rounds with entire team.
Individualized care is based on the patient’s needs and optimal level of functioning.
Expectation that patients are out of bed on day of surgery and for meals.
Enhanced availability of midlevel practitioners to nursing staff while patient is on the unit.
Nursing education on geriatric core areas including delirium, fall prevention, decubitus prevention, and pain control
Integration of programs such as HELP or NICHE into hospitals caring for patients with fragility fracture.
The Role of Administration in the Care of Fragility Fractures
Kimberlee Y. Daniels
Hospital administration plays a key role in the improvement in care for patients with fragility fracture. With the aging of the population as well as the increase in patients with the Affordable Care Act, pressure is squarely on hospital administration to decrease costs while increasing quality of care. The administrator’s role is to assist physicians in the coordination and development of systems to improve care. Operationally, this may mean structuring multidisciplinary clinics, exploring reimbursement models and building the infrastructure to support new models of care. This is of particular importance for the fragility fracture, where improvements in care may help decrease cost for a system but not generate as much revenue per patient.
Despite the projected growth in fracture care, health care systems must not allow the numbers to dilute the value of the service being provided. As payers in the health care system become more focused on quality, providers will need to be able to demonstrate the value that they provide in delivering care to patients. Payers will be looking for reductions in readmissions, reductions in infection rates, reductions in length of stay, and the elimination of duplicative services in the care cycle. The role of the administrator is to assist in the identification of the metrics that add value to the service. This requires the administrator to be knowledgeable about quality measurement. Fracture care is a strong contender for quality measurement because of the importance of the condition, the potential for quality improvement, and the degree to which health care professionals control the mechanisms for improving care.
In order to have a successful process improvement that is sustainable, it must include all members of the team, especially the physician. Without a physician champion to lead, there will likely be little to no sustainable change. Typical process improvement initiatives involve a focus on fixing specific elements of the system. The administrator must be able to start the change process, but more importantly make the change stick so that it leads to a true shift in culture. In the area of fractures, development of a strong working relationship between a physician champion and administrator is critical to success.
The typical relationship between the Administrator and the Physician Leader is a collaborative partnership where the physician establishes the vision for the practice and the Administrator coordinates the variables to carry out the vision. The Administrator explores the plan to build a solid business case to support the proposal. The business plan typically includes the financial, operational, and strategic elements involved in carrying out the vision. This process is generally followed when the vision involves building and development as growth strategies. However, as capital dollars become more limited and health care organizations are more reluctant to invest in new business, the focus must shift toward creating efficiencies in order to maximize existing resources in the system by reducing wait times, increasing access to patient care, and increasing patient satisfaction.
How does a Physician Get What They Want From Administration?
The physician must realize that administration speaks a different language. The physician must learn to speak some administrator-eze to be able to successfully convey their ideas into results. Getting buy-in from administration is critical to allow for the resources to allow for operationalization of the project. Development of a business plan is essential for success. The physician must take their PowerPoint slides and convert these into a business plan. The physician must understand the process of getting resources from administration. In general, larger projects that require significant resources must gain approval at multiple levels. First is the department level, next is approval from the institution Chief Operating Officer (COO), and finally, approval from the board of the institution is required for larger projects. Each of these levels of approval requires a concise business style presentation to a group of administrators. The presentation must show what the problem is, what the solution is, and how will revenue be generated from the project. A key with these presentations is for it to be concise and to the point. The presentation should have a small section describing the need for the project but cannot get bogged down in medical details that senior administration will not appreciate. The physician must learn to present like an administrator for senior administration to accept them as knowledgeable in their realm.
For fragility fractures, the development of a service to treat osteoporosis is a typical need. The physician must have numbers to show how many patients will need to be treated, what resources are required including the cost of the resources, and what the gains will be from the project. Barriers to success should be mentioned. The development of this business plan usually requires that the physician champion work closely with their administrative champion to get the numbers to back the plan. In this case, how may fractures will the program prevent over a year? How will you show these results to patients and insurers to prove the quality of care and the improved cost of care?
How does an Administrator Get What They Want From a Physician?
For the administrator to be successful, they must collaborate with a physician champion. A team effort is required. The administrator must help the physician to build a business case for their project. This may require investigation by the administrator to truly understand the ramifications of the physician’s plan. The administrator must educate the physician about the need for a business plan and how the plan must be pushed to senior administration for approval. Coaching and preparation for each meeting is important so that the physician learns to speak enough administrator-eze to be able to convince a board of people of the importance and need of the idea. The administrator should consider using LEAN processes with the physician to help in the operationalization of the plan. This has been successful in many areas of healthcare including joint replacement.246,247 The Lean methods have been shown to be effective in the development of a hip fracture service.248 The Lean process may require bringing in other groups necessary for the project and uncover potential roadblocks to the project that can be addressed early on. After the plan is developed, the administrator must truly be a cochampion for the project. This means bringing it up in meeting with senior administration before the board meetings so that they are familiar with the project and understand the needs of the physicians.248
In summary, for innovative care projects to succeed, collaboration must exist between a physician champion and an administrative champion.248 Each champion must learn the language of the other to achieve success. In particular the physician champion must learn how to develop a business plan and be able to precisely present this to senior administration.
Keys for the Administrator
Work closely with a physician champion.
Use Lean principles to create a structured approach to the problem.
Guide the champion to develop a business plan.
Help the physician champion present effectively to senior administration and the board.
Work independently with senior administration to inform them of the importance of the project.
Keys for the Physician Champion
Identify and work closely with your administrator
Learn how to develop a business plan
Learn about LEAN processes
Understand what your administration will support and work this into your business plan
Develop your skills so that a precise effective presentation can be made to senior administration and the board
Proximal Humerus Fractures
Brett D. Crist, MD and Gregory J. Della Rocca, MD, PhD, (Harry Hoyen, MD and Stephen L. Kates, MD)
Proximal humerus fractures are fragility fractures frequently seen in late middle-aged and older adults. A 65-year-old caucasian woman has a 5% risk of sustaining a proximal humerus fracture by the age of 90.249 Risk factors for developing proximal humerus fractures include low bone mineral density, frequent falls, diabetes, difficulty walking in dim light, poor vision, and low dietary calcium intake.
A proximal humerus fracture often occurs after a fall from standing height onto an outstretched arm or directly onto the shoulder. A careful history documents critical information that may identify modifiable risk factors that led to the injury, such as dizziness, recurrent loss of consciousness secondary to antihypertensive medication, or frequent falls due to a cluttered living environment. An understanding of the patient’s preinjury functional status, including rotator cuff function, medical comorbidities, living situation, ambulatory status, and use of ambulatory aids, will help determine their treatment and care needs.
Pathophysiology
As the proximal humerus is primarily composed of cancellous bone; it has a propensity to be affected by osteoporosis. The loss of cancellous bony trabeculae combined with decreased trabecular interconnections weaken the metaphysis more than the diaphysis, leading to higher likelihood of fracture in the metaphyseal region. In elderly patients, the bone within the articular segment is vacuous except for the subchondral bone. This makes ORIF challenging; postoperative collapse leads to articular penetration in approximately 23% of the patients.250 Although most proximal humerus fractures in these patients result from falls, fractures can also occur through a metastatic lesion, and this should be excluded when evaluating the patient. The attachments of the rotator cuff tendons onto the greater and lesser tuberosities and the pectoralis major and deltoid muscle group attachments to the humeral shaft affect fracture fragment displacement. Typical displacement patterns can be seen based upon the deforming forces associated with the fracture fragments. The greater tuberosity externally rotates posteriorly and superiorly secondary to the forces of the supraspinatus and infraspinatus. The subscapularis muscle internally rotates and medializes the lesser tuberosity fragment. The humeral shaft may be impacted into the articular surface in valgus (valgus-impacted) or displaced superiorly with articular surface in varus (varus-depressed) with apex anterior angulation in the sagittal plane as a result of the pectoralis and deltoid forces on the humeral shaft. Head-splitting fractures, fractures where the articular segment has less than 8 mm of metaphyseal extension, and fractures with a disrupted medial calcar hinge put the vascular supply to the proximal humerus at risk of avascular necrosis (AVN) and may influence the treatment method.251,252 Since a vascular necrosis of the proximal humerus may be well tolerated, it doesn’t necessarily have to direct initial treatment. However, the known risk factors for AVN allow the surgeon to discuss the risk with the patient.
Classification
The most commonly used system for classifying proximal humerus fractures was described by Neer in 1970 (Figure 9).253,254 This system incorporates Codman 4 parts of the proximal humerus, namely, the anatomic head, the lesser tuberosity, greater tuberosity, and the humeral shaft (Figure 10).255 Displacement of more than 1 cm or angulation of the part by 45° or more allows the fragment to be counted as a part. Thus, a nondisplaced fracture would be zero parts and a fracture with displacement of greater than 1 cm of all 4 parts would be a 4-part fracture.
Figure 9.
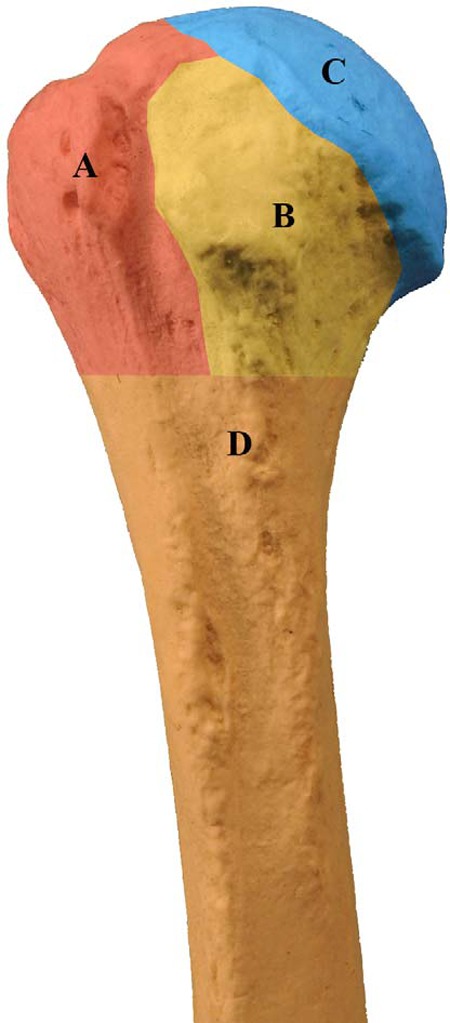
The 4 parts of the proximal humerus as described by Codman255: A, greater tuberosity. B, Lesser tuberosity. C, Anatomical head. D, Humeral shaft.
Figure 10.
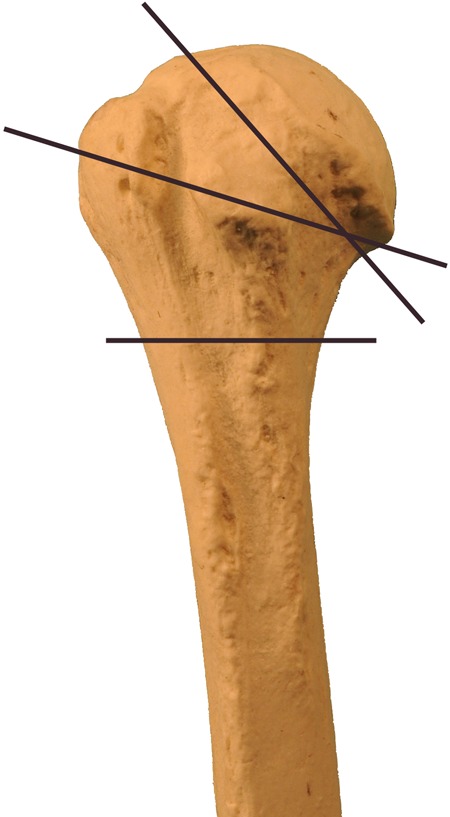
The common zones of injury in the humerus, that is, the anatomic head, tuberosity region, and surgical neck area.
In addition, the Neer classification has several special proximal humerus fracture types, such as the fracture associated with a glenohumeral dislocation and one with splitting of the articular surface (head-split). These special fractures have unfavorable prognoses, especially for osteonecrosis or traumatic arthritis. Other classification systems exist, such as the AO Foundation/Orthopaedic Trauma Association (OTA) system,256 but they are mostly used for research communication. The Neer classification is the one most commonly used in the United States.
Clinical Features
Presenting complaints are pain, swelling, tenderness, and diminished ability to move the arm. Crepitus is often present, and ecchymosis may be impressive if the patient is not seen early. Displaced fractures or fractures associated with a dislocation may have obvious deformity depending upon the patient’s size and body habitus.
Neurovascular injuries are rare but should not be overlooked. Patients may present with a neurologic deficit such as axillary nerve sensory deficit or brachial plexus injury. A thorough neurological examination should be performed and documented for all patients. The most frequently injured structures are the axillary nerve and components of the lateral cord. These are usuallya neuropraxia due to traction or compression injuries and observation is recommended. Resolution of the neurologic symptoms typically occurs within the first 3 months. Motor function of the deltoid muscle can easily be assessed when the examiner places one hand on the posterior deltoid and the other on the posterior elbow; the patient is instructed to push the elbow posteriorly, and contraction of the deltoid can be palpated. This method works even in patients unwilling to move the shoulder (the examiner asks about the elbow) due to pain from the injury.
A thorough skin evaluation should be performed to address any skin tears present to avoid missing an open fracture. Other injuries may also occur with simple falls and the patients should be assessed for ipsilateral extremity injuries and head and chest trauma (including rib fractures).
Radiographic evaluation
Conventional orthogonal radiographs are essential for diagnosis of a proximal humerus fracture. The standard views are a true shoulder (scapular) AP view (“Grashey view”), a scapular lateral “Y” view, and an axillary lateral view. Multiple alternative axillary views exist, including the Velpeaux view, to overcome the difficulty in positioning the upper extremity in the injured patient and should be considered. Most fractures can be diagnosed and classified with the 3 standard views. The relationship between the humeral head and the glenoid should be carefully studied to avoid missing a dislocation257 associated with a fracture, and the 4 anatomical parts of the humeral head should be assessed with respect to displacement and/or angulation. Full-length AP and lateral radiographs of the humerus should be done to avoid missing a noncontiguous injury. In situations involving extreme comminution, a CT scan may be necessary to fully diagnose the extent of the injury, including visualization of a head-splitting fracture. The CT scans can be helpful in determining the size of the articular segment that can accommodate screw fixation, which may determine the treatment choice.
Nonoperative Treatment
Most valgus-impacted and nearly all minimally displaced fractures are best treated with nonoperative care. Fractures in valgus alignment with an intact medial hinge (humeral calcar) tend to have a better prognosis with nonoperative treatment than those with varus alignment or medial hinge disruption.258 Nonoperative management may also be chosen in patients with displaced fractures that have low functional demands with their extremity, will not tolerate surgery, or have significant osteoporosis with a high risk of postoperative failure.
Use of a sling or shoulder immobilizer for comfort followed by early gentle range of motion has historically had a high success rate.259 Sling immobilization for 10 to 14 days is typically needed before initiation of gentle exercises for range of motion. The sling should be removed for hygiene activities and to start early wrist and elbow range of motion to avoid joint contracture and aid in edema reduction, since the shoulder may be immobilized for 3 to 4 weeks.252 The sling can be discontinued as early as their pain allows. The patient is generally kept nonweight bearing through the injured upper extremity until fracture callus is seen radiographically. The patient may start Codman pendulum exercises when pain allows. Patients should be evaluated approximately every 2 weeks with radiographs and clinical examination until there is radiographic evidence of callus at the fracture site and there is significant improvement in pain. This is usually when activities above chest level and strengthening exercises are allowed. Early involvement of physical or occupational therapists may help improve activities of daily living or if the patient has difficulty doing Codman exercises. Typically, therapists become more involved as the fracture heals and becomes less painful. Radiographic fracture healing is typically seen at 3 to 4 months with functional improvement continuing for 6 to 12 months.
Closed reduction alone is not typically successful for proximal humerus fractures. Typical circumstances for attempting a closed reduction in the ED or operating room could include an associated glenohumeral dislocation (which is occasionally successful), significant fracture displacement leading to neurovascular compromise, or an impending open fracture. Reduction may be achieved with intravenous sedation or general anesthesia, depending on the patient’s needs.
Surgical Treatment
Not all displaced proximal humerus fractures can be treated nonoperatively. Attention must be given to the type, angulation, and degree of displacement of the fracture but also to the patient’s functional demands when choosing a treatment plan. For example, an elderly patient who lives independently and fractures the dominant arm may require operative consideration in order to maintain the functional level. An elderly patient with severe dementia who resides in a nursing facility and is minimally functional may have an acceptable result with nonoperative management regardless of fracture displacement.
For 2-part fractures, surgical management includes several options based upon the parts that are displaced. Surgical neck fractures with displacement may be managed with closed reduction and percutaneous pinning, intramedullary nailing, or ORIF with plate and screws. Displaced anatomical neck fractures, uncommon in this age group, have historically been managed with prosthetic replacement due to concerns over compromised blood supply to the articular surface and risk of AVN. However, ORIF has been used successfully and may be considered.260 Although the Neer classification uses 1 cm of displacement as criteria for a part, greater tuberosity fractures with greater than 5 mm of displacement are problematic for shoulder function and may be considered for operative management. The technique used for operative management depends upon the fracture fragment size. Typically, tension band fixation either with suture, wire, or plate fixation is used based on fragment size. Occasionally screw fixation is done, but reinforcement with suture fixation into the supraspinatus bone-tendon junction is recommended to minimize risk of failure in patients with osteoporosis.
Three-part fractures may be fixed with open reduction and plate fixation, tension band wiring (mostly abandoned), closed reduction, and percutaneous pinning with terminally threaded wire fixation261 or intramedullary fixation with suture augmentation of the tuberosity fragment. Attention must be given to accurate reduction in the tuberosities and fixation sufficient to maintain fracture reduction to allow for the tuberosity fracture healing required for acceptable postoperative function. Medial bony calcar apposition, if possible, is desired in order to minimize the risk of postoperative varus collapse with concomitant articular screw penetration. Calcar support can be supplemented with an intramedullary plate or cortical allograft strut.262 In some cases, restoration of the calcar may not be possible, and intraoperative impaction of the shaft into the humeral head may improve fracture stability.263 The best remaining bone in the humeral head is typically within 1 cm of the articular surface. Placement of screws or pins into this bone gives the best purchase but carries a risk of intra-articular screw penetration.264–266
Four-part fractures and head-splitting fractures that are not reconstructable may best be managed with prosthetic replacement. As tuberosity healing is not reliable and restoration of humeral height is difficult after arthroplasty, clinical results may be mediocre.267,268 It is important to make sure the patient understands that the goal of the arthroplasty is pain relief. Generally, overhead function is not restored, and the patient must understand that there is a potential for loss of shoulder function in the long term. Depending on the patient’s extremity dominance and functional demands, these fractures could be considered for nonoperative management. For extremely comminuted 4-part fractures with nonreconstructable tuberosities, some surgeons have found good results with the reverse shoulder replacement.269
Rehabilitation
Shoulder range-of-motion exercises should begin in the early postoperative period as the patient’s pain allows. Codman exercises are usually begun within the first postoperative week. Above chest level activities should be restricted until fracture callus is evident. Overly aggressive physical therapy and exercises may increase the risk of fixation failure in the postoperative period. A sling is usually worn for comfort only and may be discarded as early as the patient’s pain allows. During the period of convalescence, range-of-motion exercises should include elbow, wrist, and hand motion. Although full shoulder functional recovery may not be expected, limited use of the rest of the upper extremity can significantly compromise activities of daily living. Scapular plane motion may substitute for some lost glenohumeral motion for activities that involve the hand-to-head function. Good strength within the limited motion arcs is often an acceptable outcome. The most commonly used tools used for postfracture functional outcomes assessment are the Disabilities of Arm, Shoulder, and Hand (DASH) and the Short Form 6D.270
Summary
Fragility fractures of the proximal humerus present many challenges. Treatment must be individualized for the patient based on the fracture characteristics as well as the patient’s functional demands. Accurate characterization of the injury and consideration of the patient’s preinjury function and postfracture needs may allow for acceptable outcomes for most patients.252,268
Treatment of Osteoporotic Proximal Humerus Fractures
Treatment of proximal humerus fractures is complex and should be designed to provide for the best functional outcome for each patient.
Nonoperative: Most fractures.
Operative: Consider for displaced 3- or 4-part fractures, indications for surgical intervention are controversial. ORIF: high complication rate due to fixation failure. Hemiarthroplasty: Outcomes related to tuberosity fixation. Reverse shoulder replacement: Failed prior treatment, pre-existing rotator cuff dysfunction.
Distal Radius Fractures
Simon C. Mears, MD, PhD (Harry Hoyen, MD)
Distal radius fractures are a very common injury in patients with reduced bone quality. Approximately 200 000 distal radius fractures occur in the United States each year, and women are approximately 4 to 6 times as likely to sustain a distal radius fracture as are men.271 The incidence of distal radius fractures begins to increase around the age of 50.272 It is thought that the overall rate of distal radius fractures is increasing.273 Care of the patient with a distal radius fracture is also costly: A cost of US$7788 (between years 2000 and 2005) has been estimated for a Medicare patient with a distal radius fracture.274 Despite their frequency, treatment of distal radius fractures in elderly patients is controversial275
Pathophysiology
Fractures of the distal radius most often occur from a fall onto the outstretched hand from a standing height. The most typical fracture pattern is with dorsal displacement of the distal radius, and it may or may not be accompanied by comminution of the radius, injury to the ulnar side of the wrist, or other wrist injuries such as injury to the scapholunate ligament. Distal radius fracture may also be open injuries. Fractures are associated with diminished bone quality in the distal metaphysis of the radius.
Classification
Many different fracture classification systems have been developed, but, in general, they have poor interobserver reliability. When evaluating radiographs of the fractured distal radius, several measurements can be helpful, including apex volar angulation, radial length, and radial inclination. Most classification schemes distinguish between fractures with and without intra-articular extension and the amount of intra-articular involvement and comminution. Measurement of fragment depression or intra-articular “step-off” should be made because depression larger than 2 mm is associated with development of traumatic arthritis.
Clinical Features
Fracture of the distal radius is usually obvious, with deformity of the wrist, pain, and swelling. Occasionally, a fracture may be nondisplaced and less painful. A skin examination is essential rule out open injury, particularly near the ulnar styloid. A thorough neurovascular examination should be performed to rule out nerve or vascular injury. The patient should be questioned to ascertain the dominant hand and the preinjury functional status. Treatment plans may be different in a patient with a poor functional status than in one who is very active. The clinician should assess the patient’s activity level and goals after the fracture has healed.
Radiographic Evaluation
AP/lateral/oblique views of the wrist should be obtained. Radiographs of the remaining forearm/elbow and potentially the shoulder should be obtained after joint-specific examinations. Other disabling conditions of the hand, such as thumb basal joint arthritis, wrist instability, preexisting deformity, and other posttraumatic conditions should be identified. These problems may cause a greater impact on hand function than does the distal radius fracture.276,277 A traction view can be helpful in determining the fracture pattern and stability.278
Nonoperative Treatment
All displaced distal radius fractures should be reduced and splinted. Reduction is often aided with the use of a hematoma block. With this block, the fracture site is infiltrated with lidocaine via a dorsal approach. Additional intravenous medication may also be needed for reduction. During reduction, a traction radiographic view should be obtained. Reduction is performed with recreation of the displacement followed by translation of the carpus volarly with traction.
A well-padded splint or cast should be then placed without excessive palmar flexion of the wrist. For fractures that require reduction, a sugar-tong splint or cast maintains the reduction better than a slab splint. The splint must be carefully placed to allow the patient to have range of motion of the metacarpophalageal joints and thumb. A splint that is too long can contribute to hand stiffness. The splint or cast must be molded using a 3-point technique to allow for fracture reduction maintenance. A splint or cast should not have a cylindrical shape; it should appear deformed—otherwise the molding is insufficient. Postreduction radiographs are then scrutinized to assess fracture reduction. The mold should be visible on the radiographs.
Based on the postreduction radiographs, patient goals, and patient activity levels, a plan for treatment can be developed. If the fracture is well reduced or the patient is nonfunctional, closed treatment can be attempted.279 This treatment should include a weekly radiograph in the splint or cast to assure maintenance of the reduction. After 3 weeks, the splint or cast may be removed and a short-arm, well-molded cast can be placed. At 6 weeks, the patient usually can be transitioned into a Velcro-applied wrist splint.
Restoring motion and reducing swelling is critical during this period. Elderly patients with distal radius fractures are susceptible to stiffness of the hand, wrist, elbow, and shoulder. Hand edema can be severe, and all rings must be removed at the time of initial evaluation. The patient and caregivers must be counseled to elevate the hand and to use a sling initially. They must be told to remove the arm from the sling frequently and to move the elbow and the shoulder. Stiffness, pain, swelling, and skin temperature changes may represent onset of a complex regional pain syndrome. Early recognition of this condition is essential to allow for early treatment with therapy and sympathetic blockade. Physical or occupational therapy can be instrumental in maintaining range of motion. The splint or cast must be checked to make sure it does not impede range of motion.
