Abstract
Context
Maternal pre-pregnancy obesity may increase the risk of childhood obesity but it is unknown whether other metabolic factors in early pregnancy such as lipid profile and hypertension are associated with offspring cardiometabolic traits.
Objective
Our objective was to investigate whether fasting lipid, glucose, and insulin levels during early pregnancy and maternal pre-pregnancy weight status, are associated with offspring adiposity measures, lipid levels and blood pressure at preschool age.
Design and Methods
The study included 618 mother-child pairs of the pregnancy cohort “Rhea” study in Crete, Greece. Pregnant women were recruited at the first prenatal visit (mean: 12weeks, SD: 0.7). A subset of 348 women provided fasting serum samples for glucose and lipid measurements. Outcomes measures were body mass index, abdominal circumference, sum of skinfold thickness, and blood pressure measurements at 4 years of age. A subsample of 525 children provided non-fasting blood samples for lipid measurements.
Results
Pre-pregnancy overweight/obesity was associated with greater risk of offspring overweight/obesity (RR: 1.83, 95%CI: 1.19, 2.81), central adiposity (RR: 1.97, 95%CI: 1.11, 3.49), and greater fat mass by 5.10mm (95%CI: 2.49, 7.71) at 4 years of age. These associations were more pronounced in girls. An increase of 40mg/dl in fasting serum cholesterol levels in early pregnancy was associated with greater skinfold thickness by 3.30mm (95%CI: 1.41, 5.20) at 4 years of age after adjusting for pre-pregnancy BMI and several other confounders. An increase of 10mmHg in diastolic blood pressure in early pregnancy was associated with increased risk of offspring overweight/obesity (RR: 1.22, 95%CI: 1.03, 1.45), and greater skinfold thickness by 1.71mm (95% CI: 0.57, 2.86) at 4 years of age.
Conclusions
Metabolic dysregulation in early pregnancy may increase the risk of obesity at preschool age.
Introduction
Childhood obesity is one of the greatest public health challenges worldwide and is having a major impact on human morbidity, mortality and quality of life [1, 2]. In Europe, its prevalence has increased dramatically in last decades, while recent estimates report that Greece has the highest prevalence of childhood obesity [3]. The commonly held causes of obesity, which are over-eating, inactivity, and genetic pre-disposition, do not fully explain the current obesity epidemic [4]. According to the developmental origins of health and disease (DOHaD) hypothesis changes in the intrauterine environment at critical or sensitive periods of the developmental process could have irreversible, lifelong consequences in offspring metabolism [4, 5]. Metabolic disorders during pregnancy like obesity, gestational diabetes, and excess gestational weight gain are well known exposures that predispose offspring to obesity [6–9]. However, the role of maternal metabolism in the first trimester of pregnancy, which is a critical developmental time window for gestational programming, is unclear [10].
Epidemiological studies indicate that higher maternal pre-pregnancy body mass index is associated with increased risk of childhood obesity [7, 8]. Few studies have examined so far its association with other cardiometabolic risk factors such as lipid levels and blood pressure in children with controversial results [11–15]. Whether these associations reflect direct intrauterine causal mechanisms or are driven in a gender-related manner remains unclear. Animal studies suggest that sex-specific vulnerabilities to an altered in utero metabolic environment may mediate sex differences in fetal growth and predisposition to adult diseases, such as cardiovascular disease [16, 17]), however evidence from human studies is scarce [18]. Moreover, it has been suggested that maternal gestational weight gain and smoking during pregnancy can act as confounders of such associations [6, 19, 20], although it can be argued that they may also act as mediators.
Studies on other maternal cardiovascular risk factors such as dyslipidemia or hypertension in pregnancy in association with offspring cardiometabolic health are scarce with conflicting results [21, 22]. Maternal non fasting lipid levels in early pregnancy were shown to be associated with increased offspring’s fat percentage and waist-to-height ratio values at preschool age [22]. Hypertension in early pregnancy has been associated with increased risk of fetal growth restriction [23, 24]), and preeclampsia [23], but there are no studies examining blood pressure in early pregnancy with offspring cardiometabolic traits.