Nondisplaced fractures are thought to be stable and can be treated with a short arm cast for 4 to 6 weeks.280 If fracture reduction is not obtained with closed reduction, or if the reduction is later lost, additional decisions must be made. If the fracture alignment is unacceptable when considering the needs of the patient, operative treatment should be considered. Repeated attempts at reduction are unlikely to result in improved final fracture alignment. The radiographic parameters for failed reduction are controversial but include radial shortening, >2 mm of intra-articular depression, volar tilt of >20°, or dorsal tilt of > 0° to 10°.
It has long been thought that fragility fractures do not require operative intervention. The parameters described for younger patients in relation to radial tilt, dorsal angulation, and ulnar variance are often in reference for the development of carpal instability patterns, posttraumatic arthritis, ulnar abutment, and long-term effects. The manner in which these factors affect carpal kinematics is less understood in the elderly population. These radiographic guides must be matched with the activity level and goals of the patient. Fractures of the palmar lip or volarly displaced fractures are typically unstable and not easily managed with closed reduction and immobilization. Similarly, if the fracture is comminuted, operative treatment may be considered. In select cases, a CT scan may be helpful for planning surgical intervention.
Surgical Treatment
If operative intervention is selected, the treatment options include percutaneous Kirschner-wire (K-wire) fixation, intrafocal K-wire fixation, external fixation, intramedullary fixation, osteobiologic supplementation, arthroscopic reduction, dorsal or volar plate stabilization, or combinations thereof.
The literature does not provide a guide for the best method of fixation, and the choice and success of the modality depend on the experience and skill of the surgeon. It is unlikely that an individual surgeon will be skilled in multiple fixation methods. Well-done fixation with 1 method is more likely to achieve a good result than poorly done fixation with a perceived (but less frequently used) superior method. Interestingly, the rate of fixation of distal radius fracture is dramatically different in different areas of the United States.281 No information is available for determining whether results are better in the geographic areas with more surgical intervention. In general, each of these methods may be successful and each has different risk associated. Certainly, pin track infection can be a risk of K-wire fixation and external fixation that is not associated with internal fixation.
The goals of surgery are to maintain reduction and improve wrist function. The objective measures are consistently better with internal fixation, but the outcome measures are very similar between operative and nonoperative treatment groups.276,282–284 A more specific outcome measure for fracture treatment is needed and is a potential for further study. The DASH outcome measure may not be appropriate for distal radius fractures in the older patient population. Volar fixed-angled plates are popular for the treatment of these fractures. The osteopenic bone is directly supported with the locking screws for volar stabilization. Risk factors for plate fixation are plate failure, fracture subsidence, and tendon irritation or rupture. Two recent studies have evaluated the outcomes of volar plate fixation versus nonoperative treatment285 and versus external fixation.286 In both studies 1-year outcomes were not different, although the internal fixation groups may recover faster than the nonoperative or external fixation groups.
Patients with complex articular fractures often present with separate dorsal and volar segments. Axial load causes complete shortening of both segments and flattening of the articular dish. This articular incongruity is better tolerated in the elderly population than in younger individuals. In the scaphoid-lunate facet region, articular fractures displaced >3 mm may lock the scaphoid and lunate from rotating with each other. This fracture type may require open reduction, and the metaphyseal angulation may also dictate the necessity for internal fixation.284,287 Fixation of the ulnar side of the wrist is debated. Although it can be achieved with K-wires or screw or wire fixation, it is unclear how much this improves wrist function, particularly in the elderly patient.
Clearly, further research is required to determine an appropriate treatment algorithm for the treatment of distal radius fractures in the elderly. This algorithm will require careful attention to standardized outcome measures, comorbidities, the needs of the patient, the skill of the surgeon, and cost.288 For most distal radius fragility fractures, strong consideration should be given to nonoperative management as 1-year results of operative fixation are similar.
Rehabilitation
After casting or surgery, early finger motion is essential to prevent edema and stiffness. When immobilization is discontinued, aggressive finger and hand motion is necessary to allow for the best possible outcomes. Hand and occupational therapists are an essential part of the patient’s recovery.
Distal radius fracture in the elderly patient should be first treated with closed reduction and immobilization.
The decision for operative management should be made after evaluation of the radiographic alignment of the fracture and a functional assessment of the patient.
The type of surgery should be based on the needs of the patient and the skill of the surgeon.
Vertebral Compression Fractures
Addisu Mesfin, MD, and Wakenda Tyler, MD
Osteoporosis of the spine is a progressive disease process that can lead to functional morbidity and severe pain, even in the absence of an acute fracture. Approximately 750 000 vertebral compression fractures (VCFs) occur yearly in the United States. These numbers will likely increase as the United States population continues to age. Vertebral compression fractures of 70 000 (˜10%) will result in a hospitalization lasting, on average, for 8 days.289 Once a person has sustained a VCF, there is a 5-fold increased risk of sustaining a second VCF and 4- to 5-fold increased risk of a subsequent hip fracture.289–291 In 2005, the estimated direct cost of osteoporotic fractures in the United States was US$18 billion a year, and if there are little changes in the epidemiology of these fractures over the next 30 to 40 years, that cost will likely double.292 Vertebral fracture comprise 27% of osteoporosis-related fractures followed by 19% of hip fractures.293 Direct costs in the first year following a vertebral fracture have been estimated at US$8380.294 Population-based prevalence studies of osteoporotic compression fractures range from 10% to 25% in women and 10% to 27% in men.295–298
Pathophysiology
The causes of osteoporosis can be explained in the vast majority of cases as a result of decreased circulating estrogen (in the case of rapid turnover postmenopausal osteoporosis) or of the natural aging process (as in the case of low turnover age-related osteoporosis). There is some evidence that mutations in the plastin 3 (PL3) gene may contribute to decreased BMD and fractures in heterozygous women.299 In either case, as the bone quality and quantity decreases throughout the body, the spine is often one of the most affected areas. As the cortical and trabecular bone of the vertebral bodies begin to decline in thickness and connectivity, the risk of fracture from physiologic compressive forces increases. Simple activities, such as standing from a sitting position, can lead to fracture through the weakened bony trabeculae. Some fractures occur as a gradual micro-fracture process that leads to progressively worsening anterior vertebral compression and wedging. These gradual fractures can often be asymptomatic. Other fractures may occur as an abrupt catastrophic failure of the trabeculae, which may be immediately painful. Once a person has a compression fracture, it alters the spine’s biomechanics, predisposing it to more compression fractures. A weak vertebral body bone is also representative of a more systemic process taking place throughout the entire body, which is why VCFs are often ominous signs of future fractures.
Classification
There are 2 main types of VCF, namely, acute and chronic. Acute VCF may present with back pain after minimal activity, as mentioned earlier; this pain can be extremely debilitating. The chronic form of VCF is often detected incidentally when the patient is being examined by a physician and is noted to have a decreased standing height or kyphotic deformity. Chronic VCF may also present with new-onset pain or postural fatigue, as the normal biomechanics of the spine become further compromised by the fractures. Some fractures may also be detected when radiographs of the lumbar or thoracic spine are obtained for unrelated reasons (such as abdominal radiographs to assess intestinal gas patterns or routine screening chest radiographs) The identification of the fracture type as acute or chronic can impact the type of medical care and surgical management (if any) chosen for the patient (see subsequently for the management of acute and chronic VCFs).
In certain instances, the patient may have a combination of chronic and acute compression fractures. Magnetic resonance imaging is useful in these instances to determine whether or not the fracture is acute. The sagittal T2 Short Tau Inversion Recovery (STIR) is ideal for differentiating between chronic and acute fracture. In acute fractures, the STIR sequence will show bony edema (high signal), whereas in the chronic fracture no edema is noted.
Although 2 common classification systems address the specific anatomic features of VCFs,300 neither has been universally accepted. In general, when relaying information about the fracture pattern, it is acceptable to describe the percentage of collapse seen on conventional lateral radiographs via Cobb angle measurements.
Clinical Features
Two-thirds of VCFs will not be noticed initially, usually because the patient has minimal symptoms at the time of the event. Patients who are initially asymptomatic may present with loss of height, kyphotic deformity in the thoracic and lumbar regions, and functional declines. The patient’s osteoporosis may go untreated because of the lack of acute symptoms, which may lead to a subsequent fracture, such as a hip fracture. The other third of patients with VCFs often present with symptoms that are detected close to the time of the initial fracture. The most common presenting symptom is acute onset of back pain after an atraumatic event, such as sneezing or standing from a sitting position. Other acute findings can include loss of height and kyphotic deformity in the spine. Patients with VCFs can also experience neurologic symptoms, such as weakness or radiating pain down the leg or across the chest wall. If such symptoms are present, one should suspect retropulsion of a fracture fragment into the spinal canal or compression of a nerve root in the neural foramen (Figure 11). An MRI is indicated in this situation (Figure 12). If the patient has a contraindication to an MRI, such as a pacemaker, then a CT can be obtained to assess for canal compromise (Figure 13). If neurologic symptoms are present then a CT myelogram can be obtained. Neurologic compromise can be a serious complication and can lead to permanent weakness or disability.301
Figure 11.
Plain radiograph of a compression fracture suspicious for foraminal involvement.
Figure 12.
Sagittal magnetic resonance imaging (MRI) scan showing a fracture with retropulsion into the spinal canal.
Figure 13.
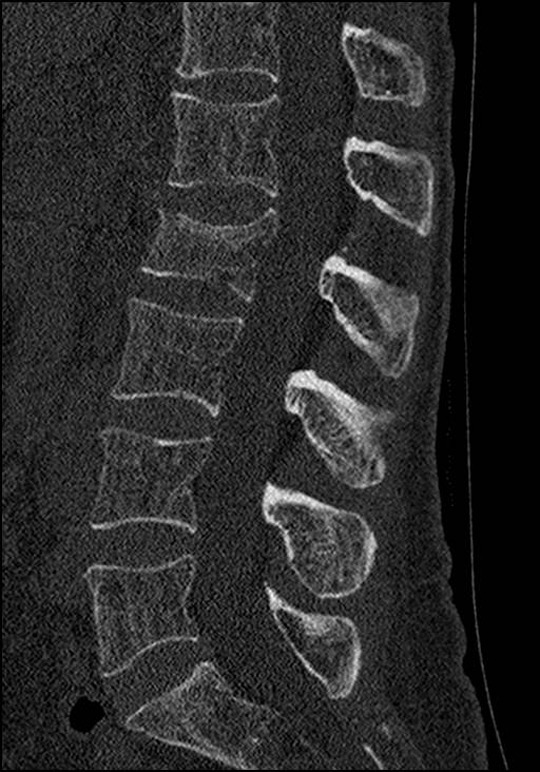
Computed tomography (CT) scan showing a vertebral compression fracture.
Radiographic Evaluation
If a VCF is suspected, conventional radiographs are a useful starting point for workup (Figure 14). AP and lateral views can be helpful. The fractures are often easily seen on the lateral view. A decrease of 4 mm or more than 20% in vertebral height compared with the baseline height of the vertebral bodies (using the normal vertebra above or below the suspected fracture site) is diagnostic of a VCF.302 Evaluation of the posterior vertebral line can also be helpful in detecting retropulsion of fracture fragments. If retropulsion or nerve compression is suspected, an MRI or CT scan should be requested.
Figure 14.
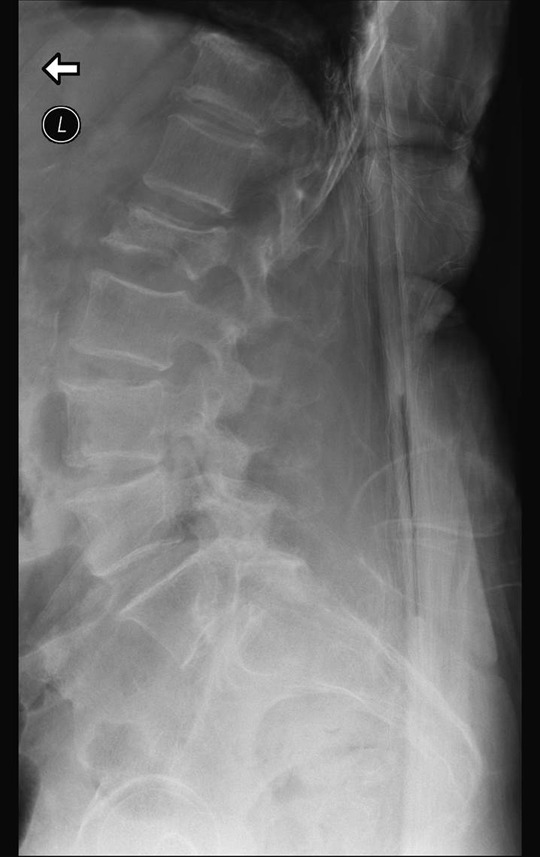
Standard radiograph showing an L1 vertebral compression fracture.
Magnetic resonance imaging is helpful in the setting of VCF for several reasons. Unlike conventional radiographs, MRI can often illustrate the acute nature of the fracture (Figure 15). The presence of marrow edema and surrounding soft-tissue edema is strongly suggestive of an acute or acute-on-chronic VCF. The MRI can also help to delineate the presence of a retropulsed fracture fragment or foraminal narrowing, which may be helpful in explaining the patient’s symptoms and in determining treatment.
Figure 15.
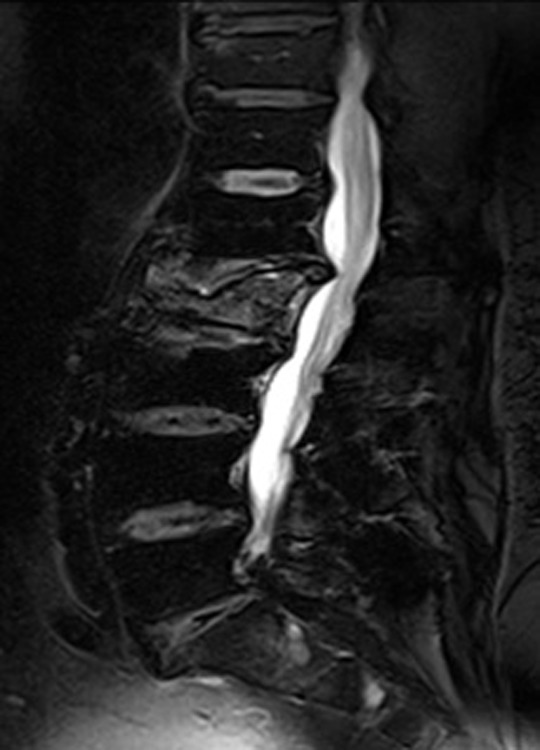
Magnetic resonance imaging (MRI) showing marrow edema with an acute vertebral compression fracture.
Patients with a history of malignancy or metastatic disease may present with a compression fracture. An MRI with and without contrast should be obtained to search for evidence of metastatic disease. The gold standard though is a CT-guided biopsy.
Finally, in some cases, when an MRI cannot be performed or is indeterminate, a bone scan can sometimes be helpful in detecting the presence of an acute fracture versus a chronic, older fracture. Bone scans may not become positive at the fracture site until about 10 days after the initial injury, and if the bone scan is obtained too early in the process, a false-negative result may occur.303
Nonoperative Treatment
Most patients with VCFs can be treated with nonsurgical options. There are 3 categories of such interventions, namely, treatment of the underlying osteoporosis, pain management, and bracing.
Treatment of the underlying osteoporosis that led to the fracture can be achieved with the use of several different medications such as calcium and vitamin D, along with calcitonin, bisphosphonates, parathyroid hormone analogue, raloxifene, and denosumab (see the section on osteoporosis for the indications for use). Pain control, maintenance of function, and correction of or stabilization of deformity are also important components in the treatment of VCF.
Pain control is an essential part of the medical treatment of VCF. Without adequate pain control, patients cannot be mobilized, which will lead to permanent functional declines and other complications frequently seen in immobile elderly individuals (e.g., decubitus ulcers, VTE, and pneumonia). NSAIDs and acetaminophen are good starting points for control of pain. However, NSAIDs should be used with caution especially in older women and in patients with a history of hypertension, gastrointestinal bleeding, ulcers, or renal disease, and acetaminophen should be used with caution in patients with advanced liver disease. If NSAID treatment is unsuccessful, a short course of narcotic medications can be considered. Narcotics can work well for pain control but can cause confusion, delirium, and constipation in elderly patients. Some medications that are used to treat the underlying osteoporosis have also been shown to improve pain related to VCFs.304,305 Calcitonin, in particular, has been associated with improvement in pain through its ability to influence beta-endorphin levels.305 Teriparatide and bisphosphonates have also been found to be associated with lessened bony pain in patients with VCF.304
Bracing can help with pain relief by reducing the amount of continued compression and micromotion at the fracture site. Bracing can also act as a supplement to muscle support for patients who experience early muscle fatigue. Bracing improves the biomechanics of the spinal column after fracture.124 Extension bracing can prevent additional collapse in the setting of an acute fracture and can help the fracture heal in a more anatomic position,306 which may in turn prevent subsequent additional fracture and pain. The Jewitt brace and the CASH brace are 2 frequently used types of braces for VCFs. They both function to provide 3-point stability to the spine and prevent flexion at the thoracic and lumbar regions.306 Both can be worn under regular clothing. The major problem with bracing is that many elderly patients are unable to tolerate it for lengthy periods of time. The braces can cause skin irritation and pressure sores. Bracing can also lead to decreased mobility if the brace is too bulky for the patient’s body type. In addition, it may also be difficult to obtain a brace that adequately fits an obese patient. The braces may contribute to further muscle atrophy. These factors, along with individual patient needs and body geometry, need to be taken into account when bracing is being considered. The patient does not need to wear the brace in bed or when in a supine position, only need to wear it when upright or walking. Bracing is recommended for 8 to 12 weeks depending on the severity of the compression fracture.
Surgical Treatment
Surgery should be reserved for patients with painful VCFs for whom nonoperative treatments have failed and those who have been shown to have an acute VCF on MRI or bone scan. The two procedures that have been approved for intervention for VCF are vertebroplasty and kyphoplasty. Vertebroplasty is the injection of polymethylmethacrylate (PMMA) bone cement, through a posterior transpedicular approach into the collapsed vertebral body. Like vertebroplasty, kyphoplasty uses PMMA to stabilize the fractured vertebral body, but it differs in that before the cement is injected, a balloon is inserted into the vertebral body and inflated to allow the vertebral body to be expanded more closely to its prefracture position. After the balloon is withdrawn, the PMMA is then injected into the expanded space and allowed to harden. Both procedures are thought to improve pain and function in patients with acute VCF307 but to have limited utility in patients with chronic back pain and chronic VCFs.
Surgical intervention for VCF is controversial, and the choice between vertebroplasty and kyphoplasty is still very much a debated topic. Advocates of kyphoplasty argue that it more accurately restores the natural anatomy of the spine (Figure 16).308,309 Advocates of vertebroplasty argue that the balloon effects on restoring the anatomy are minimal and that the pain relief experienced from both procedures is secondary to stabilization of the fracture with cement.310 Vertebroplasty advocates also argue that the risk of iatrogenically induced fracture fragment retropulsion is less with vertebroplasty.311,312 Retropulsion of bony fragments can lead to neurologic deficits and spinal cord compromise and is a major complication of either procedure.313
Figure 16.
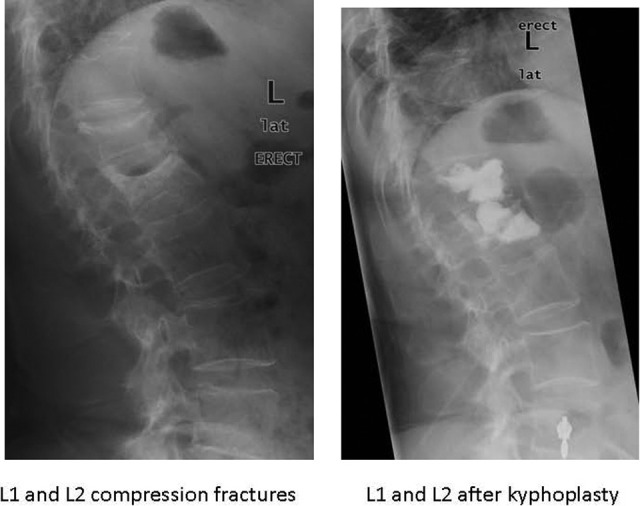
Lateral radiographs showing treatment of compression fractures with kyphoplasty cement augmentation.
Three recently published randomized placebo-controlled trials have called into question the efficacy of vertebroplasty in improving pain in patients with VCF.314–316 Kallmes et al315 found a trend toward improved pain scores over baseline in the vertebroplasty group at 1 month posttreatment, but it was not a statistically significant difference. Buchbinder et al found that at 6 months, there was no difference between groups in pain or functional scores.314 Critics of these studies point out that the analysis included patients with fractures up to 12 months old, whom many would consider beyond the window of the acute fracture period and therefore would have limited improvement from the procedure. Wardlaw et al specifically assessed the efficacy of kyphoplasty and found that patients treated with kyphoplasty had statistically significant improvements in the SF-36 scores at 1 month.316
Both vertebroplasty and kyphoplasty have potential complications, including the risk of cement extrusion into the spinal canal, retroperitoneal space, or thoracic cavity; intravascular extrusion of cement; fat embolism syndrome, which should be considered when pulmonary compromise is noted during or after the procedure; and neurologic deficits from cement causing injury to local nerve roots or the spinal cord or from subdural and epidural hematomas.312,313 Patients with bleeding disorders or on anticoagulants should have their coagulation values restored to normal before proceeding with either procedure. Patients should also be off aspirin and platelet inhibitors for 1 week before either procedure. Retropulsion of fracture fragments into the spinal canal from the pressure from the cement entering the enclosed space has been reported and can be a devastating event.317 Therefore, most advocates of these 2 procedures would argue that they should not be performed in people who already have evidence of retropulsion of the posterior vertebral body wall or an incompetent posterior vertebral body wall on MRI or conventional radiographs.
There are also concerns of adjacent-level fractures following PMMA augmentation. Low bone mineral density, low body mass index, and intradiscal cement leakage are risk factors for compression fractures adjacent to a PMMA augmented level.318
The AAOS has developed clinical practice guidelines for compression fractures (http://www.aaos.org/research/guidelines/SCFguideline.pdf). Based on a review of the literature, the AAOS is recommending against vertebroplasty and limited recommendation for kyphoplasty for compression fractures. Meanwhile the American Association of Neurological Surgeons, Congress of Neurological Surgeons, American College of Radiology, and several other radiologic societies have guidelines in support of vertebroplasty/kyphoplasty for compression fractures recalcitrant to nonsurgical management.319 For these societies, 24 hours of nonoperative management is the window prior to considering intervention. It is typical in our practice to wait a minimum of 4 weeks prior to considering vertebroplasty or kyphoplasty.
Summary
VCFs frequently occur in patients with osteoporosis and often involve complicated presentations and complex treatment decisions. Combinations of medicinal, functional, and sometimes surgical treatment options need to be considered. The vast majority of patients can be treated with medical management options, which include pain medications and bracing, but a small subset will benefit from surgical intervention. Because surgical treatment carries substantial risks, special training is suggested for those surgeons performing vertebral augmentation.
Following diagnosis of a compression fracture, referral for DEXA scan and metabolic bone specialist should be initiated.
In the setting of acute and chronic compression fractures, an MRI (T2 STIR sequence) will help in the diagnosis of the acute fracture (increased signal).
Bracing and symptomatic pain management are part of the nonoperative of acute compression fractures.
The use of vertebroplasty/kyphoplasty in the management of acute compression fractures is controversial. There may be a role for these modalities for recalcitrant pain associated with compression fractures.
In the setting of prior malignancy or metastatic disease, MRI with and without contrast should be ordered. Biopsy of the vertebra should be performed if the MRI is suspicious for a malignancy.
Fragility Fractures of the Foot and Ankle
Fernando H. Serna, Jr., MD, and Benedict F. DiGiovanni, MD
Foot and ankle injuries are among the most common orthopaedic complaints, with a published pooled incidence for ankle fractures of up to 184 fractures per 100 000 person-years; 20% to 30% of those fractures occur in the elderly patients.320 These injuries result from both high- or low-energy mechanisms, with low-energy trauma—such as slips, twists, and ground-level falls—being the more common cause in the elderly population. In recent years, the incidence and severity of ankle fractures (more unstable supination-eversion injuries) in the elderly patients has been increasing.321,322 The incidence of foot and ankle fractures in elderly, nonblack women has been reported to be 3.0 and 3.1 per 1000 woman-years, respectively, with fractures of the fifth metatarsal and distal fibula being the most common.323 The incidence of fragility fractures increases beginning in middle age.324
Risk Factors
The risk factor profiles for foot and ankle fragility fractures differ between middle-aged and older men and women.324 For men, the most commonly associated risk factors are diabetes and hospitalization for mental health problems; for women, they are diabetes, a previous fracture, and high body mass index (BMI), the last of which specifically applies to ankle fractures.324
Risk factor profiles for ankle versus foot fractures differ in elderly women.323,325 Those sustaining ankle fractures tend to be younger, have a higher BMI, participate in more vigorous physical activity, have gained weight since age 25, fallen within the previous 12 months, self-report osteoarthritis, have a blood relative who sustained a hip fracture after age 50, and get out of the house 1 time or less per week. Male and female patients sustaining foot fractures have lower distal radius and calcaneal bone mineral density values, are less physically active, more likely to have had a previous fracture, have a history of benzodiazepine use, have insulin-dependent diabetes mellitus, and have poor far-depth visual perception.326
An increasing rate of falls from baseline continues to be a risk factor for hip and proximal humerus fractures—the classic fragility fractures—but not for foot or ankle fractures.327 However, risk factor profiles for foot and ankle fractures are similar to those of other fragility fractures in that there is a significant correlation with low bone mass or density.325 Although foot and ankle fractures in the elderly patients are commonly categorized as osteoporotic fragility fractures, clinical studies have shown that the incidence of such fractures rises until the age of 65 and then plateaus or decreases thereafter, calling into question the relationship between these injuries and bone quality.328 Therefore, the increased incidence of ankle fractures may result more from an increasing number of active elderly patients and other factors such as higher BMI and frequent falls rather than the aging process and the presence of osteopenia or osteoporosis.328 Consensus appears to be shifting toward the belief that the increasing incidence of ankle fractures in the older population is secondary to increasing levels of activity and the resultant falls and trauma, while foot fractures remain largely secondary to poor bone quality.
Treatment of Foot Fractures
Fragility fractures in the foot occur most commonly in the tarsals and metatarsals. Fractures of the fifth metatarsal—the most commonly fractured foot bone323—are seen primarily in an acute or traumatic setting. Stress fractures are the most common type of foot fractures—affecting the talus, navicular, great toe sesamoids, and other metatarsals—and are defined as injuries resulting from excessive, repetitive, and submaximal loads resulting in an imbalance between bone resorption and formation. This is seen most often in the setting of intrinsic (eg, metabolic bone disease) or extrinsic (eg, muscle fatigue) factors.329 Initial conventional radiographs are often negative, but clinical suspicion and physical examination findings supporting the diagnosis should prompt appropriate initial treatment. This usually includes protected weight bearing and immobilization in a cast or fracture boot. Repeat radiographs obtained at 10 to 14 days postinjury or the onset of symptoms often show evidence of fracture lines and/or apparent callus formation that was not present or appreciated initially.
Nonoperative Treatment
The mainstay of treatment for many fractures is nonoperative intervention. Rigid cast immobilization or the use of a fracture boot for 6 to 8 weeks, with avoidance of or protected weight-bearing and aggressive treatment targeted at the causative intrinsic or extrinsic factors, allows for successful healing in most cases. However, the treating clinician must be vigilant for progression to complete fracture, delayed union or nonunion, and for the presence of any secondary complications such as skin ulceration.
Surgical Treatment
Fractures that are displaced or with chronic radiographic findings such as intramedullary sclerosis or cystic changes frequently require operative stabilization with percutaneous pinning or screw fixation, ORIF, or excision, such as sesamoidectomy.
Treatment of Ankle Fractures
The primary goals in treating these injuries are to (1) provide a functionally stable ankle joint, (2) return the patient to activities of daily living and preinjury functional levels, and (3) avoid the risks of prolonged immobilization and bed rest. Despite clear indications for treatment of such fractures in the young, there continues to be controversy regarding their optimal treatment in the elderly individuals. In this elderly population, conventional treatment modalities can be challenging secondary to poor bone quality, poor soft-tissue integrity, intrinsic instability, and difficulty in complying with weight-bearing limitations.
Nonoperative Treatment of Ankle Fractures
For nondisplaced fractures, nonoperative management with splint or cast immobilization and serial radiographic follow-up can provide satisfactory results without the risks of surgical intervention. Reported data also indicate that even displaced, but well-reduced and stable fractures in elderly patients can be managed successfully with nonoperative treatment methods.320
Surgical Treatment of Ankle Fractures
Operative stabilization should be considered for fracture dislocations and other unstable injury patterns. Although early studies recommended against this approach in the elderly individuals, recent studies have shown increasingly positive results.328 These results compared to nonoperative management can be attributed, in part, to improved postoperative rehabilitation, the use of fixed-angle devices, and an increased awareness of potential complications. A combination of internal and external fixation, at the initial surgery or in a staged fashion, has also been recommended in those cases where operative stabilization is deemed necessary, and one also wishes to avoid the potential soft-tissue complications of rigid splint, brace, or cast immobilization.
Special Considerations in Patients With Diabetes Mellitus
Compared to patients without diabetes, individuals with diabetes mellitus have a higher risk of complications with either surgical or nonoperative management of their foot or ankle injuries. Vascular disease and neuropathy (loss of protective sensation) play a role in predisposing diabetic patients to further injury, loss of reduction, delayed union, malunion, nonunion, infections, and soft-tissue or wound complications. The following is the method for testing plantar sensation using Semmes-Weinstein 5.07/10-g monofilament:
The filament should be pushed against the plantar surface of the foot (in multiple areas to map out the entire footprint).
Just enough pressure should be exerted for the filament to start bending.
It is helpful to start with the more proximal leg area (above the area of typical stocking distribution of neuropathy) and demonstrate the method and type of sensation expected. This will allow the patient to better understand the significance of the examination and the findings of potential loss of sensation.
If a patient cannot sense this pressure, the loss of protective sensation should be documented. It is important to remember that the ability to sense this pressure does not mean that neuropathy is not present but does mean that protective sensation is lost.
The result should be compared with that of the contralateral foot (may not be helpful given the tendency of bilateral involvement in diabetic neuropathy).
An assessment should be made based on how much of the plantar surface is affected.
Nondisplaced fractures can be treated nonoperatively with prolonged cast immobilization in a well-padded, nonweight-bearing cast. Patients with diabetes often have difficulty with cast immobilization and weight-bearing restrictions; close clinical and radiographic follow-up is necessary to improve outcomes. Early and aggressive operative stabilization has been recommended for displaced or unstable injuries in the diabetic elderly population.330 Treating such injuries nonoperatively results in a high rate of progression to malunion or nonunion,331 and patients may ultimately require surgical intervention in a delayed fashion. A meta-analysis of 140 diabetic ankle fractures showed an overall operative cohort complication rate of 30%, with an infection rate of 25%, a Charcot arthropathy rate of 7%, and a Charcot amputation rate of 5%.332 There are trends toward using supplemental fixation, multiple syndesmosis screws, and alternative implants (fixed-angle locking constructs) in patients of advanced age, with diabetes, comorbidities, or neuropathy; in those without comorbidities, one can expect results of operative management similar to those in patients without diabetes.333 Medical management of the patient’s diabetes should be supervised and optimized by a primary care physician or endocrinologist, as studies have shown that a hemoglobin A1C >7 is associated with increased complications.
Investigators have recommended a longer period of postoperative immobilization and subsequent protected weight-bearing and bracing in diabetic patients.333,334 As a general rule, the authors typically immobilize and protect weight-bearing for about twice as long in patients with diabetes mellitus compared to those without, especially in those patients with loss of protective sensation. Increased vigilance for complications such as loss of reduction, wound breakdown, plantar ulceration secondary to loss of protective sensation, and Charcot neuro-arthropathy is recommended.
Summary
Foot and ankle fractures are common injuries in the older population. Treating providers must identify risk factors for such injuries, know which injuries are amenable to nonoperative or operative management strategies, and have a strong focus on prevention. Appropriate medical management directed at any underlying comorbidities (eg, conditions such as osteoporosis, diabetes, peripheral vascular disease) is paramount successful management. Treating providers should consider all factors including age, functional level or capacity, bone quality, and the presence of comorbidities when outlining a treatment strategy.
Pelvic and Acetabular Fractures
Theodore Manson, MD
Pathophysiology
Fractures of the pelvis are common injuries in the elderly patient. The most common cause is a low-velocity fall, often from a standing height. The vector is most often thought to be on the side of the greater trochanter, resulting in a compression injury to the pelvis. Acetabular fractures in the elderly individuals, which may also be the result of a low-velocity fall, are becoming more common. Both of these types of fractures may also occur from higher velocity forces.
Classification
In the elderly individuals, a pelvic ring fracture typically involves 2 or more bony areas, most commonly the sacrum and one or both pubic rami. Another pattern of injury is the sacral insufficiency fracture which may be the result of little or no trauma. Sacral insufficiency fractures may be bilateral or involve a horizontal component though the sacrum. Acetabular fracture in the elderly patients may approximate the injury patterns seen in younger patients. Some patterns are more common, such as anterior wall fracture and associated both column fractures.
Clinical Features
Patients with pelvic or acetabular fractures have pain in the hip or groin region. It may be difficult to distinguish pelvic fractures from a hip fracture. Patients with sacral insufficiency fracture often present with low back pain. Both pelvic and acetabular fractures may result in bleeding, especially in the anticoagulated patient. Retroperitoneal hematoma may cause critical bleeding, and the hematocrit level should be monitored. Elderly patients can be at a higher risk of bleeding, even with relatively simple, low-energy fracture patterns.335 Elderly patients who have a pelvic fracture do not necessarily need to be observed with serial hemoglobin levels, but one baseline hemoglobin level is probably prudent for acute injuries.
Radiographic Evaluation
Standard AP pelvis and hip radiographs should be the first study ordered. If a pelvic fracture is recognized, inlet and outlet views will give better views of the pelvis. If an acetabular fracture is identified, oblique or Judet views are standard to determine the fracture type. The seagull sign, seen on oblique views, has been characterized as a poor prognostic indicator for elderly patients with acetabular fractures.336 This sign indicates substantial impaction and damage to the joint surface. For pelvic fractures, a CT scan facilitates recognition of posterior sacral injury that is often not apparent on conventional radiographs. For acetabular fractures, a CT scan provides better visualization of marginal impaction than conventional radiographs and can help the clinician classify the fracture. When a fracture cannot be visualized on radiographs or CT scans, a MRI scan can help determine whether a hip, pelvic, or acetabular fracture is present. The MRI scans also provide excellent imaging of sacral insufficiency fractures.
Which Elderly Patients With Pelvic Ring Fractures Benefit From Surgery?
Elderly patients with pelvic ring fractures are most often treated nonoperatively, as these are frequently impaction injuries of the sacrum and are very amenable to treatment without surgery.335,337,338 Surgery in pelvic ring fractures is undertaken if patients have (1) intractable pain which prevents the patient from mobilizing, (2) unstable fracture patterns, and (3) fractures which cause marked leg length discrepancy. In most cases, particularly in injuries caused by a low-energy fall, elderly patients with pelvic ring fracture should be treated nonoperatively, as the fractures are not unstable and do not produce marked leg length discrepancy. In some cases, most often from higher energy mechanisms, elderly patients may have a fracture in which the pelvic ring is unstable. In these cases, it probably benefits the patient to have percutaneous screw fixation of the pelvic ring to allow for earlier mobilization and to prevent nonunion with leg length discrepancy.338–340 Patients who cannot be mobilized due to pain, which is uncommon, should also be considered for surgery. This type of surgery usually involves percutaneous methods to stabilize the pelvis.341,342
Acetabular Fractures
Acetabular fractures in the elderly patient historically have been caused by low-energy mechanism such as a fall from standing height. However, higher energy mechanisms such as the elderly person involved in a motor vehicle collision are becoming more frequent. The decision to operate is based on both the functional status and the mental state of the patient as well as the fracture characteristics.
In general, the patients who are community ambulators do not have severe dementia and are physiologically able to undergo surgery should be considered for operative treatment of their acetabular fracture if they have an incongruent hip joint or marked fracture displacement. The majority of the patients who are surgical candidates are simply treated with the same fixation techniques as younger patients343 , 344 however; there has been a trend toward less invasive percutaneous methods in selected patients in fracture patterns.345
There are 3 radiographic signs that have been associated with higher rates of failure (progression to posttraumatic arthritis) in elderly patients with acetabular fractures. The patient with impaction of the acetabular dome called the seagull sign (Figure 17),346 a posterior wall component to their fracture347,348 (Figure 18), or impaction of the chondral surfaces of the femoral head (Figure 19) which is most often visible on the coronal reformatted CT scan.348 These have all been associated with higher rates of clinical failure with standard ORIF. In patients with these selected radiographic criteria consideration should be given to either concomitant fixation of the acetabular fracture and placement of a total hip replacement in the same surgery and through the same incision,349–355 ornon-operative treatment356 followed by delayed total hip arthroplasty.
Figure 17.
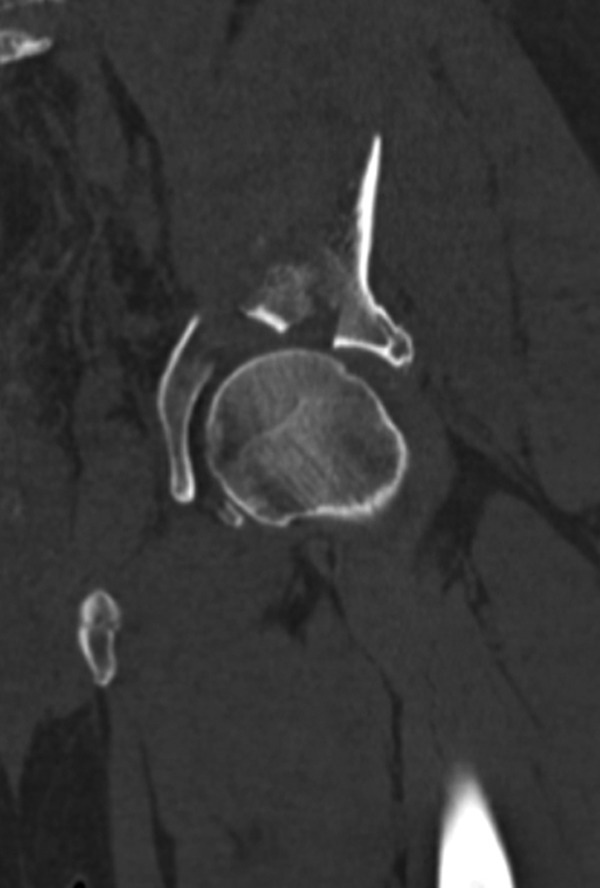
Computed tomography (CT) scan showing a comminuted acetabular fracture. The pieces of the broken acetabular surface makeup the wings of the “sea gull sign.” This is an indicator that total hip, either acute or delayed is a better treatment option.
Figure 18.
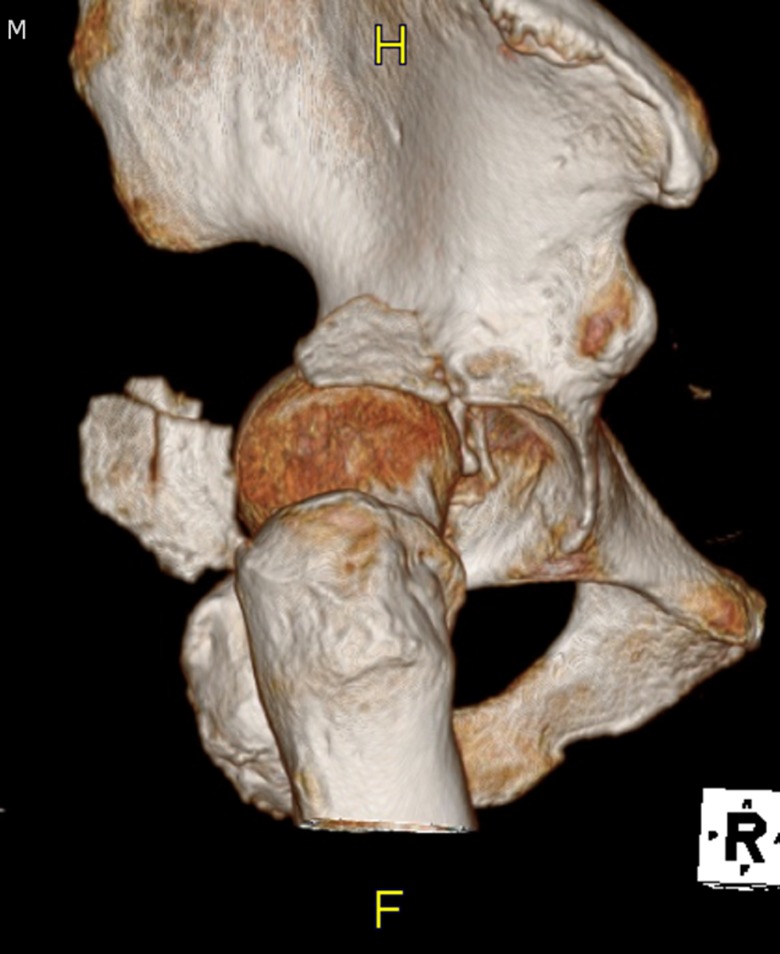
Three-dimensional computed tomography (CT) scan of an acetabular fracture in an elderly patient showing comminution of the weight-bearing area of the joint and posterior wall involvement.
Figure 19.
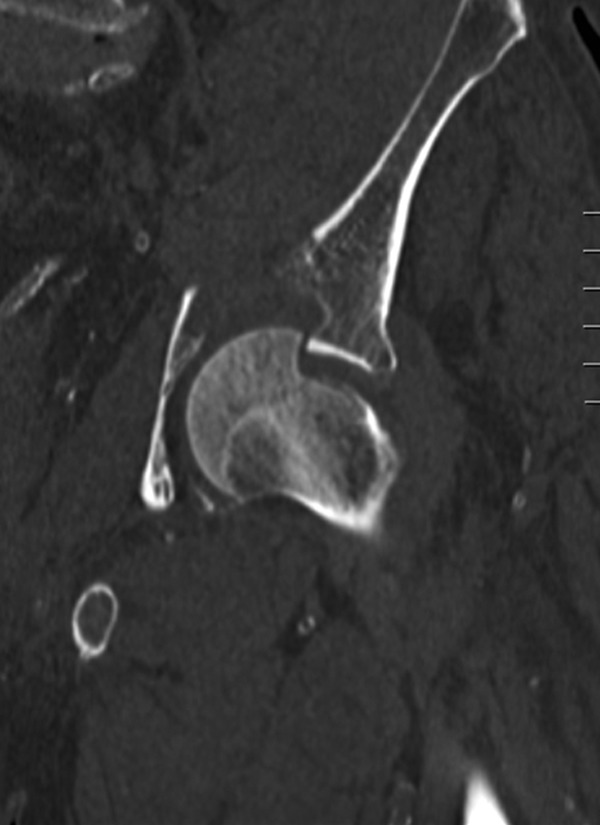
Computed tomography (CT) scan showing femoral head impaction injury which is an indicator of poor outcome for open reduction and internal fixation (ORIF) of an acetabular fracture in an elderly patient.
Patients with a posterior wall component to their fracture and a history of a hip dislocation may be difficult to keep reduced without operative treatment of their fracture. Patients with marked protrusio on their injury x-rays may have quite a bit of pain with impingement of the femoral neck on the acetabulum and may have internal organ irritation such as bladder or vessel irritation and may benefit from surgery.
Surgical Treatment
Most elderly patients with pelvic ring fractures may be treated nonoperatively; however, markedly displaced or unstable pelvic ring injuries (usually caused by high-energy mechanisms such as a motor vehicle collision) may sometimes benefit from surgical stabilization. Most often percutaneous methods are utilized for stabilization of the posterior pelvic ring in elderly patients with either percutaneous screw357 or external fixator stabilization of the anterior pelvic ring.339 , 341 , 342
Surgical treatment of acetabular fractures in the elderly individuals spans the range of treatment modalities. Less invasive methods include percutaneous stabilization of the acetabular fracture; however, only certain fracture patterns are amenable to percutaneous screw fixation and these require specialized techniques and instruments.
Standard open reduction (ORIF) of acetabular fractures is most often utilized in the elderly patients and involves restoring the bony architecture with clamps and then plate and screw fixation to hold the bony surfaces in place until the fracture is healed.343 , 344 Usually, these are larger surgeries and the patient must be carefully evaluated properly to make sure that they are physiologically able to undergo such a surgery prior to treatment. A third emerging option is stabilization of the bony components of the acetabulum with plate and screws with concomitant total hip arthroplasty through the same incision.351–355 , 358 In these instances, usually an anterior or posterior approach to the hip is used. Plate and screws are used to fix the fractured acetabulum and then a relatively standard hip replacement is placed at the same time through the same incision (Figures 20 and 21).
Figure 20.

A model showing a posterior column plate with acetabular component in place.
Figure 21.
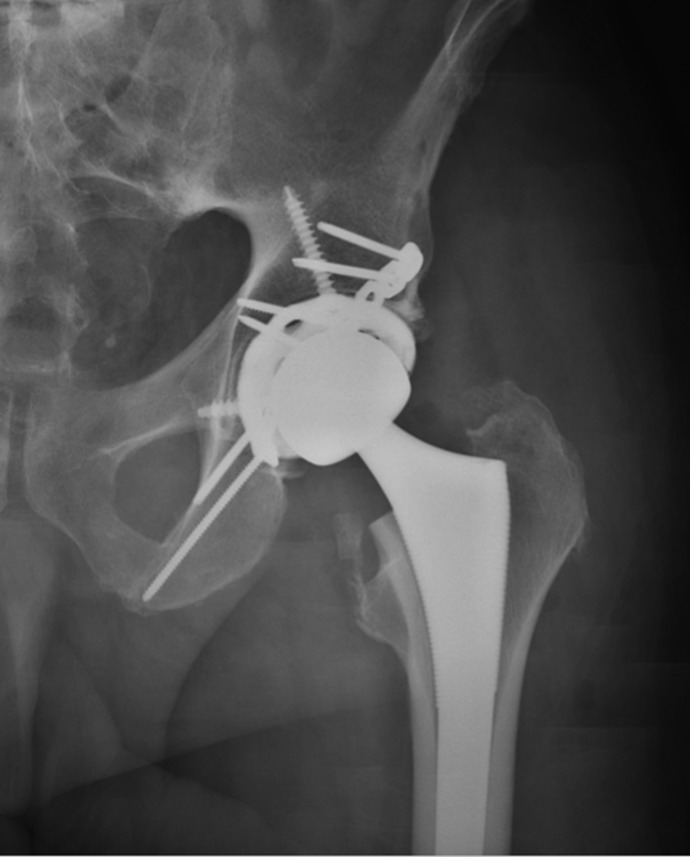
Radiographic view of simultaneous acetabular fracture fixation and total hip replacement.
All of these operative options require the patient who is physiologically able to undergo surgery and can comply with any postoperative restrictions. Usually after operative stabilization of acetabular fractures, the patient is instructed to remain nonweight-bearing for period of 6 to 12 weeks; however, these restrictions have been questioned recently with several authors moving toward earlier weight-bearing for elderly patients following operative fixation.359
Rehabilitation
Rehabilitation for a pelvic fracture is started with ambulatory aids and weight bearing as tolerated. In cases of operative fixation, weight bearing as tolerated may not be possible, and patients may be limited to a bed-to-chair status until fracture healing.
Pelvic and acetabular fractures are a common injury in elderly patients, and they often require hospitalization.
Most pelvic fractures are stable and are treated with physical therapy, weight bearing as tolerated, pain control, and thromboprophylaxis.
Treatment of acetabular fractures in the elderly patient is a controversial topic.
Stable and congruent fracture should be treated nonoperatively.
Displaced fractures may be treated with percutaneous or open fixation, or immediate or delayed hip replacement.
Osteoporosis Management
Susan V. Bukata, MD
A fragility fracture is a low-energy fracture that occurs when a patient falls from standing height or less. The vast majority of fractures are associated with a fall, although some vertebral fractures occur with simple bending or overhead lifting activities. Because these injuries occur with a fall, physicians and patients often do not recognize that the fracture is a fragility fracture. This misconception often leads to both patients and physicians to not recognize that the patient likely has osteoporosis. The most common locations of fragility fractures are vertebrae, hip, and distal radius, but pelvic, proximal humerus, distal femur, proximal tibia, and clavicle fractures can also occur from low energy falls, the majority of these patients have osteoporosis, but many patients are not diagnosed or treated.360
Defining the Disease of Osteoporosis
Osteoporosis is a metabolic bone disease characterized by low bone mass with microarchitectural and biomechanical deterioration of bone tissue that results in enhanced bone fragility and a consequent increase in fracture risk.361 Although bone mass is an important component of the disease, it is the combination of bone mass and bone quality that results in a bone’s overall strength and ability to resist fracture. Bone mass can be easily measured, but bone quality is more difficult to assess and those elements that influence bone quality are still being defined. Age is one of the most important risk factors for fracture and the reduction in bone mass and quality with age may account for much of the increased fracture risk even if bone density remains stable.
Bone density is generally measured using a dual-energy X-ray absorptiometry (DXA) scan.362 Using low doses of radiation (1-3 mrem which is about the exposure on a 2500-mile airplane trip [2.5 mrem]), an individual’s bone mass can be measured at lumbar spine, hip, and forearm. Generally, 3 sites are chosen for assessment. Any location that contains implants cannot be accurately assessed. In the spine any level that has significant arthritis, a compression fracture, a vertebroplasty or kyphoplasty, or has had a laminectomy also cannot be accurately assessed. The patient remains clothed throughout the test which takes about 15 to 20 minutes to perform. Patients are scored against race and sex-matched controls. Bone mineral density (BMD) (in gm/cm2) for each measured site is then matched to curves appropriate for race and sex to determine a patient’s score for that site.