In this study, we aimed to fill these research gaps by investigating the impact of maternal metabolic profile in early pregnancy characterized by pre-pregnancy Body Mass Index (BMI), blood pressure levels, and fasting lipids, insulin and glucose levels on offspring cardiometabolic traits in early childhood, in a prospective pregnancy cohort in Crete, Greece, after controlling for several confounding and mediator factors.
Materials and Methods
Study design and population: Rhea cohort
The present study is part of the “Rhea” project, a pregnancy cohort which examines prospectively a population-based cohort of pregnant women and their children at the prefecture of Heraklion, Crete, Greece [25]. We recruited pregnant women (Greek and immigrants) at the time of the first comprehensive ultrasound examination, around week 12 of gestation (mean: 12.1 weeks, SD: 0.7), from four prenatal clinics (two public and two private) in Heraklion city, during the twelve-month period from February 2007 until February 2008. The inclusion criteria for study participants were: residents in the study area; pregnant women aged > 16 years; 1st prenatal visit: hospitals or private clinics at Heraklion district; no communication handicap. The study was approved by the Ethical Committee of the University Hospital of Heraklion (Crete, Greece), and all participants provided written informed consent after complete description of the study.
Of 1363 singleton live births in the Rhea study, 879 children participated at the 4 years follow up, during which anthropometry and non-fasting blood samples were obtained from 785 children. From those, complete data for maternal anthropometry, follow-up interview and child anthropometric measurements were available for 631 mother–child pairs. We excluded women who had been diagnosed with preeclampsia [n = 13 (8 in this, and 5 in previous pregnancies)], since this condition is associated with a higher blood pressure and BMI in childhood and early adult life [21]. Thus, a cohort of 618 mother–child pairs was available for the present analysis. Of them a subset of 348 women provided fasting blood samples for glucose and lipid measurements, due to the timing of enrollment in the study. A subsample of 525 children provided non-fasting blood samples at the 4 year follow up (mean: 4.2 years, SD: 0.2).
Exposures: maternal pre-pregnancy BMI and metabolic profile during early pregnancy
Maternal overweight/obesity
Maternal height, measured at the first prenatal visit, and pre-pregnancy weight, as reported at the first major ultrasound visit, were used to calculate the pre-pregnant body mass index (BMI; weight (kg)/height (m)2). Women were divided into 3 categories as follows: no excess weight (pre-pregnant BMI<25 kg/m2), overweight (pre-pregnant BMI: 25–29.9 kg/m2) and obese (pre-pregnant BMI ≥30kg/m2) according to the definitions of the World Health Organization.
Maternal fasting glucose and lipid levels in early pregnancy
We measured lipids [total cholesterol (TC), triglycerides (TG), high-density lipoprotein cholesterol (HDL-C)] and glucose by standard enzymatic methods (Medicon, Greece) on an automatic analyzer (AU5400 high-volume chemistry analyzer; Olympus America, Inc., Melville, New York). Low density lipoprotein cholesterol (LDL-C) concentration was estimated by using the formula: LDL-C = TC-[(TG/5) + HDL-C]. C-reactive protein levels were measured with a high-sensitivity homogenous immunoassay (ORS 6199, Beckman Coulter, USA) on an automatic analyzer (AU5400 high-volume chemistry analyzer; Olympus America, Inc., Melville, New York). Maternal insulin concentration was measured by IMMULITE 2000 immunoassay system (Siemens Healthcare Diagnostics, Inc., Deerfield, Illinois). The inter- and intra-assay coefficients of variation were less than 5%.