T-score is used in all patients aged older than 25 and scores the patient’s bone mineral density relative to the population at the time of peak bone mass (which occurs between ages 25 and 30). Z-scores compare the patient relative to the bone density of peers their same age. The Z-score is used for children and young adults younger than age 25 because they are still gaining bone mass and have not yet reached their peak. For all other patients, T-score is used to report their level of bone density. The World Health Organization defines a T-score of ≤−2.5 as osteoporotic bone mass, and scores between −1 and −2.4 as osteopenic (low) bone mass.363 It is important to remember that these bone mass measurements alone do not define osteoporosis, but identify individuals who are at risk of osteoporosis. Bone mass measurements need to be considered in the context of other risk factors to determine when to initiate treatment. In a young patient with a T score of -2.5, fracture risk is very low, while in a 90-year-old with a T score of -1.5, the fracture risk is quite high.
Quantitative CT scan can also be used to generate T-scores and Z-scores, but radiation exposure is significantly greater. In countries and areas where there is no access to DXA scanning, quantitative CT may be used, especially for a baseline assessment. Ultrasound can be used to evaluate subcutaneous bones (tibia, calcaneus) but results do not correlate well with DXA measurements at the hip and spine and its clinical utility has not been fully defined.
Bone quality is more elusive to define and to measure; it relates to those characteristics that contribute to bone strength other than the bone mineral density. This includes not only the architectural distribution and integrity of bone substance. The rate at which the bone remodels the distribution of bone mineral and collagen, porosity of the cortex, the shape of trabeculae within the bone as well as other yet to be defined factors influence bone quality and are not easy to measure. For that reason, important risk factors for fracture are used to assess the influence these bone quality factors may make to bone strength. These factors include age, sex, body mass index (BMI), personal history of fragility fracture, parental history of hip fracture, smoking, glucocorticoid use, rheumatoid arthritis (or other chronic autoimmune inflammatory diseases), secondary causes of osteoporosis (type 1 diabetes, low Vitamin D levels, hyperthyroidism, hyperparathyroidism, hypogonadism or premature menopause (<age 45), chronic malnutrition, malabsorption, eating disorders, chronic liver disease, chronic kidney disease, HIV infection or treatments with medications that can cause any of the above-mentionedissues), or daily alcohol intake >3 units.364
FRAX (available at www.sheffield.ac.uk/FRAX) is a fracture risk assessment tool designed to predict fracture risk by combining these risk factors for reduced bone quality and includes an optional field for measurement of bone mineral density.365 FRAX evaluates the bone mineral density at the femoral neck (use the lower score if both hips are evaluated) and calculates a 10-year risk of fragility fracture at any site (major osteoporotic fracture) and of hip fracture. In the United States, it is recommended that patients with a 10-year hip fracture risk of 3% or greater, or a major osteoporotic fracture risk of 20% or greater, receive treatment for osteoporosis. Each country has its own threshold for the initiation of treatment and that information is available through the FRAX Web site. FRAX is helpful in the assessment of patients who have not yet been treated for osteoporosis.365,366 For patients who have been treated with osteoporosis medications, their fracture risk will be reduced by the medications. In these patients, the FRAX risk estimates are not valid, but the FRAX algorithm can be used to help both the physician and the patient appreciate the severity of osteoporosis for that individual patient.366 One major issue with FRAX occurs if bone mass at a patient’s spine is significantly lower than bone mass at the hip. Fracture risk for that patient may still be quite high, especially at the spine, but the algorithm may not identify this.
Fragility Fracture as a Major Risk for Future Fracture
One important risk factor for future fragility fracture is history of a fragility fracture as an adult. Regardless of bone mass, that individual’s risk of future fragility fracture is increased. Half of all patients with fragility fracture will have a second fracture, and risk of future fracture is immediately increased with 10% of patients having second fracture within 1 year of the initial fracture.367 Regardless of first fragility fracture site, a patient’s risk of hip fracture is doubled.367 Despite this knowledge and the availability of multiple medications that can reduce this fracture risk by 25% to 50%, only about 16% to 20% of patients with hip fracture and wrist fracture receive an assessment for osteoporosis. Directed fragility fracture intervention programs have dramatically improved these rates of assessment and osteoporosis treatment and have demonstrated a decrease in fracture rates. All of these programs depend upon the medical staff recognizing a fragility fracture when it occurs (or when it is picked up incidentally such as an occult VCF) and entering the patient into the intervention program.
Diagnostic Workup for Osteoporosis
Any adult with a fragility fracture should have an assessment for osteoporosis.27 For patients who have never had a fracture, current National Osteoporosis Foundation (NOF) recommendations suggest that women aged 65 and older, men age 70 and older, or men and women older than age 50 who have significant risk factors for osteoporosis, should have a bone mineral density test (DXA) and an assessment for fracture risk.368 It is estimated that 30% of patients with osteoporosis have a secondary cause for the disease.369 The rate is even higher in premenopausal women and men with osteoporosis (50%-60%) and in patients who have had a hip fracture (more than 80%).369 Patients undergoing assessment for osteoporosis, particularly those who have suffered a fragility fracture, should have additional laboratory testing to assess for these secondary causes. Serum calcium, estimated GFR, 25-hydroxy vitamin D, intact PTH, TSH, and testosterone levels (for men) should be a part of the osteoporosis assessment. For patients with known renal disease, or those with a GFR <35, levels of 1,25-dihydroxyvitamin D should be added. In patients who have not experienced a recent fracture, markers of bone turnover such as bone-specific alkaline phosphatase or P1NP and urine N-telopeptide or serum C-telopeptide can be added. These turnover markers will be elevated in the setting of a recent fracture that is healing and can remain elevated for a few months after the fracture, limiting the usefulness of these markers in the setting of an acute fracture.370
Osteoporosis Assessment
DXA scan for bone density (patients may need to wait 6 weeks after fracture to be comfortable enough to do it):
Laboratory test results include:
25 Vitamin D;
serum calcium;
intact parathyroid hormone (PTH); and
estimated glomerular filtration rate (eGFR).
Consider:
FRAX assessment if patient not taking osteoporosis therapy;
1, 25 Vitamin D level if in stage IV or Stage V renal failure;
Thyroid Stimulating Hormone level; and
Bone turnover markers (NTX, CTX, bone-specific alkaline phosphatase, and P1NP)—these will not be valid baseline for 6 to 9 months after a fragility fracture, so do not order in the setting of an acute fracture but can be used for monitoring therapy.
The Importance of Vitamin D
Vitamin D insufficiency or deficiency is common in the US population in all age ranges. Patients with hip fracture have demonstrated Vitamin D insufficiency rates as high as 70% to 90%.371 Vitamin D is important not only for bone strength and mineralization but also for lower extremity muscle strength, gait speed and performance, and balance in individuals older than 65 years.372 Vitamin D has also been shown to reduce the risk of falls in the geriatric population which is a major cause of fragility fracture. Vitamin D is a fat-soluble hormone that is produced in the skin or obtained from the diet. It is then processed by the liver (hydroxylated at the 25th carbon), and finally processed by the kidney (additional hydroxylation at the 1 carbon) to produce 1,25 dihydroxy-vitamin D. Recent evidence has shown that many other tissues are capable of 1-hydroxylating Vitamin D. Recent evidence shows373 almost 85% of vitamin D is metabolized outside the kidneys and used locally by the tissues that process it (including osteoblasts).374 These tissues do not contribute to the serum levels of 1,25 Vitamin D. For that reason, it is the serum level of 25-hydroxy vitamin D that is relevant to assess vitamin D sufficiency. Vitamin D insufficiency is defined as a serum 25-vitamin D level <32 ng/mL (<75 nmol/L). Deficiency is defined as levels <20 ng/mL (<50 nmol/L).371 Because vitamin D is a fat-soluble hormone, there is concern for accumulation and toxicity. Toxicity occurs when patients experience hypercalcemia (serum calcium levels 10.5 mg/dL or greater). Toxicity is rare and is accompanied by symptoms of anorexia, nausea, polyuria, polydipsia, weakness, and pruritis.371
Recommendations for vitamin D are evolving. Current recommendations for patients with osteoporosis or significant risk factors for osteoporosis are for 800 to 1200 IU vitamin D supplement in addition to dietary intake but can vary in need from 400 IU to 4000 IU daily.375 Baseline vitamin D level, increased age, obesity, darker skin pigmentation, certain medications, and malabsorption can all increase the dose of vitamin D needed by an individual to achieve and maintain sufficient vitamin D levels. More aggressive recommendations suggest that for adults 2000 IU vitamin D3 daily is needed for maximal effect. More vitamin D is also needed in the winter and early spring compared to the summer and fall as almost all areas of the United States do not have adequate sun strength to optimally produce vitamin D in the skin, even with extended sun exposure. Use of SPF 8 sunblock decreases the capacity of the skin to produce vitamin D by 95% and clothing used to protect the skin from sun exposure or to cover skin for cultural purposes can have a similar effect. Two forms of vitamin D are available for supplementation, namely, vitamin D2 derived from plant sources and vitamin D3 derived from animal sources. Vitamin D2 is not efficiently metabolized in humans (only 20%-40% as efficient as D3). Vitamin D2 is available in larger prescription doses (50 000 IU) and can be helpful if rapid correction of vitamin D levels is needed, such as after a fracture or in the setting of hypocalcemia. Use of vitamin D3 supplements is encouraged for long-term maintenance dosing or if correction can occur over several months. Various recommendations have been made for post-fracture patients and vitamin D supplementation.375 Vitamin D levels can be checked at the time of fracture and adjustments made to patient regimens based upon these results. If laboratory testing is not possible, the addition of 2000 IU vitamin D3 daily to the patient medication regimen and treatment with 50 000 IU of vitamin D2 once weekly for 5 to 8 weeks can be used safely as long as the patient is not hypercalcemic upon presentation.
Treatment of Osteoporosis
Many medications are now available for the treatment of osteoporosis. The goal of all therapies is to maintain bone mass, limit bone loss, and decrease fracture risk. All therapies decrease the risk of vertebral fracture by at least 50%, while changes in hip fracture risk and other nonvertebral fractures vary from drug to drug. All of the currently available medications except analogs of parathyroid hormone are antiresorptive agents that work by affecting the osteoclast to prevent additional bone loss. These agents do not stimulate new bone formation and that should not be the goal of therapy with these agents. Antiresorptive agents prevent additional bone loss and appear to affect other quality factors in the bone, making it stronger and more fracture resistant.
Bisphosphonates are analogs of hydroxyapatite that deposit into the bone and effect the development and activity of osteoclasts. This effect significantly slows the rate of bone loss. Oral agents including alendronate, risedronate, and ibandronate can be given weekly or monthly depending upon the medication. Zoledronic acid, a bisphosphonate given by once yearly intravenous infusion, has also been shown to decrease mortality after hip fractures.376 Bisphosphonates have been shown to be very effective in fracture prevention, but their use and side effects have raised some concerns recently. Oral bisphosphonates can cause gastroesophageal side effects in patients.377 Oral agents should not be used in patients with Barrett esophagitis, as these patients are at increased risk of esophageal cancer and any additional esophageal irritation should be avoided. Intravenous bisphosphonates are generally used when patients experience GI intolerance to oral agents or when patients have difficulty maintaining compliance with medication regimens (dementia and polypharmacy) and a notation of these reasons may be needed when applying for insurance approval. Fever and flu-like symptoms with muscle and joint aches, an acute-phase reaction, can be seen with the start of all bisphosphonates, especially in patients who have never been treated with any bisphosphonate in the past.378,379 This reaction occurs with the initial dosing and the intensity and duration of symptoms for this reaction appears of correlate with medications with decreased dosing frequency (yearly vs weekly). Acetaminophen and nonsteroidal drugs can help lessen the severity of the reaction. The reaction is rare with subsequent dosing. All bisphosphonates are cleared intact through the kidneys and should not be used in patients who have a GFR <35.
Estrogen and estrogen with progesterone have been used less frequently, since the Women’s Health Initiative studies raised concerned about increased heart disease and stroke as well as an increased rate of invasive breast cancer in patients using estrogen with progesterone.379 Estrogen does demonstrate reduced risk of both vertebral and hip fractures, but current recommendations suggest that it be used only in patients who also require it for vasomotor or urogenital problems. It is not recommended as a first-line therapy solely for osteoporosis treatment.
Selective estrogen receptor modulators (SERM’s) are not hormones, but they work through the estrogen receptor to produce some of the bone sparing effects of estrogen. Raloxifene is the only form available in the United States. It has been shown not only to reduce fracture risk but also to reduce the risk of developing invasive breast cancer by 50%.380
Denosumab is a fully human monoclonal antibody to RANK ligand that works by inhibiting development and activity of osteoclasts, decreasing bone resorption rates. It is given by injection twice yearly and is cleared from the body between injections. It can be used in patients regardless of renal function level, but transient hypocalcemia is more common in patients with severe renal failure.381
Odanacatib is part of an emerging treatment category and is a cathepsin K inhibitor that interferes with an enzyme pathway in osteoclasts important for bone resorption but does not appear to have an effect on bone formation rates. Odanacatib, given by pill once weekly, causes osteoclasts to stop resorbing bone but does not cause the osteoclast to undergo apoptosis.382 Odanacatib is currently in clinical trials.
Teripartide (1-34 PTH), is the only anabolic agent currently available in the United States. Full-length PTH (or 1-84 PTH) is also available in some countries. Both agents have been shown to stimulate bone formation and increase bone mass. These anabolic agents also allow the redevelopment and reconnection of some of the trabecular struts that have been lost with osteoporosis, affecting the architecture of the bone as well as the overall bone mass. Parathyroid hormone analogs are currently only available as a daily injection (5-mm 31 G needle is used for subcutaneous injection in a similar manner to insulin injection). It is indicated in patients with severe osteoporosis (T score< −3.0), patients who are intolerant of other osteoporosis agents, or in patients who have fractured despite the use of other osteoporosis medications. These reasons should be stated when seeking insurance approval for this medication. Parathyroid hormone is used for 2 years followed by an antiresorptive agent.383 Additional anabolic agents in the Wnt signaling pathway (anti-sclerostin and anti-DKK 1 agents) are in clinical development and clinical trial.
Regardless of the treatment chosen for osteoporosis, all patients should require adequate calcium and Vitamin D intake. Generally this requires supplementation. The National Academy of Sciences recommends a daily intake of 1200 mg calcium for adults aged older than 50 years.384 Most individuals only get 700 to 900 mg of calcium through their diet and require either increase in dietary intake or supplements to reach the daily intake goal of 1200 mg. Caution should be taken not to supplement patient much beyond the 1500 mg total daily goal as increased risk of kidney stones and possibly vascular calcification can be associated with high daily calcium intake (>2000 mg daily).385 Vitamin D supplements should also be given with the goal of maintaining a serum 25 Vitamin D level of 32 ng/mL (75 nmol/L) or higher. Counseling should be provided to all patients to encourage weight-bearing activities, smoking cessation, fall prevention, and activity modifications to minimize the risk of future fracture. Persistence with osteoporosis medications is a problem. Regardless of treatment type, one-third to half of patients do not take their medications as prescribed within the first year, with persistence rates for bisphosphonates as low as 20% at 24 months. Fracture protection is related to medication persistence, and patients should be reminded that they will not get the full benefit of their osteoporosis medication unless they take it as prescribed and continue to take the medication, even after their fragility fracture has healed.
Recommended Discharge Instructions for Patient With Fragility Fracture
Calcium supplementation of 500 mg daily; may need 1200 mg daily if low-calcium diet or malabsorption issues.
Vitamin D3 (cholecalciferol) at 2000 IU daily if patient not already on higher dosing.
- Vitamin D2 (ergocalciferol) if vitamin D level <32 ng /mL (75 nmol/L).
- 50,000 IU pills given for 8 weeks;
- once weekly for 25 Vit D level of 20 to 31 ng/mL (50-75 nmol/L);
- twice weekly for 25 Vit D level of 10-20 ng/mL (25-50 nmol/L); and
- three times weekly 25 Vit D level <10 ng/mL (<25 nmol/L).
Fall Prevention counseling.
Start on an osteoporosis medication or appointment for osteoporosis assessment.
Setting up an Osteoporosis Prevention Program
Kyle Jeray, MD
Why should the orthopedic surgeon be interested in osteoporosis prevention? As physicians our goal is to treat the patient with a fragility fracture with acute care. But equally important is the overall bone health for the patient and our ability to reduce the chance of another fragility fracture. The goal of the osteoporosis program is to improve patient care and outcomes. Orthopedists need to think of a fragility fracture as a sentinel event that signals the presence of a potentially frail skeleton that puts the patient at increased risk of future fractures. Much like a heart attack and the use of beta-blockers, the fragility fracture presents an opportunity for the orthopedic surgeon to get involved in the prevention. The goal is to break the fragility fracture cycle by intervening with care addressed to the problem of osteoporosis (Figure 22).
Figure 22.
Breaking the fragility fracture cycle with a multidisciplinary program.
So how does one make it work? Education, evaluation, and treatment work but how do we ensure that our patients get this care? The most important first step is to have a site champion that will be willing to drive the setup of the osteoporosis prevention program with the understanding that each hospital, clinic, or institution will have its own issues. One must recognize that this will require a multidisciplinary approach. This really represents a “community” problem requiring a multidisciplinary approach (Figure 23) but starts with a site champion. Often the site champion is the orthopedic surgeon as he or she sees the patient with the fragility fracture, but certainly this does not need to be the case at all institutions. Currently our site champion is our nurse practitioner (NP), but at other sites it may be the internist or the endocrinologist.
Figure 23.
How the Program Works: Bringing “Communities” Together.
Once someone is willing to lead the charge, he or she must work to get “buy-in” from other services within the health care system and resource support. In our institution, it was originally residents, but ideally having a nurse practitioner with dedicated time (typically 0.5 -1.0 FTE) has been the major key to the success of the program. Often administration is reluctant to support an osteoporosis prevention program. In an effort to change that, administration must understand that by reducing fragility fractures in our patients, the hospital system can actually save money.386,387 Establishing a “Bone Health” program can bring notoriety to the hospital. For example, adoption of the “Own the Bone” osteoporosis prevention program (discussed subsequently) brings the institution a “Best Hospital” rating from US News and World Report.386
As performance and outcomes data are helpful to convince a reluctant administration to support a new program, creating a local, regional, or national registry will also help. A registry will promote gathering and recording pertinent data, provide educational tools to assist compliance against measures, and provide benchmarking reports for sites to compare their results with others. An example of this is the Kaiser Permanente Healthy Bones Program, designed to establish quality care for osteoporosis after a fragility fracture. Their program and registry have improved the care, improved patient safety, and demonstrated cost-effectiveness with their osteoporosis prevention program. A national program, “Own the Bone” is a Web-based, evidence-based quality improvement initiative developed by the American Orthopedic Association to improve the care and prevention of osteoporotic fractures.388,389 This program, available to all centers across the country, provides extensive educational materials, a Web-based registry, and guidance for development of a comprehensive program to improve osteoporosis care postfragility fracture. Both of these programs collect data and provide documentation to satisfy the NOF’s Clinical Guidelines and CMS/PQRI measures which in the near future will be tied to the physician/hospital reimbursement from Medicare. Most recently, the NQF released 3 measures specific to osteoporosis management and prevention (laboratory investigation for secondary causes of fracture, risk assessment/treatment after fracture, and discharge instructions/emergency department), all of which are addressed with the development of an osteoporosis prevention program.390
The next step is to recognize the patients with fragility fractures. Using a daily orthopedic census in the hospital, the NP or designated staff (resident or physician extender) can identify the patients with fragility fracture and generate a consult by the admitting service to the osteoporosis fracture program. Sites can identify patients for inclusion based on what works for their community. At our institution, we include all fractures in patients older than 50 years old and evaluate any other consults from other services within the hospital regarding the evaluation and/or treatment of osteoporosis. Not surprisingly as the word gets out in the hospital of an “osteoporosis prevention program,” consults for this specific service will increase outside the fragility fractures. Much like the hip fracture order sets mentioned within this guide, some standard orders addressing the need for the osteoporosis prevention program can be incorporated. An order to consult the osteoporosis NP at our institution meeting the inclusion criterion mentioned earlier sits on all the electronic order sets.
The patients admitted into the hospital are essentially “captured” and provide an opportunity for a teachable moment. The consultation is a billable service using the osteoporosis codes and includes patient education on nutrition, physical activity, lifestyle (smoking cessation and limiting excessive alcohol intake), pharmacology, laboratory testing (including DEXA), and finally communication. This is the time to provide your patients with educational materials that are available online or create your own. Depending on your own situation, the communication piece may be a letter to the patient’s primary care physician or more directly to the orthopedic surgeon or NP that may manage the osteoporosis postfracture. Within a closed system, the communication may actually be an appointment (made by the consultation service) with a physician or extender that is designated to manage the osteoporosis. Without question the communication piece is the most difficult and time consuming for the NP.
Capturing the outpatient fracture patients is another part of the program. In an effort to do so, education regarding the inclusion criteria and the select osteoporotic fractures is outlined to all the staff at the outpatient clinics. The staff are then charged with recognition of these patients and required to generate a consult to the physician or physician extender managing the program. From here, much like the inpatient process, the patients are either seen or called, then education, evaluation, and if indicated treatment are initiated. If the patient has a primary care physician who is involved, a form letter is sent indicating the patients’ condition with regard to the fragility fracture and plan for secondary preventative measures. Often the primary care physicians will agree to assume care for osteoporosis prevention.
In summary, developing an osteoporosis prevention program is an integral part in the care of the patient with fragility fracture. Keys in development of a successful program are identifying a site champion, developing buy-in from administration, determining a trigger to capture patients, developing a database to manage patients, coordinating care of treatment using mid-level practitioners or primary care physicians, and further follow-up to ensure continued osteoporosis prevention.
Keys to Success With Implementing a Prevention Program
identify a Site Champion;
developing buy-in from administration;
determine a trigger system to identify and capture the patients;
use/develop a database/registry to manage patients;
develop coordinated plan with communication for preventative secondary measures (often NP or primary care physician); and
follow-up to determine whether care directed to osteoporosis occurring.
Falls Assessment
Deborah Kado, MD, MS
In most cases, a fragility fracture results from a fall. Thus, when working toward the goal of secondary fracture prevention, falls assessment is a key component in developing an effective treatment strategy. While US life expectancy has increased by about 3 years from 1990 to 2010, unfortunately, so has the age-standardized years of life lost due to premature mortality from falls.391 Falls assessment and falls risk reduction are as important to fracture prevention as treatment of osteoporosis.
Epidemiology
The incidence and health impact of falls vary depending on age, sex, and living status. With increasing age, fall risk increases. Approximately 30% of people older than 65 years and 50% of those older than 80 years of age fall each year.392 Up to 50% of falls result in some type of injury, the most serious of which include hip fractures, head trauma, and cervical spine fractures.393 Men and women tend to fall in equal proportions, but women are more likely to suffer an injury. It has been reported that 50% of residents in long-term care settings fall each year.394 Although the majority of falls do not result in subsequent fall.395 Of those older persons who have had at least 1 fall, 50% will be unable to get up without help, potentially resulting in dehydration, pressure sores, and rhabdomyolysis.396 Thus, many who experience a fall develop a fear of falling, and up to 40% will restrict their activities as a result.397 Becoming less active leads to further disability and heightened risk of falling and, of course, fracture.
Of fall-related injuries in older adults, hip fractures are among the most common and costly. More than 90% of all hip fractures occur as a result of a fall.398 Falls resulting in hip fracture are known to roughly double the 1-year mortality rate compared to matched seniors without hip fractures. The 1-year mortality rate ranges from 12% to 37%, and approximately half of those who fall and fracture a hip are unable to regain the ability to live independently.399
Falls Risk Assessment
Falls in older persons are most often not due to a single cause, but result from the accumulated effects of multiple impairments. As an example, an age-related decline in balance, challenges to postural control and environmental factors such as uneven flooring may result in a fall. Seventeen independent risk factors for injurious falls among community-dwelling older adults have been reported in the literature, of which 3 are not modifiable (age, sex, and previous fall history).400 Of the remaining 14 risk factors, 3 are modifiable, 7 may be modifiable, and 4 are unlikely to be modifiable. The 3 modifiable risk factors include (1) balance impairment, (2) muscle weakness, and (3) use of more than 4 medications or use of psychoactive medication. Potentially modifiable risk factors include (1) visual impairment, (2) gait impairment or walking difficulty, (3) depression, (4) dizziness or orthostatic hypotension, (5) urinary incontinence, (6) arthritis, and (7) pain. Risk factors very difficult to modify are (1) functional limitations (ADL disabilities), (2) low body mass index, (3) cognitive impairment, and (4) diabetes.
In the clinical evaluation of a geriatric patient, the above fall risk factors should be integrated into the history and physical examination. Beginning with the history, an inquiry should be made regarding the patient’s history of falls in the past year. Details with regard to the activity that lead to the fall, any prodromal symptoms (eg, lightheadedness, imbalance), and where the fall occurred should be obtained. Assessing the number of falls, whether any resulted in injury, a history of fear of falling and whether the patient has any difficulties with walking or balance will also inform the particular patient’s risk of falls. The greater the number of risk factors, the higher the risk of recurrent falls. With recurrent falls, the risk of fracture becomes higher.