Maternal abnormal lipid profile in early pregnancy was defined as triglyceride levels ≥150 mg/dL, or total cholesterol levels ≥200 mg/dL, or high density lipoprotein cholesterol (HDL-C) levels <50 mg/dL, or low density lipoprotein cholesterol (LDL-C) levels ≥130 mg/dl [26]. Maternal hyperglycemia in early pregnancy was defined as a maternal fasting blood glucose level ≥92 mg/dl [27].
Maternal blood pressure in early pregnancy
Systolic (SBP) and diastolic (DBP) blood pressure were measured at the ultrasound examination. Measurements were taken by using an electronic blood pressure monitor after 10 minutes of rest in a sitting position. All readings were replicated 3 times in the right arm for each woman. The mean value obtained from the second and third readings was used in the analysis [25].
Outcomes: Offspring cardiometabolic traits during early childhood
Child adiposity outcomes at 4 years of age
Child anthropometric measures at 4 years of age (mean: 4.2 years, SD: 0.2), were taken by specially trained research assistants according to standard procedures at the University Hospital of Heraklion, Crete, Greece. Body weight was measured once by a digital scale (Seca Bellisima 841) to the nearest 0.1kg with subjects standing without shoes and in light clothing. Height was measured to the nearest 0.1 cm with the use of a commercial stadiometer (Seca 213). Overweight/obesity were defined using age-and sex-specific BMI thresholds proposed by the International Obesity Task Force [28].
Waist circumference (WC) was measured in duplicate to the nearest 0.1 cm in the standing position, at the high point of the iliac crest at the end of a gentle expiration, using a flexible tape measure (Seca 201). We used age-and sex-specific 90th waist circumference percentiles based on national references [29], as a cut-off point to define central adiposity.
Skin fold thickness was measured to the nearest 0.1mm at four anatomical sites (triceps, thigh, subscapular and suprailiac) on the right side of the body, using calibrated calipers (Harpenden HSK- BI, CE-0120).
Child non fasting lipid profile at 4 years of age
We measured non fasting total cholesterol (TC) and high-density lipoprotein cholesterol (HDL-C) with the same biochemical methods used for maternal lipids measurements. As there is no standard definition for lipid disorders at preschool age, we used the 75th percentile of the study cohort distribution for total cholesterol (≥173.9 mg/dL), LDL-C- (≥111.5 mg/dL) and the 25th percentile for HDL-C levels (<40 mg/dL) as a cut-off point to denote abnormal lipid levels in children [30].
Child blood pressure at 4 years of age
At 4 years of age, trained research assistants measured systolic (SBP) and diastolic blood pressure (DBP) after 5 minutes rest in the seated position, at the child right arm with a cuff of appropriate size for arm circumference using a Dinamap Pro Care 400, which utilizes an oscillometric method. We used the average of five consecutive measurements, taken with 1 minute intervals [31]. We then calculated blood pressure percentiles specific for age, sex, and height, as blood pressure measurements in children are suggested to differ according to these characteristics.
Potential confounders
Potential confounders included characteristics that have an established or potential association with maternal metabolic profile in early pregnancy or cardiometabolic risk in childhood including: maternal age at delivery (years); maternal education (low level: ≤6 yrs. of school; medium level: ≤12 yrs. of school; high level: university or technical college degree); maternal origin (Greek/other); marital status (married/other); physical activity before pregnancy (yes/no); parity (primiparous/multiparous); type of delivery (vaginal/caesarean); smoking during pregnancy (yes/no); gestational weight gain, categorized according to 2009 Institute of Medicine guidelines [32]; family history of dyslipidemia (yes/no); family history of diabetes (yes/no); gestational diabetes (yes/no); gestational hypertension (yes/no); gestational age (weeks); birth weight (kg); child’s sex (male/female); duration of breastfeeding (months); day of care attendance at the first 2 years of life (yes/no); TV viewing at 4 years of age (hours/day); child’s energy intake (Kcals/day) at 4 years of age based on a validated food frequency questionnaire [33].
Statistical analysis
Differences in distributions of normally distributed variables were tested with t-test, non-normally distributed continuous variables were tested by using non parametric tests (i.e., Mann-Whitney, Kruskal-Wallis, and Spearman non parametric statistical tests), whereas categorical variables were tested with chi-square test (Pearson’s or Fisher exact test with Monte-Carlo correction). The possibility of nonlinear associations was tested by generalized additive models (GAMs) indicating linear relationships for all exposure-outcomes associations.
Multivariable log-Poisson regression models with robust standard errors were used for dichotomous outcomes, as these are more appropriate than logistic regression when the incidence of the outcome is 10% or more [34]. Linear regression models were performed for continuous outcomes. Estimated associations were described as relative risks (RR) with 95% confidence intervals (CIs) or β-coefficients with 95% CIs accordingly. We examined the associations of maternal metabolic profile in early pregnancy with childhood cardiometabolic traits at 4 years of age in 3 models: The first model (crude model) was adjusted for the child’s sex (except models using offspring systolic and diastolic blood pressure percentiles as an outcome); the second model (confounder model) was additionally adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI (only for models using maternal fasting lipid levels or blood pressure as an exposure variable). In a third model (mediation model), we additionally adjusted for maternal weight gain during pregnancy, birth weight, breastfeeding duration, child’s anthropometry at age of outcome assessment, and child lifestyle characteristics [TV viewing (hours/day)]. Because relations of pre-pregnancy BMI with offspring cardiometabolic traits could be moderated by paternal BMI we also examined associations after adjusting for paternal BMI.
We examined potential effect modification by child’s sex, maternal smoking during pregnancy, and gestational weight gain by including the interaction term in the models (statistically significant effect modification if p-value<0.05) and stratified analyses accordingly. We also examined potential effect modification by child BMI in the models using child lipid levels as an outcome variable.
All hypotheses testing were conducted assuming a 0.05 significance level and a 2-sided alternative hypothesis. We used Stata S.E. version 11.2 for the statistical analyses (Stata Corp, Texas, USA).
Results
Participants’ characteristics
Maternal and child demographic characteristics according to maternal overweight/obesity status are shown in Table 1. A total of 209 (34%) women were overweight/obese pre-pregnancy, while 77 (12.5%) women were obese prior to gestation. Overweight/obese women prior to gestation were more likely to be multiparous, less educated, to gain excessive weight during pregnancy and to breastfeed their children for shorter durations compared with women with no excess weight (Table 1). S1 Table in the supporting information material shows that mothers without offspring follow-up data were more likely to be younger, smokers, less educated, and of non-Greek ethnicity. There were no significant differences in socio- demographic characteristics between women who provided fasting blood samples and those who did not (S2 Table).
Table 1. Mother- child characteristics by pre-pregnancy overweight/obesity status, Rhea pregnancy cohort, Crete, Greece.