As part of the clinical history, chronic medical conditions associated with an increased fall risk should be ascertained and include cognitive impairment, dementia, chronic musculoskeletal pain, knee osteoarthritis, urinary incontinence, stroke, Parkinson disease, and diabetes. In addition, physicians should perform a careful review of all of the patient’s medications, including over-the-counter medications. In one study of 4260 older community-dwelling men, investigators found that 82.3% report improper medication use defined as polypharmacy (greater than 4 medications), inappropriate medication consumption, and underutilization.401 Psychoactive medications that include sedatives, antipsychotics, and antidepressants along with anticonvulsants and antihypertensive medications are the most strongly associated with increased fall risk and should be minimized, if possible.402,403
As part of the physical examination in someone who has fallen, orthostatic vital signs, visual acuity, cognitive status, and cardiac system evaluation should be performed. And, perhaps most importantly, a gait and balance evaluation should be done. Although there are formal assessments that are done in the clinical research setting, a busy clinician can perform an efficient evaluation by simply asking the patient to rise from a seated position in a chair without his or her arms. Once standing, the patient’s initial standing balance, semitandem and full-tandem balance can be assessed and his or her gait be evaluated by asking him or her to walk across the examination room. Difficulty manifested during any one of these steps is an indication for further evaluation and treatment by a physical therapist for gait and balance training.
Finally, attention should be made to the feet and footwear. Moderate bunions, toe and nail deformities, and foot ulcers are associated with fall risk in the elderly patients.396 Making recommendations to have patients optimize their foot care, avoid high-heels, floppy slippers, shoes with slick sole,s and ill-fitting footwear can result in a decreased fall rate.
To complete the medical workup in an older patient who has fallen, further diagnostic testing should include laboratory tests for hemoglobin to rule out clinically significant anemia, electrolytes to rule out significant imbalances, thyroid-stimulating hormone (TSH) to rule out hypothyroidism, vitamin B12 to rule out B12 deficiency (linked to proprioceptive problems), and 25-hydroxy vitamin D levels to rule to vitamin D deficiency (linked to falls and fractures). As a fall may be a symptom of an underlying medical illness such as pneumonia or urinary tract infection, a standard urinalysis and chest X-ray should be obtained, particularly if the patient has significant cognitive impairment or dementia.
Fewer than 10% of falls are caused by a loss of consciousness. If a loss of consciousness is reported, additional investigation is warranted. An ECG can screen for significant cardiac pathology, and echocardiogram may help identify clinically significant aortic stenosis. As carotid sensitivity has been linked to falls, pacemaker placement might be considered in patients who experience carotid sinus induced heart rate pauses of >3 seconds. Carotid sinus message should be avoided. Underlying neurologic causes of falls should be suspected in patients who have new or unexplained focal neurologic findings on examination. Head CT or MRI can rule out stroke, mass, normal pressure hydrocephalus, or other structural abnormality. If the patient has significant gait abnormalities, spine radiographs or MRI may help exclude cervical spondylosis or lumbar stenosis as a cause of falls.
Treatment and Prevention
Secondary fall prevention measures need to be tailored to the particular patient’s situation. Focus should be placed on the patient’s modifiable risk factors that can be classified as intrinsic and extrinsic to the individual. Recommended treatment of modifiable risk factors are included in Table 2. Of the interventions, medication reduction, physical therapy, and home safety modifications have demonstrated the best efficacy in fall prevention.
Table 2.
Recommended Treatments for Modifiable Risk Factors.
| Risk Factors | Management |
|---|---|
| Intrinsic | |
| Vision | Check acuity and for cataracts, refer to ophthalmology if indicated; advise to avoid multifocal lenses while walking |
| Postural hypotension | Reduce medications, rule out dehydration, advise to change positions slowly, consider fludrocortisone if above 3 interventions don’t work |
| Cardiovascular | Medical management, consider dual chamber cardiac pacing if carotid induced hypersensitivity >3 second pauses |
| Neurologic | Consider neuroimaging with MRI/CT, medical management as indicated |
| Arthritis | Medical management, consider PT/OT referral, assistive devices as appropriate |
| Balance or gait impairment | Referral to physical and/or occupational therapy for progressive strength, balance and gait training |
| Vitamin D insufficiency/deficiency | Replete vitamin D with a minimum of 800 IU daily |
| Other medical conditions (cognitive impairment, depression, etc) | Medical management as indicated |
| Psychoactive medications | Eliminate or reduce dose of as many sedatives, antidepressants, anxiolytics, and antipsychotics as possible as these are associated with an increased fall risk |
| Other medications | Eliminate or reduce dose of as many medications as possible, paying close attention to (1) antihypertensive medications that can lead to orthostatic hypotension/lightheadedness and (2) antihistamines, anticonvulsants, and opioids that can lead to confusion or impaired alertness |
| Extrinsic | |
| Home environmental hazards | Ideally, PT/OT referral can assess home safety and make recommendations for safety improvements (ie, grab bars in shower, reaching devices, adequate lighting) |
| Footwear | Advise to wear well-fitting shoes with low heel height and high surface contact area |
Abbreviations: CT, computed tomography; MRI, magnetic resonance imaging.
Physical therapy deserves special mention in a patient who has fractured and is at increased fall risk. Progressive standing balance and strength exercise, transfer practice, and gait interventions with use of appropriate assistive devices should be undertaken. Patients should be trained on how to get up from the floor after a fall. Once the therapy sessions have been completed, clinicians should recommend an individually tailored exercise program to maintain function, possibly increase endurance, and decrease fall risk. In a review that included 43 exercise trials to decrease fall risk, 14 that employed gait training, balancing, and strengthening had a 17% risk reduction in falling (95% confidence interval: 0.72-0.97). Four trials included Tai-Chi with an even more impressive risk reduction of 37% (95% confidence interval: 0.51-0.82).404
Home safety modification entails checking the home environment to remove obvious fall hazards. Removal of clutter to minimize tripping hazards, ensuring adequate lighting and installation of safety measures like shower bars and/or raised toilet seats can decrease fall risk.
Finally, patient education and information programs are helpful in falls prevention. Fall prevention strategies appear to work in both community and institutional settings.404,405 Single and multifactorial intervention randomized controlled trials have been conducted, and the preponderance of evidence demonstrate benefit with fall rate decline. Since 2004, the United States National Council on Aging has lead a Falls Free Initiative, culminating in coalition workgroups that have provided step-by-step processes of decision making and interventions to manage falls among older persons assessed to be at high fall risk (http://www.medcats.com/FALLS/frameset.htm).
Summary
While recurrent falls, inability to get up after a fall, and the psychological effects following a fall are associated with a poor prognosis, each is potentially modifiable. Thus, in a patient who has fallen, careful attention should be given to underlying medical disorders, medication use, and home environment that may contribute to an individual’s fall risk. In one randomized controlled trial, a focused history and physical assessment by nurse practitioners following a fall resulted in identification of modifiable medical conditions and decreased hospitalization rates over 2 years of follow-up.406 There is an overwhelming consensus that fall prevention strategies work.Those patients who have already had a fragility fracture deserve proper evaluation, education, and treatment.
Modifiable Risk Factors for Falls
Balance impairment;
muscle weakness; and
use of more than 4 medications or psychoactive medication use.
Potentially Modifiable Risk Factors for Falls
Visual impairment;
gait impairment or walking difficulty;
depression;
dizziness or orthostatic hypotension;
urinary incontinence;
arthritis; and
pain.
Nonmodifiable risk factors
Functional limitations (ADL disabilities);
low body mass index;
cognitive impairment; and
diabetes.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Sathiyakumar V, Greenberg SE, Molina CS, Thakore RV, Obremskey WT, Sethi MK. Hip fractures are risky business: an analysis of the NSQIP data[published online October 22, 2014]. Injury. 2014. [DOI] [PubMed] [Google Scholar]
- 2. Foster K. Hip fractures in adults. 2014; Web site http://www.uptodate.com/contents/hip-fractures-in-adults Accessed February 21, 2015, 2015.
- 3. Weinstein J. The Dartmouth Atlas of Musculoskeletal Health Care. Chicago AHA Press; 2000. [PubMed] [Google Scholar]
- 4. Frey WH. The National Center for Health Statistics. Teaching/Training Modules on Trends in Health and Aging. Web site http://www.asaging.org/blog/aging-community-communitarian-alternative-aging-place-alone Accessed February 21, 2015.
- 5. Amin S, Achenbach SJ, Atkinson EJ, Khosla S, Melton LJ., III Trends in fracture incidence: a population-based study over 20 years. J Bone Miner Res. 2014;29(3):581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Costa AG, Wyman A, Siris ES, et al. When, Where and How Osteoporosis-Associated Fractures Occur: An Analysis from the Global Longitudinal Study of Osteoporosis in Women (GLOW). PLoS One. 2013;8(12):e83306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. U. S. Department of Health and Human Services. The 2004 Surgeon General's Report on Bone Health and Osteoporosis. Web site http://www.ncbi.nlm.nih.gov/books/NBK45513/ Accessed February 21, 2015.
- 8. Su H, Aharonoff GB, Zuckerman JD, Egol KA, Koval KJ. The relation between discharge hemoglobin and outcome after hip fracture. Am J Orthop (Belle Mead NJ). 2004;33(11):576–580. [PubMed] [Google Scholar]
- 9. Melton LJ, III, Amin S. Is there a specific fracture ‘cascade’? Bonekey Rep. 2013;2:367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ekman EF. The role of the orthopaedic surgeon in minimizing mortality and morbidity associated with fragility fractures. J Am Acad Orthop Surg. 2010;18(5):278–285. [DOI] [PubMed] [Google Scholar]
- 11. Kates SL, Blake D, Bingham KW, Kates OS, Mendelson DA, Friedman SM. Comparison of an organized geriatric fracture program to United States government data. J Geratir Orth Surg. 2010;1(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. British Orthopaedic Association. The Care of Patients with Fragility Fracture. London: British Orthopaedic Association; 2007. [Google Scholar]
- 13. Agency for Healthcare Research and Quality. 2005 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. Rockville (MD: ): US Department of Health and Human Services; 2008. [Google Scholar]
- 14. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–370. [DOI] [PubMed] [Google Scholar]
- 15. Diamantopoulos AP, Hoff M, Skoie IM, Hochberg M, Haugeberg G. Short- and long-term mortality in males and females with fragility hip fracture in Norway. a population-based study. Clin Interv Aging. 2013;8:817–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Orces CH. In-hospital hip fracture mortality trends in older adults: the National Hospital Discharge Survey, 1988-2007. J Am Geriatr Soc. 2013;61(12):2248–2249. [DOI] [PubMed] [Google Scholar]
- 17. Gosch M, Wortz M, Nicholas JA, Doshi HK, Kammerlander C, Lechleitner M. Inappropriate prescribing as a predictor for long-term mortality after hip fracture. Gerontology. 2014;60(2):114–122. [DOI] [PubMed] [Google Scholar]
- 18. Youm T, Koval KJ, Zuckerman JD. The economic impact of geriatric hip fractures. The Am J Orthop. 1999;28(7):423–428. [PubMed] [Google Scholar]
- 19. Barrett M WE, Whalen D. 2007 HCUP Nationwide Inpatient Sample (NIS) Comparison Report. HCUP Methods Series Report # 2010-03. Online 2010; 2010: Web site http://www.hcup-us.ahrq.gov/reports/methods/2010_03.pdf Accessed February 21, 2015.
- 20. DeFrances CJ, Lucas CA, Buie VC, Golosinskiy A. 2006 National Hospital Discharge Survey. Nat Health Stat Rep. 2008;(5):1–20. [PubMed] [Google Scholar]
- 21. Solomon D, Johnston S, Boytsov N, McMorrow D, Lane J, Krohn K. Osteoporosis Medication Use after Hip Fracture in US patients between 2002 and 2011. J Bone Miner Res. 2014;29(9):1929–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Adams A, Black M, Zhang J, Shi J, Jacobson S. Proton – pump inhibitor use and hip fractures in men: a population-based case-control study. Ann Epidemiol. 2014;24(4):286–290. [DOI] [PubMed] [Google Scholar]
- 23. Baumgarten M, Margolis DJ, Localio AR, et al. Pressure ulcers among elderly patients early in the hospital stay. J Gerontol A Biol Sci Med Sci. 2006;61(7):749–754. [DOI] [PubMed] [Google Scholar]
- 24. Hwang U, Richardson LD, Sonuyi TO, Morrison RS. The effect of emergency department crowding on the management of pain in older adults with hip fracture. J Am Geriatr Soc. 2006;54(2):270–275. [DOI] [PubMed] [Google Scholar]
- 25. Rashid A, Brooks TR, Bessman E, Mears SC. Factors associated with emergency department length of stay for patients with hip fracture. Geriatr Orthop Surg Rehabil. 2013;4(3):78–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inouye SK. Delirium in hospitalized older patients: recognition and risk factors. J Geriatr Psychiatry Neurol. 1998;11(3):118–125; discussion 157-118. [DOI] [PubMed] [Google Scholar]
- 27. AAOS. MANAGEMENT OF HIP FRACTURES IN THE ELDERLY. 2014; September, 2014:17. Web site http://www.aaos.org/Research/guidelines/HipFxSummaryofRecommendations.pdf Accessed February 21, 2015.
- 28. Levine MB, Moore AB, Franck C, Li J, Kuehl DR. Variation in use of all types of computed tomography by emergency physicians. Am J Emerg Med. 2013;31(10):1437–1442. [DOI] [PubMed] [Google Scholar]
- 29. Robertson BD, Robertson TJ. Postoperative delirium after hip fracture. J Bone Joint Surg Am. 2006;88(9):2060–2068. [DOI] [PubMed] [Google Scholar]
- 30. Elkhodair S, Mortazavi J, Chester A, Pereira M. Single fascia iliaca compartment block for pain relief in patients with fractured neck of femur in the emergency department: a pilot study. Eur J Emerg Med. 2011;18(6):340–343. [DOI] [PubMed] [Google Scholar]
- 31. Rosen JE, Chen FS, Hiebert R, Koval KJ. Efficacy of preoperative skin traction in hip fracture patients: a prospective, randomized study. J Orthop Trauma. 2001;15(2):81–85. [DOI] [PubMed] [Google Scholar]
- 32. Friedman SM, Mendelson DA, Bingham KW, Kates SL. Impact of a comanaged Geriatric Fracture Center on short-term hip fracture outcomes. Arch Intern Med. 2009;169(18):1712–1717. [DOI] [PubMed] [Google Scholar]
- 33. Friedman SM, Mendelson DA, Kates SL, McCann RM. Geriatric co-management of proximal femur fractures: total quality management and protocol-driven care result in better outcomes for a frail patient population. J Am Geriatr Soc. 2008;56(7):1349–1356. [DOI] [PubMed] [Google Scholar]
- 34. Holte K. Pathophysiology and clinical implications of peroperative fluid management in elective surgery. Dan Med Bull. 2010;57(7):B4156. [PubMed] [Google Scholar]
- 35. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ. 2010;182(15):1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Orosz GM, Magaziner J, Hannan EL, et al. Association of timing of surgery for hip fracture and patient outcomes. JAMA. 2004;291(14):1738–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Al-Ani AN, Samuelsson B, Tidermark J, et al. Early operation on patients with a hip fracture improved the ability to return to independent living. A prospective study of 850 patients. J Bone Joint Surg Am. 2008;90(7):1436–1442. [DOI] [PubMed] [Google Scholar]
- 38. Bottle A, Aylin P. Mortality associated with delay in operation after hip fracture: observational study. BMJ. 2006;332(7547):947–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schultz-Larsen K, Kreiner S, Lomholt RK. Mini-Mental Status Examination: mixed Rasch model item analysis derived two different cognitive dimensions of the MMSE. J Clin Epidemiol. 2007;60(3):268–279. [DOI] [PubMed] [Google Scholar]
- 40. Borson S, Scanlan JM, Chen P, Ganguli M. The Mini-Cog as a screen for dementia: validation in a population-based sample. J Am Geriatr Soc. 2003;51(10):1451–1454. [DOI] [PubMed] [Google Scholar]
- 41. Fleisher LA, Beckman JA, Brown KA, et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation. 2007;116(17):e418–e499. [DOI] [PubMed] [Google Scholar]
- 42. Carson JL, Terrin ML, Noveck H, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marsland D, Mears SC, Kates SL. Venous thromboembolic prophylaxis for hip fractures. Osteoporos Int. 2010;21(suppl 4):S593–S604. [DOI] [PubMed] [Google Scholar]
- 44. Vitale MA, Vanbeek C, Spivack JH, Cheng B, Geller JA. Pharmacologic reversal of warfarin-associated coagulopathy in geriatric patients with hip fractures: a retrospective study of thromboembolic events, postoperative complications, and time to surgery. Geriatr Orthop Surg Rehabil. 2011;2(4):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gleason LJ, Mendelson DA, Kates SL, Friedman SM. Anticoagulation management in individuals with hip fracture. J Am Geriatr Soc. 2014;62(1):159–164. [DOI] [PubMed] [Google Scholar]
- 46. Baron TH, Kamath PS, McBane RD. Management of antithrombotic therapy in patients undergoing invasive procedures. N Engl J Med. 2013;368(22):2113–2124. [DOI] [PubMed] [Google Scholar]
- 47. Collinge CA, Kelly KC, Little B, Weaver T, Schuster RD. The effects of clopidogrel (Plavix) and other oral anticoagulants on early hip fracture surgery. J Orthop Trauma. 2012;26(10):568–573. [DOI] [PubMed] [Google Scholar]
- 48. Bouri S, Shun-Shin MJ, Cole GD, Mayet J, Francis DP. Meta-analysis of secure randomised controlled trials of beta-blockade to prevent perioperative death in non-cardiac surgery. Heart. 2014;100(6):456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bapoje SR, Whitaker JF, Schulz T, Chu ES, Albert RK. Preoperative evaluation of the patient with pulmonary disease. Chest. 2007;132(5):1637–1645. [DOI] [PubMed] [Google Scholar]
- 50. White SM, Moppett IK, Griffiths R. Outcome by mode of anaesthesia for hip fracture surgery. An observational audit of 65 535 patients in a national dataset. Anaesthesia. 2014;69(3):224–230. [DOI] [PubMed] [Google Scholar]
- 51. Neuman MD, Rosenbaum PR, Ludwig JM, Zubizarreta JR, Silber JH. Anesthesia technique, mortality, and length of stay after hip fracture surgery. JAMA. 2014;311(24):2508–2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Memtsoudis SG, Sun X, Chiu YL, et al. Perioperative comparative effectiveness of anesthetic technique in orthopedic patients. Anesthesiology. 2013;118(5):1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neuman MD, Silber JH, Elkassabany NM, Ludwig JM, Fleisher LA. Comparative effectiveness of regional versus general anesthesia for hip fracture surgery in adults. Anesthesiology. 2012;117(1):72–92. [DOI] [PubMed] [Google Scholar]
- 54. Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450–455. [DOI] [PubMed] [Google Scholar]
- 55. Enneking FK, Chan V, Greger J, Hadzic A, Lang SA, Horlocker TT. Lower-extremity peripheral nerve blockade: essentials of our current understanding. Reg Anesth Pain Med. 2005;30(1):4–35. [DOI] [PubMed] [Google Scholar]
- 56. Modig J. Regional anaesthesia and blood loss. Acta Anaesthesiol Scand Suppl. 1988;89:44–48. [DOI] [PubMed] [Google Scholar]
- 57. Bryson GL, Wyand A. Evidence-based clinical update: general anesthesia and the risk of delirium and postoperative cognitive dysfunction. Can J Anaesth. 2006;53(7):669–677. [DOI] [PubMed] [Google Scholar]
- 58. Vanzetto G, Machecourt J, Blendea D, et al. Additive value of thallium single-photon emission computed tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol. 1996;77(2):143–148. [DOI] [PubMed] [Google Scholar]
- 59. Sieber FE, Zakriya KJ, Gottschalk A, et al. Sedation depth during spinal anesthesia and the development of postoperative delirium in elderly patients undergoing hip fracture repair. Mayo Clin Proc. 2010;85(1):18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Barash PG CB, Stoelting RK. ed Clinical anesthesia. Philadelphia: Lippincott Williams & Wilkins; 2009. Bernards C, ed. [Google Scholar]
- 61. Horlocker TT, Wedel DJ, Rowlingson JC, et al. Regional anesthesia in the patient receiving antithrombotic or thrombolytic therapy: American Society of Regional Anesthesia and Pain Medicine Evidence-Based Guidelines (Third Edition). Reg Anesth Pain Med. 2010;35(1):64–101. [DOI] [PubMed] [Google Scholar]
- 62. Lowell JD. Fractures of the hip. N Engl J Med. 1966;274(26):1480–1490. [DOI] [PubMed] [Google Scholar]
- 63. Raaymakers EL. The non-operative treatment of impacted femoral neck fractures. Injury. 2002;33(suppl 3):S–C8- S–C14. [DOI] [PubMed] [Google Scholar]
- 64. Yang JJ, Lin LC, Chao KH, et al. Risk factors for nonunion in patients with intracapsular femoral neck fractures treated with three cannulated screws placed in either a triangle or an inverted triangle configuration. J Bone Joint Surg Am. 2013;95(1):61–69. [DOI] [PubMed] [Google Scholar]
- 65. Oakey JW, Stover MD, Summers HD, Sartori M, Havey RM, Patwardhan AG. Does screw configuration affect subtrochanteric fracture after femoral neck fixation? Clin Orthop Relat Res. 2006;443:302–306. [DOI] [PubMed] [Google Scholar]
- 66. Lee KB, Howe TS, Chang HC. Cancellous screw fixation for femoral neck fractures: one hundred and sixteen patients. Ann Acad Med Singapore. 2004;33(2):248–251. [PubMed] [Google Scholar]
- 67. Gjertsen JE, Fevang JM, Matre K, Vinje T, Engesaeter LB. Clinical outcome after undisplaced femoral neck fractures. Acta Orthop. 2011;82(3):268–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lapidus LJ, Charalampidis A, Rundgren J, Enocson A. Internal fixation of garden I and II femoral neck fractures: posterior tilt did not influence the reoperation rate in 382 consecutive hips followed for a minimum of 5 years. J Orthop Trauma. 2013;27(7):386–390; discussion 390-381. [DOI] [PubMed] [Google Scholar]
- 69. Callaghan JJ, Liu SS, Haidukewych GJ. Subcapital fractures: a changing paradigm. J Bone Joint Surg Br. 2012;94(11 suppl A):19–21. [DOI] [PubMed] [Google Scholar]
- 70. Rogmark C, Carlsson A, Johnell O, Sernbo I. A prospective randomised trial of internal fixation versus arthroplasty for displaced fractures of the neck of the femur. Functional outcome for 450 patients at two years. J Bone Joint Surg Br. 2002;84(2):183–188. [DOI] [PubMed] [Google Scholar]
- 71. Gjertsen JE, Vinje T, Engesaeter LB, et al. Internal screw fixation compared with bipolar hemiarthroplasty for treatment of displaced femoral neck fractures in elderly patients. J Bone Joint Surg Am. 2010;92(3):619–628. [DOI] [PubMed] [Google Scholar]
- 72. Hedbeck CJ, Inngul C, Blomfeldt R, Ponzer S, Tornkvist H, Enocson A. Internal fixation versus cemented hemiarthroplasty for displaced femoral neck fractures in patients with severe cognitive dysfunction: a randomized controlled trial. J Orthop Trauma. 2013;27(12):690–695. [DOI] [PubMed] [Google Scholar]
- 73. Waaler Bjornelv GM, Frihagen F, Madsen JE, Nordsletten L, Aas E. Hemiarthroplasty compared to internal fixation with percutaneous cannulated screws as treatment of displaced femoral neck fractures in the elderly: cost-utility analysis performed alongside a randomized, controlled trial. Osteoporos Int. 2012;23(6):1711–1719. [DOI] [PubMed] [Google Scholar]
- 74. Parker MJ, Gurusamy K. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev. 2006;3:1–65. [DOI] [PubMed] [Google Scholar]
- 75. Parker MJ, Gurusamy KS, Azegami S. Arthroplasties (with and without bone cement) for proximal femoral fractures in adults. Cochrane Database Syst Rev. 2010;6:CD001706. [DOI] [PubMed] [Google Scholar]
- 76. Viberg B, Overgaard S, Lauritsen J, Ovesen O. Lower reoperation rate for cemented hemiarthroplasty than for uncemented hemiarthroplasty and internal fixation following femoral neck fracture: 12- to 19-year follow-up of patients aged 75 years or more. Acta Orthop. 2013;84(3):254–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Taylor F, Wright M, Zhu M. Hemiarthroplasty of the hip with and without cement: a randomized clinical trial. J Bone Joint Surg Am. 2012;94(7):577–583. [DOI] [PubMed] [Google Scholar]
- 78. Langslet E, Frihagen F, Opland V, Madsen JE, Nordsletten L, Figved W. Cemented versus uncemented hemiarthroplasty for displaced femoral neck fractures: 5-year followup of a randomized trial. Clin Orthop Relat Res. 2014;472(4):1291–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Gjertsen JE, Lie SA, Vinje T, et al. More re-operations after uncemented than cemented hemiarthroplasty used in the treatment of displaced fractures of the femoral neck: an observational study of 11,116 hemiarthroplasties from a national register. J Bone Joint Surg Br. 2012;94(8):1113–1119. [DOI] [PubMed] [Google Scholar]
- 80. Raia FJ, Chapman CB, Herrera MF, Schweppe MW, Michelsen CB, Rosenwasser MP. Unipolar or bipolar hemiarthroplasty for femoral neck fractures in the elderly? Clin Orthop Relat Res. 2003;414:259–265. [DOI] [PubMed] [Google Scholar]
- 81. Hedbeck CJ, Blomfeldt R, Lapidus G, Tornkvist H, Ponzer S, Tidermark J. Unipolar hemiarthroplasty versus bipolar hemiarthroplasty in the most elderly patients with displaced femoral neck fractures: a randomised, controlled trial. Int Orthop. 2011;35(11):1703–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Baker RP, Squires B, Gargan MF, Bannister GC. Total hip arthroplasty and hemiarthroplasty in mobile, independent patients with a displaced intracapsular fracture of the femoral neck. A randomized, controlled trial. J Bone Joint Surg Am. 2006;88(12):2583–2589. [DOI] [PubMed] [Google Scholar]
- 83. Blomfeldt R, Tornkvist H, Eriksson K, Soderqvist A, Ponzer S, Tidermark J. A randomised controlled trial comparing bipolar hemiarthroplasty with total hip replacement for displaced intracapsular fractures of the femoral neck in elderly patients. J Bone Joint Surg Br. 2007;89(2):160–165. [DOI] [PubMed] [Google Scholar]
- 84. Leonardsson O, Karrholm J, Akesson K, Garellick G, Rogmark C. Higher risk of reoperation for bipolar and uncemented hemiarthroplasty. Acta Orthop. 2012;83(5):459–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Muller ME, Nazarian S, Koch P, Schatzker J. The Comprehensive Classification of Fractures of Long Bones. Berlin: Springer-Verlag; 1990. [Google Scholar]
- 86. Gotfried Y. Integrity of the lateral femoral wall in intertrochanteric hip fractures: an important predictor of a reoperation [letter]. J Bone Joint Surg Am. 2007;89(11):2552–2553; author reply 2553. [DOI] [PubMed] [Google Scholar]
- 87. Baumgaertner MR, Curtin SL, Lindskog DM, Keggi JM. The value of the tip-apex distance in predicting failure of fixation of peritrochanteric fractures of the hip. J Bone Joint Surg Am. 1995;77(7):1058–1064. [DOI] [PubMed] [Google Scholar]
- 88. Haidukewych GJ, Israel TA, Berry DJ. Reverse obliquity fractures of the intertrochanteric region of the femur. J Bone Joint Surg Am. 2001;83(5):643–650. [DOI] [PubMed] [Google Scholar]
- 89. Bojan AJ, Beimel C, Speitling A, Taglang G, Ekholm C, Jonsson A. 3066 consecutive Gamma Nails. 12 years experience at a single centre. BMC Musculoskelet Disord. 2010;11:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Koval KJ, Sala DA, Kummer FJ, Zuckerman JD. Postoperative weight-bearing after a fracture of the femoral neck or an intertrochanteric fracture. J Bone Joint Surg Am. 1998;80(3):352–356. [DOI] [PubMed] [Google Scholar]
- 91. Hoffmann R, Haas NP. Femur: proximal. In: Ruedi TP, Murphy WM. eds. AO Principles of Fracture Management. New York: Thieme; 2000:441–454. [Google Scholar]
- 92. Broderick JM, Bruce-Brand R, Stanley E, Mulhall KJ. Osteoporotic hip fractures: the burden of fixation failure. ScientificWorldJournal. 2013;2013:515197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Calderwood MS, Ma A, Khan YM, et al. Use of Medicare diagnosis and procedure codes to improve detection of surgical site infections following hip arthroplasty, knee arthroplasty, and vascular surgery. Infect Control Hosp Epidemiol. 2012;33(1):40–49. [DOI] [PubMed] [Google Scholar]
- 94. Wolf BR, Lu X, Li Y, Callaghan JJ, Cram P. Adverse outcomes in hip arthroplasty: long-term trends. J Bone Joint Surg Am. 2012;94(14):e103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rogmark C, Fenstad AM, Leonardsson O, et al. Posterior approach and uncemented stems increases the risk of reoperation after hemiarthroplasties in elderly hip fracture patients. Acta Orthop. 2014;85(1):18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Della Valle C, Parvizi J, Bauer TW, et al. American Academy of Orthopaedic Surgeons clinical practice guideline on: the diagnosis of periprosthetic joint infections of the hip and knee. J Bone Joint Surg Am. 2011;93(14):1355–1357. [DOI] [PubMed] [Google Scholar]
- 97. Duncan CP, Masri BA. Fractures of the femur after hip replacement. Instr Course Lect. 1995;44:293–304. [PubMed] [Google Scholar]
- 98. Marsland D, Mears SC. A review of periprosthetic femoral fractures associated with total hip arthroplasty. Geriatr Orthop Surg Rehabil. 2012;3(3):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. [DOI] [PubMed] [Google Scholar]
- 100. Haidukewych GJ, Berry DJ. Hip arthroplasty for salvage of failed treatment of intertrochanteric hip fractures. J Bone Joint Surg Am. 2003;85-A(5):899–904. [DOI] [PubMed] [Google Scholar]
- 101. DeHaan AM, Groat T, Priddy M, et al. Salvage hip arthroplasty after failed fixation of proximal femur fractures. J Arthroplasty. 2013;28(5):855–859. [DOI] [PubMed] [Google Scholar]
- 102. Pui CM, Bostrom MP, Westrich GH, et al. Increased complication rate following conversion total hip arthroplasty after cephalomedullary fixation for intertrochanteric hip fractures: a multi-center study. J Arthroplasty. 2013;28(8 suppl):45–47. [DOI] [PubMed] [Google Scholar]
- 103. Gibson SJ, Helme RD. Age-related differences in pain perception and report. Clin Geriatr Med. 2001;17(3):433–456, v–vi. [DOI] [PubMed] [Google Scholar]
- 104. Chai E, Horton JR. Managing pain in the elderly population: pearls and pitfalls. Curr Pain Headache Rep. 2010;14(6):409–417. [DOI] [PubMed] [Google Scholar]
- 105. Hadjistavropoulos T, Herr K, Turk DC, et al. An interdisciplinary expert consensus statement on assessment of pain in older persons. Clin J Pain. 2007;23(1 suppl):S1–S43. [DOI] [PubMed] [Google Scholar]
- 106. Abou-Setta AM, Beaupre LA, Rashiq S, et al. Comparative effectiveness of pain management interventions for hip fracture: a systematic review. Ann Intern Med. 2011;155(4):234–245. [DOI] [PubMed] [Google Scholar]
- 107. Newman B, McCarthy L, Thomas PW, May P, Layzell M, Horn K. A comparison of pre-operative nerve stimulator-guided femoral nerve block and fascia iliaca compartment block in patients with a femoral neck fracture. Anaesthesia. 2013;68(9):899–903. [DOI] [PubMed] [Google Scholar]
- 108. Beaudoin FL, Haran JP, Liebmann O. A comparison of ultrasound-guided three-in-one femoral nerve block versus parenteral opioids alone for analgesia in emergency department patients with hip fractures: a randomized controlled trial. Acad Emerg Med. 2013;20(6):584–591. [DOI] [PubMed] [Google Scholar]
- 109. Fong HK, Sands LP, Leung JM. The role of postoperative analgesia in delirium and cognitive decline in elderly patients: a systematic review. Anesth Analg. 2006;102(4):1255–1266. [DOI] [PubMed] [Google Scholar]
- 110. Marcantonio ER, Juarez G, Goldman L, et al. The relationship of postoperative delirium with psychoactive medications. JAMA. 1994;272(19):1518–1522. [PubMed] [Google Scholar]
- 111. Stein C. The control of pain in peripheral tissue by opioids. N Engl J Med. 1995;332(25):1685–1690. [DOI] [PubMed] [Google Scholar]
- 112. Grass JA. Patient-controlled analgesia. Anesth Analg. 2005;101(5 suppl):S44–S61. [DOI] [PubMed] [Google Scholar]
- 113. Montamat SC, Cusack BJ, Vestal RE. Management of drug therapy in the elderly. N Engl J Med. 1989;321(5):303–309. [DOI] [PubMed] [Google Scholar]
- 114. Gloth FM., III Geriatric pain. Factors that limit pain relief and increase complications. Geriatrics. 2000;55(10):46–48, 51–44. [PubMed] [Google Scholar]
- 115. Aubrun F, Marmion F. The elderly patient and postoperative pain treatment. Best Pract Res Clin Anaesthesiol. 2007;21(1):109–127. [DOI] [PubMed] [Google Scholar]
- 116. Elvir-Lazo OL, White PF. The role of multimodal analgesia in pain management after ambulatory surgery. Curr Opin Anaesthesiol. 2010;23(6):697–703. [DOI] [PubMed] [Google Scholar]
- 117. Fick DM, Semla TP. 2012 American Geriatrics Society Beers Criteria: new year, new criteria, new perspective. J Am Geriatr Soc. 2012;60(4):614–615. [DOI] [PubMed] [Google Scholar]
- 118. Kissin I, Lee SS, Bradley EL., Jr Effect of prolonged nerve block on inflammatory hyperalgesia in rats: prevention of late hyperalgesia. Anesthesiology. 1998;88(1):224–232. [DOI] [PubMed] [Google Scholar]
- 119. Strike SA, Sieber FE, Gottschalk A, Mears SC. Role of fracture and repair type on pain and opioid use after hip fracture in the elderly. Geriatr Orthop Surg Rehabil. 2013;4(4):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Valentine RJ, Weigelt JA, Dryer D, Rodgers C. Effect of remote infections on clean wound infection rates. Am J Infect Control. 1986;14(2):64–67. [DOI] [PubMed] [Google Scholar]
- 121. Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for prevention of surgical site infection, 1999. Hospital Infection Control Practices Advisory Committee. Infect Control Hosp Epidemiol. 1999;20(4):250–278; quiz 279-280. [DOI] [PubMed] [Google Scholar]
- 122. Urquhart DM, Hanna FS, Brennan SL, et al. Incidence and risk factors for deep surgical site infection after primary total hip arthroplasty: a systematic review. J Arthroplasty. 2010;25(8):1216–1222 e1211-1213. [DOI] [PubMed] [Google Scholar]
- 123. Matar WY, Jafari SM, Restrepo C, Austin M, Purtill JJ, Parvizi J. Preventing infection in total joint arthroplasty. J Bone Joint Surg Am. 2010;92(suppl 2):36–46. [DOI] [PubMed] [Google Scholar]
- 124. Wilson MG, Kelley K, Thornhill TS. Infection as a complication of total knee-replacement arthroplasty. Risk factors and treatment in sixty-seven cases. J Bone Joint Surg Am. 1990;72(6):878–883. [PubMed] [Google Scholar]
- 125. Zerr KJ, Furnary AP, Grunkemeier GL, Bookin S, Kanhere V, Starr A. Glucose control lowers the risk of wound infection in diabetics after open heart operations. Ann Thorac Surg. 1997;63(2):356–361. [DOI] [PubMed] [Google Scholar]
- 126. APIC. Guide to Elimination of Orthopaedic Surgical Site Infections. Washington, DC: APIC; 2010. 2010. [Google Scholar]
- 127. Lavernia CJ, Sierra RJ, Baerga L. Nutritional parameters and short term outcome in arthroplasty. J Am Coll Nutr. 1999;18(3):274–278. [DOI] [PubMed] [Google Scholar]
- 128. Hacek DM, Robb WJ, Paule SM, Kudrna JC, Stamos VP, Peterson LR. Staphylococcus aureus nasal decolonization in joint replacement surgery reduces infection. Clin Orthop Relat Res. 2008;466(6):1349–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rao N, Cannella B, Crossett LS, Yates AJ, Jr, McGough R., III A preoperative decolonization protocol for staphylococcus aureus prevents orthopaedic infections. Clin Orthop Relat Res. 2008;466(6):1343–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Harrison WJ. Open tibia fractures in HIV positive patients. Malawi Med J. 2009;21(4):174–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Kawakami K, Ikari K, Kawamura K, et al. Complications and features after joint surgery in rheumatoid arthritis patients treated with tumour necrosis factor-alpha blockers: perioperative interruption of tumour necrosis factor-alpha blockers decreases complications? Rheumatology (Oxford). 2010;49(2):341–347. [DOI] [PubMed] [Google Scholar]
- 132. James RC, Macleod CJ. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br J Exp Pathol. 1961;42:266–277. [PMC free article] [PubMed] [Google Scholar]
- 133. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis. 1998;27(5):1247–1254. [DOI] [PubMed] [Google Scholar]
- 134. Enninghorst N, McDougall D, Hunt JJ, Balogh ZJ. Open tibia fractures: timely debridement leaves injury severity as the only determinant of poor outcome. J Trauma. 2011;70(2):352–356; discussion 356-357. [DOI] [PubMed] [Google Scholar]
- 135. Anderson DJ, Kaye KS, Classen D, et al. Strategies to prevent surgical site infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S51–S61. [DOI] [PubMed] [Google Scholar]
- 136. Classen DC, Evans RS, Pestotnik SL, Horn SD, Menlove RL, Burke JP. The timing of prophylactic administration of antibiotics and the risk of surgical-wound infection. N Engl J Med. 1992;326(5):281–286. [DOI] [PubMed] [Google Scholar]
- 137. Fletcher N, Sofianos D, Berkes MB, Obremskey WT. Prevention of perioperative infection. J Bone Joint Surg Am. 2007;89(7):1605–1618. [DOI] [PubMed] [Google Scholar]
- 138. Fry DE, Fry RV. Surgical site infection: the host factor. AORN J. 2007;86(5):801–810; quiz 811-804. [DOI] [PubMed] [Google Scholar]
- 139. Darouiche RO, Wall MJ, Jr, Itani KM, et al. Chlorhexidine-Alcohol versus Povidone-Iodine for Surgical-Site Antisepsis. N Engl J Med. 2010;362(1):18–26. [DOI] [PubMed] [Google Scholar]
- 140. Bosco JA, III, Slover JD, Haas JP. Perioperative strategies for decreasing infection: a comprehensive evidence-based approach. J Bone Joint Surg Am. 2010;92(1):232–239. [PubMed] [Google Scholar]
- 141. Levy JH, Nagle DM, Curling PE, Waller JL, Kopel M, Tobia V. Contamination reduction during central venous catheterization. Crit Care Med. 1988;16(2):165–167. [DOI] [PubMed] [Google Scholar]
- 142. Johnston DH, Fairclough JA, Brown EM, Morris R. Rate of bacterial recolonization of the skin after preparation: four methods compared. Br J Surg. 1987;74(1):64. [DOI] [PubMed] [Google Scholar]
- 143. Jacobson C, Osmon DR, Hanssen A, et al. Prevention of wound contamination using DuraPrep solution plus Ioban 2 drapes. Clin Orthop Relat Res. 2005;439:32–37. [DOI] [PubMed] [Google Scholar]
- 144. Hasselgren PO, Hagberg E, Malmer H, Saljo A, Seeman T. One instead of two knives for surgical incision. Does it increase the risk of postoperative wound infection? Arch Surg. 1984;119(8):917–920. [DOI] [PubMed] [Google Scholar]
- 145. Bhandari M, Schemitsch EH, Adili A, Lachowski RJ, Shaughnessy SG. High and low pressure pulsatile lavage of contaminated tibial fractures: an in vitro study of bacterial adherence and bone damage. J Orthop Trauma. 1999;13(8):526–533. [DOI] [PubMed] [Google Scholar]
- 146. Anglen JO, Gainor BJ, Simpson WA, Christensen G. The use of detergent irrigation for musculoskeletal wounds. Int Orthop. 2003;27(1):40–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Tarbox BB, Conroy BP, Malicky ES, et al. Benzalkonium chloride. A potential disinfecting irrigation solution for orthopaedic wounds. Clin Orthop Relat Res. 1998(346):255–261. [PubMed] [Google Scholar]
- 148. Allo MD, Tedesco M. Operating room management: operative suite considerations, infection control. Surg Clin North Am. 2005;85(6):1291–1297, xii. [DOI] [PubMed] [Google Scholar]
- 149. Olsen MA, Nepple JJ, Riew KD, et al. Risk factors for surgical site infection following orthopaedic spinal operations. J Bone Joint Surg Am. 2008;90(1):62–69. [DOI] [PubMed] [Google Scholar]
- 150. Babkin Y, Raveh D, Lifschitz M, et al. Incidence and risk factors for surgical infection after total knee replacement. Scand J Infect Dis. 2007;39(10):890–895. [DOI] [PubMed] [Google Scholar]
- 151. Confalonieri N, Manzotti A, Pullen C. Is closed-suction drain necessary in unicompartmental knee replacement? A prospective randomised study. Knee. 2004;11(5):399–402. [DOI] [PubMed] [Google Scholar]
- 152. Lang GJ, Richardson M, Bosse MJ, et al. Efficacy of surgical wound drainage in orthopaedic trauma patients: a randomized prospective trial. J Orthop Trauma. 1998;12(5):348–350. [DOI] [PubMed] [Google Scholar]
- 153. Tjeenk RM, Peeters MP, van den Ende E, Kastelein GW, Breslau PJ. Wound drainage versus non-drainage for proximal femoral fractures. A prospective randomised study. Injury. 2005;36(1):100–104. [DOI] [PubMed] [Google Scholar]
- 154. Hutchinson JJ, McGuckin M. Occlusive dressings: a microbiologic and clinical review. Am J Infect Control. 1990;18(4):257–268. [DOI] [PubMed] [Google Scholar]
- 155. Cho CY, Lo JS. Dressing the part. Dermatol Clin. 1998;16(1):25–47. [DOI] [PubMed] [Google Scholar]
- 156. Moretti B, Larocca AM, Napoli C, et al. Active warming systems to maintain perioperative normothermia in hip replacement surgery: a therapeutic aid or a vector of infection? J Hosp Infect. 2009;73(1):58–63. [DOI] [PubMed] [Google Scholar]
- 157. Doufas AG. Consequences of inadvertent perioperative hypothermia. Best Pract Res Clin Anaesthesiol. 2003;17(4):535–549. [DOI] [PubMed] [Google Scholar]
- 158. AORN, ed Perioperative Standards and recommended practices: AORN; 2011. AORN I, ed; No. 1. [Google Scholar]
- 159. Schimpff SC. Improving operating room and perioperative safety: background and specific recommendations. Surg Innov. 2007;14(2):127–135. [DOI] [PubMed] [Google Scholar]
- 160. Ong KL, Kurtz SM, Lau E, Bozic KJ, Berry DJ, Parvizi J. Prosthetic joint infection risk after total hip arthroplasty in the Medicare population. J Arthroplasty. 2009;24(6 suppl):105–109. [DOI] [PubMed] [Google Scholar]
- 161. Wymenga AB, van Horn JR, Theeuwes A, Muytjens HL, Slooff TJ. Perioperative factors associated with septic arthritis after arthroplasty. Prospective multicenter study of 362 knee and 2,651 hip operations. Acta Orthop Scand. 1992;63(6):665–671. [DOI] [PubMed] [Google Scholar]
- 162. Keegan AD. Hospital bed occupancy: more than queuing for a bed. Med J Aust. 2010;193(5):291–293. [DOI] [PubMed] [Google Scholar]
- 163. Cunningham JB, Kernohan WG, Rush T. Bed occupancy, turnover intervals and MRSA rates in English hospitals. Br J Nurs. 2006;15(12):656–660. [DOI] [PubMed] [Google Scholar]
- 164. Davidson J, Griffin R, Higgs S. Introducing a clinical pathway in fluid management. J Perioper Pract. 2007;17(6):248–250, 255–246. [DOI] [PubMed] [Google Scholar]
- 165. Lewis JR, Hassan SK, Wenn RT, Moran CG. Mortality and serum urea and electrolytes on admission for hip fracture patients. Injury. 2006;37(8):698–704. [DOI] [PubMed] [Google Scholar]
- 166. Bierbaum BE, Callaghan JJ, Galante JO, Rubash HE, Tooms RE, Welch RB. An analysis of blood management in patients having a total hip or knee arthroplasty. J Bone Joint Surg Am. 1999;81(1):2–10. [DOI] [PubMed] [Google Scholar]
- 167. Gruber-Baldini AL, Marcantonio E, Orwig D, et al. Delirium outcomes in a randomized trial of blood transfusion thresholds in hospitalized older adults with hip fracture. J Am Geriatr Soc. 2013;61(8):1286–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Comfort EH. Reducing pressure ulcer incidence through Braden Scale risk assessment and support surface use. Adv Skin Wound Care. 2008;21(7):330–334. [DOI] [PubMed] [Google Scholar]
- 169. Xakellis GC, Frantz RA, Arteaga M, Nguyen M, Lewis A. A comparison of patient risk for pressure ulcer development with nursing use of preventive interventions. J Am Geriatr Soc. 1992;40(12):1250–1254. [DOI] [PubMed] [Google Scholar]
- 170. Gumieiro DN, Rafacho BP, Gradella LM, et al. Handgrip strength predicts pressure ulcers in patients with hip fractures. Nutrition. 2012;28(9):874–878. [DOI] [PubMed] [Google Scholar]
- 171. Remaley DT, Jaeblon T. Pressure ulcers in orthopaedics. The Journal of the American Academy of Orthopaedic Surgeons. 2010;18(9):568–575. [DOI] [PubMed] [Google Scholar]
- 172. Comfort EH. Reducing pressure ulcer incidence through Braden Scale risk assessment and support surface use. Adv Skin Wound Care. 2008;21(7):330–334. [DOI] [PubMed] [Google Scholar]
- 173. Baumgarten M, Margolis D, Berlin JA, et al. Risk factors for pressure ulcers among elderly hip fracture patients. Wound Repair Regen. 2003;11(2):96–103. [DOI] [PubMed] [Google Scholar]
- 174. Baumgarten M, Rich SE, Shardell MD, et al. Care-related risk factors for hospital-acquired pressure ulcers in elderly adults with hip fracture. J Am Geriatr Soc. 2012;60(2):277–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Rich SE, Margolis D, Shardell M, et al. Frequent manual repositioning and incidence of pressure ulcers among bed-bound elderly hip fracture patients. Wound Repair Regen. 2011;19(1):10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Rich SE, Shardell M, Hawkes WG, et al. Pressure-redistributing support surface use and pressure ulcer incidence in elderly hip fracture patients. J Am Geriatr Soc. 2011;59(6):1052–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. McNamara I, Sharma A, Prevost T, Parker M. Symptomatic venous thromboembolism following a hip fracture. Acta Orthop. 2009;80(6):687–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Hung WW, Egol KA, Zuckerman JD, Siu AL. Hip fracture management: tailoring care for the older patient. JAMA. 2012;307(20):2185–2194. [DOI] [PubMed] [Google Scholar]
- 179. Fisher CG, Blachut PA, Salvian AJ, Meek RN, O’Brien PJ. Effectiveness of pneumatic leg compression devices for the prevention of thromboembolic disease in orthopaedic trauma patients: a prospective, randomized study of compression alone versus no prophylaxis. J Orthop Trauma. 1995;9(1):1–7. [DOI] [PubMed] [Google Scholar]
- 180. Handoll HHG, Farrar MJ, McBirnie J, Tytherleigh-Strong G, Milne AA, Gillespie WJ. Heparin, low molecular weight heparin and physical methods for preventing deep vein thrombosis and pulmonary embolism following surgery for hip fractures. Cochrane Database Syst Rev. 2002;4:CD000305 doi:000310.001002/14651858.CD14000305. [DOI] [PubMed] [Google Scholar]
- 181. Sasaki S, Miyakoshi N, Matsuura H, et al. Prospective study on the efficacies of fondaparinux and enoxaparin in preventing venous thromboembolism after hip fracture surgery. J Orthop Sci. 2011;16(1):64–70. [DOI] [PubMed] [Google Scholar]
- 182. National Institute for Health and Clinical Excellence. Reducing the risk of venous thromboembolism (deep vein thrombosis and pulmonary embolism) in patients admitted to hospital. Clinical Guideline 92. Web site. http://www.nice.org.uk/guidance/cg92 Accessed February 21, 2015.