| Pre-pregnancy obesity status | |||
|---|---|---|---|
| No excess weight | Overweight/Obese | P- value a | |
| (n = 409) | (n = 209) | ||
| Maternal characteristics | |||
| Maternal age at delivery (yr), mean (SD) | 29.87 (0.2) | 29.98 (0.3) | 0.891 |
| Education, n (%) | <0.001 | ||
| Low | 49 (12.0) | 54 (25.8) | |
| Medium | 208 (50.9) | 102 (48.8) | |
| High | 152 (37.2) | 53 (25.4) | |
| Greek origin, n (%) | 384 (93.9) | 199 (95.2) | 0.499 |
| Primiparous, n (%) | 194 (47.4) | 70 (33.5) | 0.001 |
| Smoking during pregnancy, n (%) | 126 (30.8) | 70 (33.5) | 0.497 |
| Gestational diabetes, n (%) | 32 (8.4) | 18 (9.2) | 0.758 |
| Gestational hypertension, n (%) | 15 (4.0) | 14 (7.1) | 0.106 |
| Gestational weight gain (kg), n (%) | <0.001 | ||
| Inadequate | 114 (27.9) | 15 (7.2) | |
| Adequate | 155 (37.9) | 81 (38.8) | |
| Excessive | 140 (34.2) | 113 (54.1) | |
| Caesarian section, n (%) | 195 (48.0) | 115 (55.0) | 0.100 |
| Metabolic profile in early pregnancy (n = 348) | |||
| Glucose (mg/dL), mean (SD) | 74.93 (0.7) | 76.10 (1.2) | 0.341 |
| Insulin (mg/dL), mean (SD) | 9.33 (0.9) | 12.75 (1.5) | <0.001 |
| TC (mg/dL), mean (SD) | 195.49 (2.3) | 203.87 (3.7) | 0.025 |
| LDL-C (mg/dL), mean (SD) | 113.87 (1.8) | 123.14 (3.0) | 0.008 |
| HDL-C (mg/dL), mean (SD) | 60.18 (0.9) | 55.42 (1.3) | 0.007 |
| TG (mg/dL), mean (SD) | 108.04 (2.8) | 126.23 (4.8) | <0.001 |
| SBP (mmHg), mean (SD) | 105.06 (0.5) | 109.92 (0.8) | <0.001 |
| DBP (mmHg), mean (SD) | 69.27 (0.5) | 71.31 (0.7) | 0.013 |
| Child characteristics in infancy | |||
| Sex, girl, n (%) | 201 (49.1) | 93 (44.5) | 0.274 |
| Birth weight (kg), mean (SD) | 3.20 (0.02) | 3.21 (0.03) | 0.887 |
| Gestational age (weeks), mean (SD) | 38.32 (0.07) | 38.08 (0.11) | 0.123 |
| Duration of breastfeeding (months), mean (SD) | 4.69 (0.2) | 3.44 (0.3) | <0.001 |
| Day care attendance in the first 2 years of life, n (%) | 79 (19.5) | 36 (17.3) | 0.518 |
| Child characteristics at 4 years of age | |||
| BMI (Kg/m2), mean (SD) | 16.11 (0.08) | 16.93 (0.15) | <0.001 |
| Overweight/obese, n (%) | 74 (18.1) | 60 (28.7) | 0.002 |
| Waist circumference (cm), mean (SD) | 52.90 (0.2) | 54.66 (0.4) | <0.001 |
| Waist circumference ≥90th pct, n (%) | 35 (8.7) | 37 (17.9) | <0.001 |
| Sum of skinfolds (mm), mean (SD) | 37.78 (0.7) | 43.29 (1.1) | <0.001 |
| TC (mg/dL), mean (SD) | 156.39 (1.5) | 158.97 (2.0) | 0.247 |
| LDL-C (mg/dL), mean (SD) | 69.98 (27.4) | 69.58 (26.7) | 0.856 |
| HDL-C (mg/dL), mean (SD) | 47.17 (0.6) | 47.78 (0.8) | 0.518 |
| TG (mg/dL), mean (SD) | 69.69 (1.5) | 70.40 (2.1) | 0.690 |
| Time spent watching TV (hours/day), n (%) | 0.019 | ||
| Almost never | 124 (30.5) | 52 (25.0) | |
| 1–2 hours/day | 251 (61.8) | 126 (60.6) | |
| More than 3 hours/day | 31 (7.6) | 30 (14.4) | |
| Energy intake (Kcals/day), mean (SD) | 1583.5 (23.0) | 1594.3 (32.1) | 0.752 |
BMI, Body Mass Index; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density
Lipoprotein Cholesterol; TG, Triglyceride; CRP, C-reactive protein; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; pct, percentile
a P-values obtained by Mann-Whitney U test for two independent samples, and χ2 test or Fisher exact test with Monte-Carlo correction.
Numbers may not correspond to the total due to missing numbers.
In the subset of pregnant women with available fasting serum samples in early pregnancy, dyslipidemia was the most frequent metabolic disorder, as 49.7% women had total cholesterol levels ≥ 200 mg/dL, 26.8% had HDL-C levels< 50 mg/dL, and 18.7% had TG levels ≥ 150 mg/dL. Only 22 (6.3%) women were suffering from hyperglycemia in the first trimester of pregnancy. Overweight/obesity prior to gestation was associated with higher fasting total cholesterol, LDL-C, triglycerides, and insulin levels at the first trimester of pregnancy, lower HDL-C levels, and higher systolic and diastolic blood pressure (Table 1).
The prevalence of overweight/obesity and central adiposity (WC ≥ 90th percentile) at 4 years of age was 21.7% (n = 134) and 11.8% (n = 72), respectively (Table 1). Mean (±SD) non fasting total cholesterol, LDL-C and HDL-C were 198.1 (±36.2), 116.7 (±29.4), and 58.7 (±14.1) respectively. Children whose mothers were overweight/obese prior to gestation had higher BMI, waist circumference, and fat mass at 4 years of age compared to children whose mothers had no excess weight pre-pregnancy (Table 1).
Overweight/obesity pre-pregnancy in association with offspring cardiometabolic traits at 4 years of age
Generalised additive models examining the shape of the relationships of metabolic profile in early pregnancy with offspring cardiometabolic traits at 4 years of age showed no significant departures from linearity overall. Pre-pregnancy overweight/obesity showed a positive linear relationship with the probability of overweight/obesity at 4 years of age (Fig 1). Pre-pregnancy overweight/obesity was also positively associated with all other offspring adiposity outcomes at 4 years of age (Table 2). We found no association between pre-pregnancy BMI and offspring non-fasting lipid levels or blood pressure percentiles at 4 years of age (Table 2). Further adjustment for paternal BMI did not attenuate the observed associations (S3 Table).
Fig 1. Relationship between pre-pregnancy BMI and the estimated probability for overweight/obesity (A) and cholesterol levels ≥75th percentile (B) at 4 years of age.
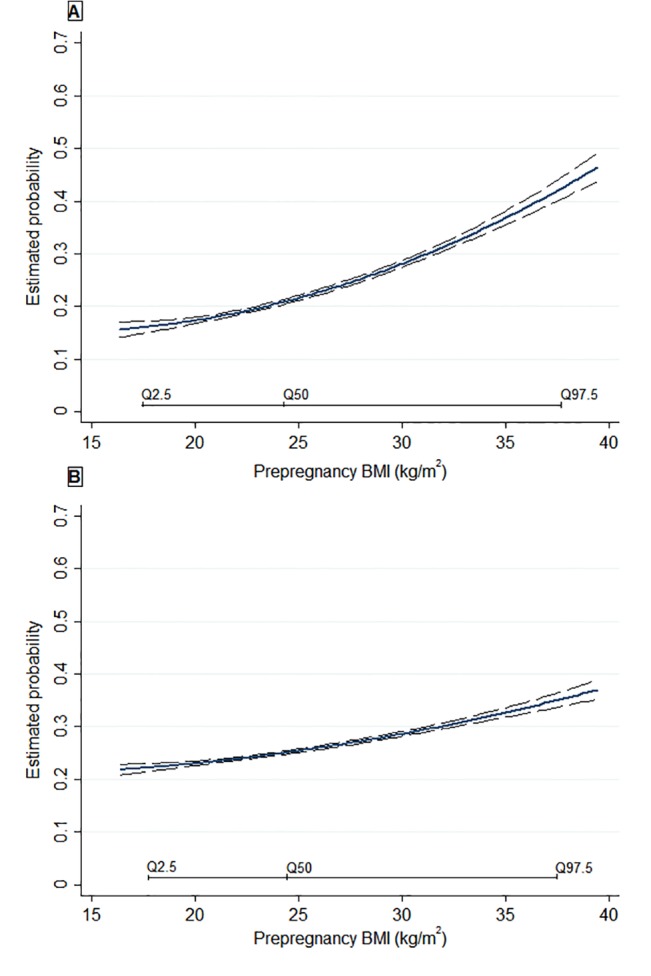
Estimated probability is based on multivariable models adjusted for maternal age, education, parity, smoking during pregnancy, gestational weight gain, birth weight, breastfeeding duration and TV watching at 4 years of age. Q2.5, Q50, Q97.5 represent the 2.5th, 50.0th, and the 97.5th percentiles of the studied population. Long-dashes represent the 95%CIs.