- 183. Long A, Zhang L, Zhang Y, et al. Efficacy and safety of rivaroxaban versus low-molecular-weight heparin therapy in patients with lower limb fractures. J Thromb Thrombolysis. 2014;38(3):299–305. [DOI] [PubMed] [Google Scholar]
- 184. Ji HM, Lee YK, Ha YC, Kim KC, Koo KH. Little impact of antiplatelet agents on venous thromboembolism after hip fracture surgery. J Korean Med Sci. 2011;26(12):1625–1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Stewart DW, Freshour JE. Aspirin for the prophylaxis of venous thromboembolic events in orthopedic surgery patients: a comparison of the AAOS and ACCP guidelines with review of the evidence. Ann Pharmacother. 2013;47(1):63–74. [DOI] [PubMed] [Google Scholar]
- 186. Fisher WD, Agnelli G, George DJ, et al. Extended venous thromboembolism prophylaxis in patients undergoing hip fracture surgery - the SAVE-HIP3 study. Bone Joint J. 2013;95-B(4):459–466. [DOI] [PubMed] [Google Scholar]
- 187. Sobieraj DM, Coleman CI, Tongbram V, et al. Mar 2012. [DOI] [PubMed]
- 188. Kaiser M, Bandinelli S, Lunenfeld B. Frailty and the role of nutrition in older people. A review of the current literature. Acta Bio-medica: Atenei Parmensis. 2010;81 Suppl 1:37–45. [PubMed] [Google Scholar]
- 189. Bell JJ, Bauer JD, Capra S, Pulle RC. Quick and Easy Is Not without Cost: Implications of Poorly Performing Nutrition Screening Tools in Hip Fracture. J Am Geriatr Soc. 2014;62(2):237–243. [DOI] [PubMed] [Google Scholar]
- 190. Pioli G, Barone A, Giusti A, et al. Predictors of mortality after hip fracture: results from 1-year follow-up. Aging Clin Exp Res. 2006;18(5):381–387. [DOI] [PubMed] [Google Scholar]
- 191. Fiatarone Singh MA. Exercise, nutrition and managing hip fracture in older persons. Curr Opin Clin Nutr Metab Care. 2014;17(1):12–24. [DOI] [PubMed] [Google Scholar]
- 192. Bell JJ, Bauer JD, Capra S, Pulle RC. Multidisciplinary, multi-modal nutritional care in acute hip fracture inpatients - Results of a pragmatic intervention. Clin Nutr. 2014;33(6):1101–1107. [DOI] [PubMed] [Google Scholar]
- 193. Gumieiro DN, Rafacho BP, Goncalves AF, et al. Mini Nutritional Assessment predicts gait status and mortality 6 months after hip fracture. Br J Nutr. 2013;109(9):1657–1661. [DOI] [PubMed] [Google Scholar]
- 194. Wyers CE, Reijven PL, Evers SM, et al. Cost-effectiveness of nutritional intervention in elderly subjects after hip fracture. A randomized controlled trial. Osteoporos Int. 2013;24(1):151–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Mallinson T, Deutsch A, Bateman J, et al. Comparison of discharge functional status after rehabilitation in skilled nursing, home health, and medical rehabilitation settings for patients after hip fracture repair. Arch Phys Med Rehabil. 2014;95(2):209–217. [DOI] [PubMed] [Google Scholar]
- 196. Popejoy LL, Dorman Marek K, Scott-Cawiezell J. Patterns and problems associated with transitions after hip fracture in older adults. J Gerontol Nurs. 2013;39(9):43–52. [DOI] [PubMed] [Google Scholar]
- 197. Chin RP, Ho CH, Cheung LP. Scheduled analgesic regimen improves rehabilitation after hip fracture surgery. Clin Orthop Relat Res. 2013;471(7):2349–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Buddingh S, Liang J, Allen J, Koziak A, Buckingham J, Beaupre LA. Rehabilitation for long-term care residents following hip fracture: a survey of reported rehabilitation practices and perceived barriers to delivery of care. J Geriatr Phys Ther. 2013;36(1):39–46. [DOI] [PubMed] [Google Scholar]
- 199. Gialanella B, Prometti P, Monguzzi V, Ferlucci C. Neuropsychiatric symptoms and rehabilitation outcomes in patients with hip fracture. Am J Phys Med Rehabil. 2014;93(7):562–569. [DOI] [PubMed] [Google Scholar]
- 200. Allen J, Koziak A, Buddingh S, Liang J, Buckingham J, Beaupre LA. Rehabilitation in patients with dementia following hip fracture: a systematic review. Physiother Can. 2012;64(2):190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Binder EF, Brown M, Sinacore DR, Steger-May K, Yarasheski KE, Schechtman KB. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292(7):837–846. [DOI] [PubMed] [Google Scholar]
- 202. Auais MA, Eilayyan O, Mayo NE. Extended exercise rehabilitation after hip fracture improves patients’ physical function: a systematic review and meta-analysis. Phys Ther. 2012;92(11):1437–1451. [DOI] [PubMed] [Google Scholar]
- 203. Latham NK, Harris BA, Bean JF, et al. Effect of a home-based exercise program on functional recovery following rehabilitation after hip fracture: a randomized clinical trial. JAMA. 2014;311(7):700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Handoll HH, Sherrington C, Mak JC. Interventions for improving mobility after hip fracture surgery in adults. Cochrane Database Syst Rev. 2011;(3):CD001704. [DOI] [PubMed] [Google Scholar]
- 205. Schnell S, Friedman SM, Mendelson DA, Bingham KW, Kates SL. The 1-Year mortality of patients treated in a hip fracture program for elders. Geriatr Orthop Surg Rehabil. 2010;1(1):6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Dell R, Greene D, Schelkun SR, Williams K. Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am. 2008;90(suppl 4):188–194. [DOI] [PubMed] [Google Scholar]
- 207. Della Rocca GJ, Moylan KC, Crist BD, Volgas DA, Stannard JP, Mehr DR. Comanagement of geriatric patients with hip fractures: a retrospective, controlled, cohort study. Geriatr Orthop Surg Rehabil. 2013;4(1):10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Biber R, Singler K, Curschmann-Horter M, Wicklein S, Sieber C, Bail HJ. Implementation of a co-managed Geriatric Fracture Center reduces hospital stay and time-to-operation in elderly femoral neck fracture patients. Arch Orthop Trauma Surg. 2013;133(11):1527–1531. [DOI] [PubMed] [Google Scholar]
- 209. Collinge CA, McWilliam-Ross K, Beltran MJ, Weaver T. Measures of clinical outcome before, during, and after implementation of a comprehensive geriatric hip fracture program: is there a learning curve? J Orthop Trauma. 2013;27(12):672–676. [DOI] [PubMed] [Google Scholar]
- 210. Kammerlander C, Gosch M, Blauth M, Lechleitner M, Luger TJ, Roth T. The Tyrolean Geriatric Fracture Center: an orthogeriatric co-management model. Z Gerontol Geriatr. 2011;44(6):363–367. [DOI] [PubMed] [Google Scholar]
- 211. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma. 2014;28(3):e49–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Cutler D, Wikler E, Basch P. Reducing administrative costs and improving the health care system. N Engl J Med. 2012;367(20):1875–1878. [DOI] [PubMed] [Google Scholar]
- 213. Kates SL. Lean Business Model and Implementation of a Geriatric Fracture Center. Clin Geriatr Med. 2014;30(2):191–205. [DOI] [PubMed] [Google Scholar]
- 214. Kates SL, Mendelson DA, Friedman SM. Co-managed care for fragility hip fractures (Rochester model). Osteoporos Int. 2010;21(suppl 4):S621–S625. [DOI] [PubMed] [Google Scholar]
- 215. Kates SL, Blake D, Bingham KW, Kates OS, Mendelson DA, Friedman SM. Comparison of an organized geriatric fracture program to United States government data. Geriatr Orthop Surg Rehabil. 2010;1(1):15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Kates SL, Mendelson DA, Friedman SM. The value of an organized fracture program for the elderly: early results. J Orthop Trauma. 2011;25(4):233–237. [DOI] [PubMed] [Google Scholar]
- 217. Goodman DC, Fisher ES, Chang CH. After Hospitalization: A Dartmouth Atlas Report on Post-Acute Care for Medicare Beneficiaries. Hanover, NH: Dartmouth; 2011. [PubMed] [Google Scholar]
- 218. Zidel TG. Lean Guide to Transforming Healthcare. Vol 1. 1 ed Milwaukee: ASQ Quality Press; 2006. [Google Scholar]
- 219. Chalice R. Improving Healthcare Using Toyota Lean Production Methods. Vol 1. 2 ed Milwaukee: ASQ Quality Press; 2007. [Google Scholar]
- 220. Clement RC, Ahn J, Mehta S, Bernstein J. Economic viability of geriatric hip fracture centers. Orthopedics. 2013;36(12): e1509–1514. [DOI] [PubMed] [Google Scholar]
- 221. Berwick DM, James B, Coye MJ. Connections between quality measurement and improvement. Med Care. 2003;41(1 Suppl): I30–38. [DOI] [PubMed] [Google Scholar]
- 222. Agel J, Swiontkowski MF. Guide to outcomes instruments for musculoskeletal trauma research. J Orthop Trauma. 2006;20(8 suppl):S1–146. [PubMed] [Google Scholar]
- 223. Adair CE, Simpson E, Casebeer AL, Birdsell JM, Hayden KA, Lewis S. Performance measurement in healthcare: part I—concepts and trends from a State of the Science Review. Healthcare policy = Politiques de sante. 2006;1(4):85–104. [PMC free article] [PubMed] [Google Scholar]
- 224. Porter ME. What is value in health care? N Engl J Med. 2010;363(26):2477–2481. [DOI] [PubMed] [Google Scholar]
- 225. Kaplan RS, Porter ME. How to solve the cost crisis in health care. Harv Bus Rev. 2011;89(9):46–52, 54,, 56–61 passim. [PubMed] [Google Scholar]
- 226. Mouzopoulos G, Vasiliadis G, Lasanianos N, Nikolaras G, Morakis E, Kaminaris M. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo-controlled study. J Orthop Traum. 2009;10(3):127–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Foss NB, Kristensen BB, Bundgaard M, et al. Fascia iliaca compartment blockade for acute pain control in hip fracture patients: a randomized, placebo-controlled trial. Anesthesiology. 2007;106(4):773–778. [DOI] [PubMed] [Google Scholar]
- 228. Olson SA, Mather RC., III Understanding how orthopaedic surgery practices generate value for healthcare systems. Clin Orthop Relat Res. 2013;471(6):1801–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229. Bilimoria NM. CMS “never events” and other new trends in quality health care standards for hospitals. Health Care Law Mon. 2008;2008(12):2–10. [PubMed] [Google Scholar]
- 230. Kohn LT, Corrigan J, Donaldson MS. To Err is Human: Building a Safer Health System. Washington, D.C: National Academy Press; 2000. [PubMed] [Google Scholar]
- 231. Forum NQ. Web site http://www.qualityforum.org/Measuring_Performance/Measuring_Performance.aspx Accessed February 21, 2015.
- 232. Services USDoHaH. National Quality Measures Clearlinghouse Hip Fracture Measures. Web site http://www.qualitymeasures.ahrq.gov/search/search.aspx?term=hip+fracture Accessed February 21, 2015.
- 233. Senior Editors: Richard E. Gliklich MD, Nancy A. Dreyer, M.P.H., Ph.D Registries for Evaluating Patient Outcomes: A User’s Guide: AHRQ; 2007:235. [Google Scholar]
- 234. Centre HHSCI. National Hip Fracture Database. Web site http://www.hscic.gov.uk/hipfracture Accessed January 24, 2014.
- 235. Dell R. Fracture prevention in Kaiser Permanente Southern California. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. 2011;22(suppl 3):457–460. [DOI] [PubMed] [Google Scholar]
- 236. Dell RM, Greene D, Anderson D, Williams K. Osteoporosis disease management: What every orthopaedic surgeon should know. J Bone Joint Surg Am. 2009;91(suppl 6):79–86. [DOI] [PubMed] [Google Scholar]
- 237. Fernandez-Moyano A, Fernandez-Ojeda R, Ruiz-Romero V, Garcia-Benitez B, Palmero-Palmero C, Aparicio-Santos R. Comprehensive care program for elderly patients over 65 years with hip fracture. Rev Clin Esp. 2014;214(1):17–23. [DOI] [PubMed] [Google Scholar]
- 238. De Rui M, Veronese N, Manzato E, Sergi G. Role of comprehensive geriatric assessment in the management of osteoporotic hip fracture in the elderly: an overview. Disabil Rehabil. 2013;35(9):758–765. [DOI] [PubMed] [Google Scholar]
- 239. Toscan J, Manderson B, Santi SM, Stolee P. “Just another fish in the pond”: the transitional care experience of a hip fracture patient. Int J Integr Care. 2013;13:e023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Geary S, Cale DD, Quinn B, Winchell J. Daily rapid rounds: decreasing length of stay and improving professional practice. J Nurs Adm. 2009;39(6):293–298. [DOI] [PubMed] [Google Scholar]
- 241. Poe SS, Cvach MM, Gartrelu DG, Radzik BR, Joy TL. An evidence-based approach to fall risk assessment, prevention, and management: lessons learned. J Nurs Care Qual. 2005;20(2):107–116; quiz 117-108. [DOI] [PubMed] [Google Scholar]
- 242. Inouye SK, Bogardus ST, Jr, Baker DI, Leo-Summers L, Cooney LM., Jr The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc. 2000;48(12):1697–1706. [DOI] [PubMed] [Google Scholar]
- 243. Maher AB, Meehan AJ, Hertz K, et al. Acute nursing care of the older adult with fragility hip fracture: an international perspective (part 1). Int J Orthop Trauma Nurs. 2012;16(4):177–194. [Google Scholar]
- 244. Maher AB, Meehan AJ, Hertz K, et al. Acute nursing care of the older adult with fragility hip fracture: an international perspective (Part 2). Int J Orthop Trauma Nurs. 2013;17(1):4–18. [Google Scholar]
- 245. CMS. Medicare Policy Manual. [Web documnet]. 2014; Web site http://www.cms.gov/Regulations-and-Guidance/Guidance/Manuals/Downloads/bp102c08.pdf Accessed February 21, 2015.
- 246. Gayed B, Black S, Daggy J, Munshi IA. Redesigning a joint replacement program using Lean Six Sigma in a Veterans Affairs hospital. JAMA Surg. 2013;148(11):1050–1056. [DOI] [PubMed] [Google Scholar]
- 247. Van Citters AD, Fahlman C, Goldmann DA, et al. Developing a pathway for high-value, patient-centered total joint arthroplasty. Clin Orthop Relat Res. 2014;472(5):1619–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Kates SL. Lean business model and implementation of a geriatric fracture center. Clin Geriatr Med. 2014;30(2):191–205. [DOI] [PubMed] [Google Scholar]
- 249. Barrett JA, Baron JA, Karagas MR, Beach ML. Fracture risk in the U.S. Medicare population. J Clin Epidemiol. 1999;52(3):243–249. [DOI] [PubMed] [Google Scholar]
- 250. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233–240. [DOI] [PubMed] [Google Scholar]
- 251. Hettrich CM, Boraiah S, Dyke JP, Neviaser A, Helfet DL, Lorich DG. Quantitative assessment of the vascularity of the proximal part of the humerus. J Bone Joint Surg Am. 2010;92(4):943–948. [DOI] [PubMed] [Google Scholar]
- 252. Hertel R, Hempfing A, Stiehler M, Leunig M. Predictors of humeral head ischemia after intracapsular fracture of the proximal humerus. J Shoulder Elbow Surg. 2004;13(4):427–433. [DOI] [PubMed] [Google Scholar]
- 253. Neer CS., II Displaced proximal humeral fractures. II. Treatment of three- part and four-part displacement. J Bone Joint Surg Am. 1970;52(6):1090–1103. [PubMed] [Google Scholar]
- 254. Neer CS., II Displaced proximal humeral fractures. I. Classification and evaluation. J Bone Joint Surg Am. 1970;52(6):1077–1089. [PubMed] [Google Scholar]
- 255. Codman EA. Fractures in Relation to the Subacromial Bursa. The Shoulder. Rupture of the Supraspinatus Tendon and Other Lesions in or about the Subacromial Bursa. Boston: Thomas Todd; 1934:313–331. [Google Scholar]
- 256. Orthopaedic Trauma Association Committee for Coding and Classification. Fracture and dislocation compendium. J Orthop Trauma. 1996;10(suppl 1):v–ix, 154. [PubMed] [Google Scholar]
- 257. Hawkins RJ. Unrecognized dislocations of the shoulder. Instr Course Lect. 1985;34:258–263. [PubMed] [Google Scholar]
- 258. Hertel R, Hempfing A, Stiehler M, Leunig M. Predictors of humeral head ischemia after intracapsular fracture of the proximal humerus. J Shoulder Elbow Surg. 2004;13(4):427–433. [DOI] [PubMed] [Google Scholar]
- 259. Court-Brown CM, Garg A, McQueen MM. The translated two-part fracture of the proximal humerus. Epidemiology and outcome in the older patient. J Bone Joint Surg Br. 2001;83(6):799–804. [DOI] [PubMed] [Google Scholar]
- 260. Nho SJ, Brophy RH, Barker JU, Cornell CN, MacGillivray JD. Innovations in the management of displaced proximal humerus fractures. J Am Acad Orthop Surg. 2007;15(1):12–26. [DOI] [PubMed] [Google Scholar]
- 261. Dimakopoulos P, Panagopoulos A, Kasimatis G. Transosseous suture fixation of proximal humeral fractures. Surgical technique. J Bone Joint Surg Am. 2009;91(suppl 2 pt 1):8–21. [DOI] [PubMed] [Google Scholar]
- 262. Gardner MJ, Boraiah S, Helfet DL, Lorich DG. Indirect medial reduction and strut support of proximal humerus fractures using an endosteal implant. J Orthop Trauma. 2008;22(3):195–200. [DOI] [PubMed] [Google Scholar]
- 263. Wachtl SW, Marti CB, Hoogewoud HM, Jakob RP, Gautier E. Treatment of proximal humerus fracture using multiple intramedullary flexible nails. Arch Orthop Trauma Surg. 2000;120(3-4):171–175. [DOI] [PubMed] [Google Scholar]
- 264. Konrad G, Bayer J, Hepp P, et al. Open reduction and internal fixation of proximal humeral fractures with use of the locking proximal humerus plate. Surgical technique. J Bone Joint Surg Am. 2010;92(suppl 1 pt 1):85–95. [DOI] [PubMed] [Google Scholar]
- 265. Owsley KC, Gorczyca JT. Fracture displacement and screw cutout after open reduction and locked plate fixation of proximal humeral fractures [corrected]. J Bone Joint Surg Am. 2008;90(2):233–240. [DOI] [PubMed] [Google Scholar]
- 266. Guy P, Slobogean GP, McCormack RG. Treatment preferences for displaced three- and four-part proximal humerus fractures. J Orthop Trauma. 2010;24(4):250–254. [DOI] [PubMed] [Google Scholar]
- 267. Cazeneuve JF, Cristofari DJ. The reverse shoulder prosthesis in the treatment of fractures of the proximal humerus in the elderly. J Bone Joint Surg Br. 2010;92(4):535–539. [DOI] [PubMed] [Google Scholar]
- 268. Den Hartog D, Van Lieshout EMM, Tuinebreijer WE, et al. Primary hemiarthroplasty versus conservative treatment for comminuted fractures of the proximal humerus in the elderly (ProCon): a multicenter randomized controlled trial. BMC Musculoskelet Disord. 2010;11:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Martin TG, Iannotti JP. Reverse total shoulder arthroplasty for acute fractures and failed management after proximal humeral fractures. Orthop Clin North Am. 2008;39(4):451–457. [DOI] [PubMed] [Google Scholar]
- 270. Slobogean GP, Noonan VK, O’Brien PJ. The reliability and validity of the Disabilities of Arm, Shoulder, and Hand, EuroQol-5D, Health Utilities Index, and Short Form-6D outcome instruments in patients with proximal humeral fractures. J Shoulder Elbow Surg. 2010;19(3):342–348. [DOI] [PubMed] [Google Scholar]
- 271. Centers for Disease Control and Prevention. Incidence and Costs to Medicare of Fractures Among Medicare Beneficiaries Aged Greater Than or Equal to 65 Years — United States, July 1991–June 1992. MMWR Morb Mortal Wkly Rep. 1996;45(41):877–900. [PubMed] [Google Scholar]
- 272. Brogren E, Petranek M, Atroshi I. Incidence and characteristics of distal radius fractures in a southern Swedish region. BMC Musculoskelet Disord. 2007;8:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Nellans KW, Kowalski E, Chung KC. The epidemiology of distal radius fractures. Hand Clin. 2012;28(2):113–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Kilgore ML, Morrisey MA, Becker DJ, et al. Health care expenditures associated with skeletal fractures among Medicare beneficiaries, 1999-2005. J Bone Miner Res. 2009;24(12):2050–2055. [DOI] [PubMed] [Google Scholar]
- 275. Diaz-Garcia RJ, Oda T, Shauver MJ, Chung KC. A systematic review of outcomes and complications of treating unstable distal radius fractures in the elderly. J Hand Surg Am. 2011;36(5):824–835 e822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Matullo KS, Dennison DG. Lateral tilt wrist radiograph using the contralateral hand to position the wrist after volar plating of distal radius fractures. J Hand Surg. 2010;35(6):900–904. [DOI] [PubMed] [Google Scholar]
- 277. Soong M, Got C, Katarincic J, Akelman E. Fluoroscopic evaluation of intra-articular screw placement during locked volar plating of the distal radius: a cadaveric study. J Hand Surg. 2008;33(10):1720–1723. [DOI] [PubMed] [Google Scholar]
- 278. Avery DM, III, Matullo KS. Distal radial traction radiographs: interobserver and intraobserver reliability compared with computed tomography. J Bone Joint Surg Am. 2014;96(7):582–588. [DOI] [PubMed] [Google Scholar]
- 279. Anzarut A, Johnson JA, Rowe BH, Lambert RGW, Blitz S, Majumdar SR. Radiologic and patient-reported functional outcomes in an elderly cohort with conservatively treated distal radius fractures. J Hand Surg. 2004;29(6):1121–1127. [DOI] [PubMed] [Google Scholar]
- 280. Roth KM, Blazar PE, Earp BE, Han R, Leung A. Incidence of displacement after nondisplaced distal radial fractures in adults. J Bone Joint Surg Am. 2013;95(15):1398–1402. [DOI] [PubMed] [Google Scholar]
- 281. Fanuele J, Koval KJ, Lurie J, Zhou W, Tosteson A, Ring D. Distal radial fracture treatment: what you get may depend on your age and address. J Bone Joint Surg Am. 2009;91(6):1313–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Arora R, Gabl M, Gschwentner M, Deml C, Krappinger D, Lutz M. A comparative study of clinical and radiologic outcomes of unstable Colles type distal radius fractures in patients older than 70 years: nonoperative treatment versus volar locking plating. J Orthop Trauma. 2009;23(4):237–242. [DOI] [PubMed] [Google Scholar]
- 283. Marcheix PS, Dotzis A, Benko PE, Siegler J, Arnaud JP, Charissoux JL. Extension fractures of the distal radius in patients older than 50: a prospective randomized study comparing fixation using mixed pins or a palmar fixed-angle plate. J Hand Surg Eur Vol. 2010;35(8):646–651. [DOI] [PubMed] [Google Scholar]
- 284. Schmelzer-Schmied N, Wieloch P, Martini AK, Daecke W. Comparison of external fixation, locking and non-locking palmar plating for unstable distal radius fractures in the elderly. Int Orthop. 2009;33(3):773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285. Arora R, Lutz M, Deml C, Krappinger D, Haug L, Gabl M. A prospective randomized trial comparing nonoperative treatment with volar locking plate fixation for displaced and unstable distal radial fractures in patients sixty-five years of age and older. J Bone Joint Surg Am. 2011;93(23):2146–2153. [DOI] [PubMed] [Google Scholar]
- 286. Karantana A, Downing ND, Forward DP, et al. Surgical treatment of distal radial fractures with a volar locking plate versus conventional percutaneous methods: a randomized controlled trial. J Bone Joint Surg Am. 2013;95(19):1737–1744. [DOI] [PubMed] [Google Scholar]
- 287. Figl M, Weninger P, Jurkowitsch J, Hofbauer M, Schauer J, Leixnering M. Unstable distal radius fractures in the elderly patient—volar fixed-angle plate osteosynthesis prevents secondary loss of reduction. J Trauma. 2010;68(4):992–998. [DOI] [PubMed] [Google Scholar]
- 288. Rozental TD, Blazar PE, Franko OI, Chacko AT, Earp BE, Day CS. Functional outcomes for unstable distal radial fractures treated with open reduction and internal fixation or closed reduction and percutaneous fixation. A prospective randomized trial. J Bone Joint Surg Am. 2009;91(8):1837–1846. [DOI] [PubMed] [Google Scholar]
- 289. Kim DH, Vaccaro AR. Osteoporotic compression fractures of the spine; current options and considerations for treatment. Spine J. 2006;6(5):479–487. [DOI] [PubMed] [Google Scholar]
- 290. Compston J. Osteoporosis: social and economic impact. Radiologic Clinics of North America. 2010;48(3):477–482. [DOI] [PubMed] [Google Scholar]
- 291. Ismail AA, Cockerill W, Cooper C, et al. Prevalent vertebral deformity predicts incident hip though not distal forearm fracture: results from the European Prospective Osteoporosis Study. Osteoporos Int. 2001;12(2):85–90. [DOI] [PubMed] [Google Scholar]
- 292. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Mineral Res. 2007;22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 293. Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res. 2007;22(3):465–475. [DOI] [PubMed] [Google Scholar]
- 294. Tosteson AN, Melton LJ, III, Dawson-Hughes B, et al. Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19(4):437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Waterloo S, Ahmed LA, Center JR, et al. Prevalence of vertebral fractures in women and men in the population-based Tromso Study. BMC Musculoskelet Disord. 2012;13:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Johnell O, Kanis JA. An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int. 2006;17(12):1726–1733. [DOI] [PubMed] [Google Scholar]
- 297. Tsai K, Twu S, Chieng P, Yang R, Lee T. Prevalence of vertebral fractures in chinese men and women in urban Taiwanese communities. Calcif Tissue Int. 1996;59(4):249–253. [DOI] [PubMed] [Google Scholar]
- 298. Naves M, Diaz-Lopez JB, Gomez C, Rodriguez-Rebollar A, Rodriguez-Garcia M, Cannata-Andia JB. The effect of vertebral fracture as a risk factor for osteoporotic fracture and mortality in a Spanish population. Osteoporos Int. 2003;14(6):520–524. [DOI] [PubMed] [Google Scholar]
- 299. van Dijk FS, Zillikens MC, Micha D, et al. PLS3 mutations in X-linked osteoporosis with fractures. N Engl J Med. 2013;369(16):1529–1536. [DOI] [PubMed] [Google Scholar]
- 300. Wood KB, Khanna G, Vaccaro AR, Arnold PM, Harris MB, Mehbod AA. Assessment of two thoracolumbar fracture classification systems as used by multiple surgeons. J Bone Joint Surg Am. 2005;87(7):1423–1429. [DOI] [PubMed] [Google Scholar]
- 301. Nguyen HV, Ludwig S, Gelb D. Osteoporotic vertebral burst fractures with neurologic compromise. J Spinal Disord Tech. 2003;16(1):10–19. [DOI] [PubMed] [Google Scholar]
- 302. Nevitt MC, Ettinger B, Black DM, et al. The association of radiographically detected vertebral fractures with back pain and function: a prospective study. Ann Intern Med. 1998;128(10):793–800. [DOI] [PubMed] [Google Scholar]
- 303. Karam M, Lavelle WF, Cheney R. The role of bone scintigraphy in treatment planning, and predicting pain relief after kyphoplasty. Nucl Med Commun. 2008;29(3):247–253. [DOI] [PubMed] [Google Scholar]
- 304. Francis RM, Aspray TJ, Hide G, Sutcliffe AM, Wilkinson P. Back pain in osteoporotic vertebral fractures. Osteoporos Int. 2008;19(7):895–903. [DOI] [PubMed] [Google Scholar]
- 305. Porter RW, Hibbert C. Calcitonin treatment for neurogenic claudication. Spine (Phila Pa 1976). 1983;8(6):585–592. [DOI] [PubMed] [Google Scholar]
- 306. Prather H, Watson JO, Gilula LA. Nonoperative management of osteoporotic vertebral compression fractures. Injury. 2007;38(suppl 3):S40–S48. [DOI] [PubMed] [Google Scholar]
- 307. Yan D, Duan L, Li J, Soo C, Zhu H, Zhang Z. Comparative study of percutaneous vertebroplasty and kyphoplasty in the treatment of osteoporotic vertebral compression fractures. Arch Orthop Trauma Surg. 2011;131(5):645–650. [DOI] [PubMed] [Google Scholar]
- 308. Ledlie JT, Renfro MB. Kyphoplasty treatment of vertebral fractures: 2-year outcomes show sustained benefits. Spine (Phila Pa 1976). 2006;31(1):57–64. [DOI] [PubMed] [Google Scholar]
- 309. Shindle MK, Gardner MJ, Koob J, Bukata S, Cabin JA, Lane JM. Vertebral height restoration in osteoporotic compression fractures: kyphoplasty balloon tamp is superior to postural correction alone. Osteoporos Int. 2006;17(12):1815–1819. [DOI] [PubMed] [Google Scholar]
- 310. Diamond TH, Bryant C, Browne L, Clark WA. Clinical outcomes after acute osteoporotic vertebral fractures: a 2-year non-randomised trial comparing percutaneous vertebroplasty with conservative therapy. Med J Aust. 2006;184(3):113–117. [DOI] [PubMed] [Google Scholar]
- 311. Barr JD, Barr MS, Lemley TJ, McCann RM. Percutaneous vertebroplasty for pain relief and spinal stabilization. Spine (Phila Pa 1976). 2000;25(8):923–928. [DOI] [PubMed] [Google Scholar]
- 312. Hochmuth K, Proschek D, Schwarz W, Mack M, Kurth AA, Vogl TJ. Percutaneous vertebroplasty in the therapy of osteoporotic vertebral compression fractures: a critical review. Eur Radiol. 2006;16(5):998–1004. [DOI] [PubMed] [Google Scholar]
- 313. Kasperk C, Hillmeier J, Noldge G, et al. Treatment of painful vertebral fractures by kyphoplasty in patients with primary osteoporosis: a prospective nonrandomized controlled study. J Bone Miner Res. 2005;20(4):604–612. [DOI] [PubMed] [Google Scholar]
- 314. Buchbinder R, Osborne RH, Ebeling PR, et al. A randomized trial of vertebroplasty for painful osteoporotic vertebral fractures. N Engl J Med. 2009;361(6):557–568. [DOI] [PubMed] [Google Scholar]
- 315. Kallmes DF, Comstock BA, Heagerty PJ, et al. A randomized trial of vertebroplasty for osteoporotic spinal fractures. N Engl J Med. 2009;361(6):569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373(9668):1016–1024. [DOI] [PubMed] [Google Scholar]
- 317. Hiwatashi A, Westesson PL. Vertebroplasty for osteoporotic fractures with spinal canal compromise. AJNR Am J Neuroradiol. 2007;28(4):690–692. [PMC free article] [PubMed] [Google Scholar]
- 318. Zhang Z, Fan J, Ding Q, Wu M, Yin G. Risk factors for new osteoporotic vertebral compression fractures after vertebroplasty: a systematic review and meta-analysis. J Spinal Disord Tech. 2013;26(4): E150–157. [DOI] [PubMed] [Google Scholar]
- 319. Barr JD, Jensen ME, Hirsch JA, et al. Position statement on percutaneous vertebral augmentation: a consensus statement developed by the Society of Interventional Radiology (SIR), American Association of Neurological Surgeons (AANS) and the Congress of Neurological Surgeons (CNS), American College of Radiology (ACR), American Society of Neuroradiology (ASNR), American Society of Spine Radiology (ASSR), Canadian Interventional Radiology Association (CIRA), and the Society of NeuroInterventional Surgery (SNIS). J Vasc Interv Radiol. 2014;25(2):171–181. [DOI] [PubMed] [Google Scholar]
- 320. Salai M, Dudkiewicz I, Novikov I, Amit Y, Chechick A. The epidemic of ankle fractures in the elderly—is surgical treatment warranted? Arch Orthop Trauma Surg. 2000;120(9):511–513. [DOI] [PubMed] [Google Scholar]
- 321. Bauer M, Bengner U, Johnell O, Redlund-Johnell I. Supination-eversion fractures of the ankle joint: changes in incidence over 30 years. Foot Ankle. 1987;8(1):26–28. [DOI] [PubMed] [Google Scholar]
- 322. Kannus P, Palvanen M, Niemi S, Parkkari J, Jarvinen M. Increasing number and incidence of low-trauma ankle fractures in elderly people: Finnish statistics during 1970-2000 and projections for the future. Bone. 2002;31(3):430–433. [DOI] [PubMed] [Google Scholar]
- 323. Hasselman CT, Vogt MT, Stone KL, Cauley JA, Conti SF. Foot and ankle fractures in elderly white women. Incidence and risk factors. J Bone Joint Surg Am. 2003;85(5):820–824. [DOI] [PubMed] [Google Scholar]
- 324. Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Akesson K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos Int. 2006;17(7):1065–1077. [DOI] [PubMed] [Google Scholar]
- 325. Seeley DG, Kelsey J, Jergas M, Nevitt MC. Study of Osteoporotic Fractures Research Group. Predictors of ankle and foot fractures in older women. J Bone Miner Res. 1996;11(9):1347–1355. [DOI] [PubMed] [Google Scholar]
- 326. Adami S. Bone health in diabetes: considerations for clinical management. Curr Med Res Opin. 2009;25(5):1057–1072. [DOI] [PubMed] [Google Scholar]
- 327. Schwartz AV, Nevitt MC, Brown BW, Jr, Kelsey JL. Increased falling as a risk factor for fracture among older women: the study of osteoporotic fractures. Am J Epidemiol. 2005;161(2):180–185. [DOI] [PubMed] [Google Scholar]
- 328. Strauss EJ, Egol KA. The management of ankle fractures in the elderly. Injury. 2007;38(suppl 3):S2–S9. [DOI] [PubMed] [Google Scholar]
- 329. Boden BP, Osbahr DC. High-risk stress fractures: evaluation and treatment. J Am Acad Orthop Surg. 2000;8(6):344–353. [DOI] [PubMed] [Google Scholar]
- 330. Robinson AH, Pasapula C, Brodsky JW. Surgical aspects of the diabetic foot. J Bone Joint Surg Br. 2009;91(1):1–7. [DOI] [PubMed] [Google Scholar]
- 331. Schon LC, Easley ME, Weinfeld SB. Charcot neuroarthropathy of the foot and ankle. Clin Orthop Relat Res. 1998;(349):116–131. [DOI] [PubMed] [Google Scholar]
- 332. Lillmars SA, Meister BR. Acute trauma to the diabetic foot and ankle. Curr Opin Orthop. 2001;12:100–105. [Google Scholar]
- 333. Wukich DK, Kline AJ. The management of ankle fractures in patients with diabetes. J Bone Joint Surg Am. 2008;90(7):1570–1578. [DOI] [PubMed] [Google Scholar]
- 334. Jones KB, Maiers-Yelden KA, Marsh JL, Zimmerman MB, Estin M, Saltzman CL. Ankle fractures in patients with diabetes mellitus. J Bone Joint Surg Br. 2005;87(4):489–495. [DOI] [PubMed] [Google Scholar]
- 335. Henry SM, Pollak AN, Jones AL, Boswell S, Scalea TM. Pelvic fracture in geriatric patients: a distinct clinical entity. J Trauma. 2002;53(1):15–20. [DOI] [PubMed] [Google Scholar]
- 336. Anglen JO, Burd TA, Hendricks KJ, Harrison P. The “Gull Sign.” A harbinger of failure for internal fixation of geriatric acetabular fractures. J Orthop Trauma. 2003;17(9):625–634. [DOI] [PubMed] [Google Scholar]
- 337. Gaski GE, Manson TT, Castillo RC, Slobogean GP, O’Toole RV. Nonoperative treatment of intermediate severity lateral compression type 1 pelvic ring injuries with minimally displaced complete sacral fracture. J Orthop Trauma. 2014;28(12):674–680. [DOI] [PubMed] [Google Scholar]
- 338. Burgess AR, Eastridge BJ, Young JW, et al. Pelvic ring disruptions: effective classification system and treatment protocols. J Trauma. 1990;30(7):848–856. [PubMed] [Google Scholar]
- 339. Bellabarba C, Ricci WM, Bolhofner BR. Distraction external fixation in lateral compression pelvic fractures. J Orthop Trauma. 2006;20(1 suppl):S7–S14. [PubMed] [Google Scholar]
- 340. Vaccaro AR, Kim DH, Brodke DS, et al. Diagnosis and management of sacral spine fractures. Instr Course Lect. 2004;53:375–385. [PubMed] [Google Scholar]
- 341. Tucker MC, Nork SE, Simonian PT, Routt ML., Jr Simple anterior pelvic external fixation. J Trauma. 2000;49(6):989–994. [DOI] [PubMed] [Google Scholar]
- 342. Gansslen A, Hildebrand F, Kretek C. Supraacetabular external fixation for pain control in geriatric type B pelvic injuries. Acta Chir Orthop Traumatol Cech. 2013;80(2):101–105. [PubMed] [Google Scholar]
- 343. Helfet DL, Borrelli J, Jr, DiPasquale T, Sanders R. Stabilization of acetabular fractures in elderly patients. J Bone Joint Surg Am. 1992;74(5):753–765. [PubMed] [Google Scholar]
- 344. Carroll EA, Huber FG, Goldman AT, et al. Treatment of acetabular fractures in an older population. J Orthop Trauma. 2010;24(10):637–644. [DOI] [PubMed] [Google Scholar]
- 345. Gary JL, VanHal M, Gibbons SD, Reinert CM, Starr AJ. Functional outcomes in elderly patients with acetabular fractures treated with minimally invasive reduction and percutaneous fixation. J Orthop Trauma. 2012;26(5):278–283. [DOI] [PubMed] [Google Scholar]
- 346. Anglen JO, Burd TA, Hendricks KJ, Harrison P. The “Gull Sign”: a harbinger of failure for internal fixation of geriatric acetabular fractures. J Orthop Trauma. 2003;17(9):625–634. [DOI] [PubMed] [Google Scholar]
- 347. O’Toole RV, Hui E, Chandra A, Nascone JW. How often does open reduction and internal fixation of geriatric acetabular fractures lead to hip arthroplasty? J Orthop Trauma. 2014;28(3):148–153. [DOI] [PubMed] [Google Scholar]
- 348. Tannast M, Najibi S, Matta JM. Two to twenty-year survivorship of the hip in 810 patients with operatively treated acetabular fractures. J Bone Joint Surg Am. 2012;94(17):1559–1567. [DOI] [PubMed] [Google Scholar]
- 349. Beaule PE, Griffin DB, Matta JM. The Levine anterior approach for total hip replacement as the treatment for an acute acetabular fracture. J Orthop Trauma. 2004;18(9):623–629. [DOI] [PubMed] [Google Scholar]
- 350. Sermon A, Broos P, Vanderschot P. Total hip replacement for acetabular fractures. Results in 121 patients operated between 1983 and 2003. Injury. 2008;39(8):914–921. [DOI] [PubMed] [Google Scholar]
- 351. Cornell CN. Hip fractures in the elderly: on the acetabular side. Orthopedics. 2004;27(9):931–932. [DOI] [PubMed] [Google Scholar]
- 352. Boraiah S, Ragsdale M, Achor T, Zelicof S, Asprinio DE. Open reduction internal fixation and primary total hip arthroplasty of selected acetabular fractures. J Orthop Trauma. 2009;23(4):243–248. [DOI] [PubMed] [Google Scholar]
- 353. Herscovici D, Jr, Lindvall E, Bolhofner B, Scaduto JM. The combined hip procedure: open reduction internal fixation combined with total hip arthroplasty for the management of acetabular fractures in the elderly. J Orthop Trauma. 2010;24(5):291–296. [DOI] [PubMed] [Google Scholar]
- 354. Mears DC, Shirahama M. Stabilization of an acetabular fracture with cables for acute total hip arthroplasty. J Arthroplasty. 1998;13(1):104–107. [DOI] [PubMed] [Google Scholar]
- 355. Mears DC, Velyvis JH. Acute total hip arthroplasty for selected displaced acetabular fractures: two to twelve-year results. J Bone Joint Surg Am. 2002;84-A(1):1–9. [DOI] [PubMed] [Google Scholar]
- 356. Spencer RF. Acetabular fractures in older patients. J Bone Joint Surg Br. 1989;71(5):774–776. [DOI] [PubMed] [Google Scholar]
- 357. Routt ML, Jr, Simonian PT. Closed reduction and percutaneous skeletal fixation of sacral fractures. Clin Orthop Relat Res. 1996(329):121–128. [DOI] [PubMed] [Google Scholar]
- 358. Sarkar SD. Delayed open reduction of traumatic dislocation of the hip. A case report and historical review. Clin Orthop Relat Res. 1984(186):38–41. [PubMed] [Google Scholar]
- 359. Rickman M, Young J, Trompeter A, Pearce R, Hamilton M. Managing acetabular fractures in the elderly with fixation and primary arthroplasty: aiming for early weightbearing. Clin Orthop Relat Res. 2014;472(11):3375–3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Rozental TD, Makhni EC, Day CS, Bouxsein ML. Improving evaluation and treatment for osteoporosis following distal radial fractures. A prospective randomized intervention. J Bone Joint Surg Am. 2008;90(5):953–961. [DOI] [PubMed] [Google Scholar]
- 361. Kanis JA, Gluer CC. An update on the diagnosis and assessment of osteoporosis with densitometry. Committee of Scientific Advisors, International Osteoporosis Foundation. Osteoporos Int. 2000;11(3):192–202. [DOI] [PubMed] [Google Scholar]
- 362. Blake GM, Fogelman I. An update on dual-energy x-ray absorptiometry. Semin Nucl Med. 2010;40(1):62–73. [DOI] [PubMed] [Google Scholar]
- 363. Lewiecki EM. Bone density measurement and assessment of fracture risk. Clin Obstet Gynecol. 2013;56(4):667–676. [DOI] [PubMed] [Google Scholar]
- 364. Milte R, Crotty M. Musculoskeletal health, frailty and functional decline. Best Pract Res Clin Rheumatol. 2014;28(3):395–410. [DOI] [PubMed] [Google Scholar]
- 365. Kanis JA, Johansson H, Oden A, McCloskey EV. Assessment of fracture risk. Eur J Radiol. 2009;71(3):392–397. [DOI] [PubMed] [Google Scholar]
- 366. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int. 2011;22(9):2395–2411. [DOI] [PubMed] [Google Scholar]
- 367. Nakamura K, Takahashi S, Oyama M, et al. Prior nonhip limb fracture predicts subsequent hip fracture in institutionalized elderly people. Osteoporos Int. 2010;21(8):1411–1416. [DOI] [PubMed] [Google Scholar]
- 368. Dawson-Hughes B, Looker AC, Tosteson AN, Johansson H, Kanis JA, Melton LJ., III The potential impact of new National Osteoporosis Foundation guidance on treatment patterns. Osteoporos Int. 2010;21(1):41–52. [DOI] [PubMed] [Google Scholar]
- 369. Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement. 2000;17(1):1–45. [PubMed] [Google Scholar]
- 370. Bukata SV, Kates SL, O’Keefe RJ. Short-term and long-term orthopaedic issues in patients with fragility fractures. Clin Orthop Relat Res. 2011;469(8):2225–2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Holick M. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. [DOI] [PubMed] [Google Scholar]
- 372. Bischoff-Ferrari HA, Orav EJ, Willett WC, Dawson-Hughes B. The effect of vitamin D supplementation on skeletal, vascular, or cancer outcomes. Lancet Diabetes Endocrinol. 2014;2(5):363–364. [DOI] [PubMed] [Google Scholar]
- 373. Bikle DD. Vitamin D metabolism, mechanism of action, and clinical applications. Chem Biol. 2014;21(3):319–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374. Wang Y, Zhu J, DeLuca HF. Identification of the vitamin D receptor in osteoblasts and chondrocytes but not osteoclasts in mouse bone. J Bone Miner Res. 2014;29(3):685–692. [DOI] [PubMed] [Google Scholar]
- 375. Bischoff-Ferrari HA. Optimal serum 25-hydroxyvitamin D levels for multiple health outcomes. Adv Exp Med Biol. 2014;810:500–525. [DOI] [PubMed] [Google Scholar]
- 376. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic Acid in Reducing Clinical Fracture and Mortality after Hip Fracture. N Engl J Med. 2007;357: nihpa40967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377. Knopp-Sihota JA, Cummings GG, Homik J, Voaklander D. The association between serious upper gastrointestinal bleeding and incident bisphosphonate use: a population-based nested cohort study. BMC Geriatr. 2013;13:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med. 2007;357(18):1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Bhupathiraju SN, Manson JE. Menopausal hormone therapy and chronic disease risk in the women's health initiative: is timing everything? Endocr Pract. 2014;20(11):1201–1213. [DOI] [PubMed] [Google Scholar]
- 380. Komm BS, Mirkin S. An overview of current and emerging SERMs. J Steroid Biochem Mol Biol. 2014;143:207–222. [DOI] [PubMed] [Google Scholar]
- 381. Bogado CE, Zanchetta MB, Boailchuk JA, Massari FE, Zanchetta JR. Denosumab: What's new? Curr Osteoporos Rep. 2011;9(1):12–19. [DOI] [PubMed] [Google Scholar]
- 382. Bone HG, Dempster DW, Eisman JA, et al. Odanacatib for the treatment of postmenopausal osteoporosis: development history and design and participant characteristics of LOFT, the Long-Term Odanacatib Fracture Trial[published online November 29, 2014]. Osteoporos Int. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Bukata SV, Puzas JE. Orthopedic uses of teriparatide. Curr Osteoporos Rep. 2010;8(1):28–33. [DOI] [PubMed] [Google Scholar]
- 384. Ross AC, Taylor CL, Yaktine AL, DelValle HB. DRI Calcium Vitamin D. Washington D.C: The national academies press 2011; http://www.nap.edu/openbook.php?record_id=13050&page=7. [PubMed] [Google Scholar]
- 385. Neupane S, Knohl SJ. The Institute of Medicine, the Food and Drug Administration, and the calcium conundrum. Public Health Nutr. 2014;17(8):1865–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386. Hill A, Althausen PL, O’Mara TJ, Bray TJ. Why veteran orthopaedic trauma surgeons are being fired and what we can do about it? J Orthop Trauma. 2013;27(6):355–362. [DOI] [PubMed] [Google Scholar]
- 387. Solomon DH, Patrick AR, Schousboe J, Losina E. The potential economic benefits of improved postfracture care: a cost-effectiveness analysis of a fracture liaison service in the US health-care system. J Bone Miner Res. 2014;29(7):1667–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388. Association AO. American Orthopaedic Association initiative for improving osteoporosis management “Own The Bone”. 2014; Web site www.ownthebone.org Accessed February 21, 2015.
- 389. Tosi LL, Gliklich R, Kannan K, Koval KJ. The American Orthopaedic Association's “own the bone” initiative to prevent secondary fractures. J Bone Joint Surg Am. 2008;90(1):163–173. [DOI] [PubMed] [Google Scholar]
- 390. Forum NQ. National Quality Forum Post-Fracture Performance Measures # 2416, 2417, and 2418; 2014; Web site www.qualityforum.org Accessed February 21, 2015.
- 391. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 392. Tinetti ME, Speechley M, Ginter SF. Risk factors for falls among elderly persons living in the community. N Engl J Med. 1988;319(26):1701–1707. [DOI] [PubMed] [Google Scholar]
- 393. Nachreiner NM, Findorff MJ, Wyman JF, McCarthy TC. Circumstances and consequences of falls in community-dwelling older women. J Womens Health (Larchmt). 2007;16(10):1437–1446. [DOI] [PubMed] [Google Scholar]
- 394. Public health and aging: nonfatal injuries among older adults treated in hospital emergency departments—United States, 2001. MMWR Morb Mortal Wkly Rep. 2003;52(42):1019–1022. [PubMed] [Google Scholar]
- 395. Nevitt MC, Cummings SR, Hudes ES. Risk factors for injurious falls: a prospective study. J Gerontol. 1991;46(5):M164–M170. [DOI] [PubMed] [Google Scholar]
- 396. Tinetti ME, Liu WL, Claus EB. Predictors and prognosis of inability to get up after falls among elderly persons. JAMA. 1993;269(1):65–70. [PubMed] [Google Scholar]
- 397. Zijlstra GA, van Haastregt JC, van Eijk JT, van Rossum E, Stalenhoef PA, Kempen GI. Prevalence and correlates of fear of falling, and associated avoidance of activity in the general population of community-living older people. Age Ageing. 2007;36(3):304–309. [DOI] [PubMed] [Google Scholar]
- 398. Grisso JA, Kelsey JL, Strom BL, et al. Risk factors for falls as a cause of hip fracture in women. The Northeast Hip Fracture Study Group. N Engl J Med. 1991;324(19):1326–1331. [DOI] [PubMed] [Google Scholar]
- 399. Morrison RS, Chassin MR, Siu AL. The medical consultant's role in caring for patients with hip fracture. Ann Intern Med. 1998;128(12 pt 1):1010–1020. [DOI] [PubMed] [Google Scholar]
- 400. Tinetti ME, Kumar C. The patient who falls: “It's always a trade-off”. JAMA. 2010;303(3):258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 401. Beer C, Hyde Z, Almeida OP, et al. Quality use of medicines and health outcomes among a cohort of community dwelling older men: an observational study. Br J Clin Pharmacol. 2011;71(4):592–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402. Ensrud KE, Blackwell T, Mangione CM, et al. Central nervous system active medications and risk for fractures in older women. Arch Intern Med. 2003;163(8):949–957. [DOI] [PubMed] [Google Scholar]
- 403. Woolcott JC, Richardson KJ, Wiens MO, et al. Meta-analysis of the impact of 9 medication classes on falls in elderly persons. Arch Intern Med. 2009;169(21):1952–1960. [DOI] [PubMed] [Google Scholar]
- 404. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2009(2):CD007146. [DOI] [PubMed] [Google Scholar]
- 405. Cameron ID, Murray GR, Gillespie LD, et al. Interventions for preventing falls in older people in nursing care facilities and hospitals. Cochrane Database Syst Rev. 2010;(1):CD005465. [DOI] [PubMed] [Google Scholar]
- 406. Rubenstein LZ, Robbins AS, Josephson KR, Schulman BL, Osterweil D. The value of assessing falls in an elderly population. A randomized clinical trial. Ann Intern Med. 1990;113(4):308–316. [DOI] [PubMed] [Google Scholar]