Table 2. Association of maternal pre-pregnancy obesity status with offspring cardiometabolic traits at 4 years of age, Rhea pregnancy cohort Crete, Greece.
| Pre-pregnancy overweight/obese (≥25 kg/m2) | ||||
|---|---|---|---|---|
| (n = 209) | ||||
| Cardiometabolic traits at 4 years of age | n | Model 1 | Model 2 | Model 3 |
| Adiposity outcomes | ||||
| RR (95%CI) | RR (95%CI) | RR (95%CI) | ||
| Overweight/obese | 134 | 1.59 (1.17, 2.15) | 1.53 (1.11, 2.09) | 1.83 (1.19, 2.81) |
| WC (cm) ≥ 90th pct | 72 | 2.03 (1.32, 3.14) | 1.89 (1.20, 2.96) | 1.97 (1.11, 3.49) |
| β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | ||
| Child BMI | 618 | 0.80 (0.45, 1.14) | 0.78 (0.44, 1.12) | 0.79 (0.36, 1.06) |
| WC (cm) | 606 | 1.75 (0.87, 2.63) | 1.76 (0.89, 2.64) | 1.36 (0.55, 2.17) |
| Sum of 4 Skinfolds (mm) | 601 | 5.74 (3.17, 8.30) | 5.37 (2.75, 7.99) | 5.10 (2.49, 7.71) |
| Non-fasting lipid levels | β-coeff. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | |
| TC(mg/dl) | 525 | 2.64 (-2.21, 7.50) | 2.52 (-2.50. 7.55) | 2.18 (-3.04. 7.41) |
| HDL-C(mg/dl) | 525 | 0.59 (-1.38, 2.56) | 0.43 (-1.63, 2.49) | 0.59 (-1.54, 2.73) |
| Blood pressure levels | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | |
| SBP percentiles | 488 | 0.26 (-0.17, 0.69) | 0.30 (-0.13, 0.75) | 0.21 (-0.24, 0.67) |
| DBP percentiles | 488 | -0.07 (-0.49, 0.34) | -0.11 (-0.53, 0.31) | -0.10 (-0.54, 0.33) |
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; pct, percentile
Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome)
Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy
Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4
years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child
height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05.
Fasting lipid, glucose and insulin levels in early pregnancy in association with offspring cardiometabolic traits at 4 years of age
Maternal fasting cholesterol levels showed a positive linear relationship with the probability of overweight/obesity at 4 years of age (Fig 2). An increase of 40mg/dl in total cholesterol levels was associated with 42% increased risk of overweight/obesity (RR: 1.42, 95% CI: 1.03, 1.95) and greater skinfold thickness by 3.30 mm (95%CI: 1.41, 5.20) at 4 years of age after adjustment for several covariates and pre-pregnancy BMI (Model 3). A positive association was also observed between maternal fasting cholesterol levels and offspring cholesterol levels at 4 years of age, but the associations were attenuated when we further adjusted for potential mediators (Table 3, model 3). No association was found between maternal fasting cholesterol levels and offspring blood pressure percentiles at 4 years of age (Table 3). No association was also observed among maternal fasting glucose and insulin serum levels in early pregnancy and offspring cardiometabolic traits at 4 years of age (data not shown).
Fig 2. Relationship between first-trimester fasting maternal cholesterol levels and the estimated probability for overweight/obesity (A) and cholesterol levels ≥75th percentile (B) at 4 years of age.
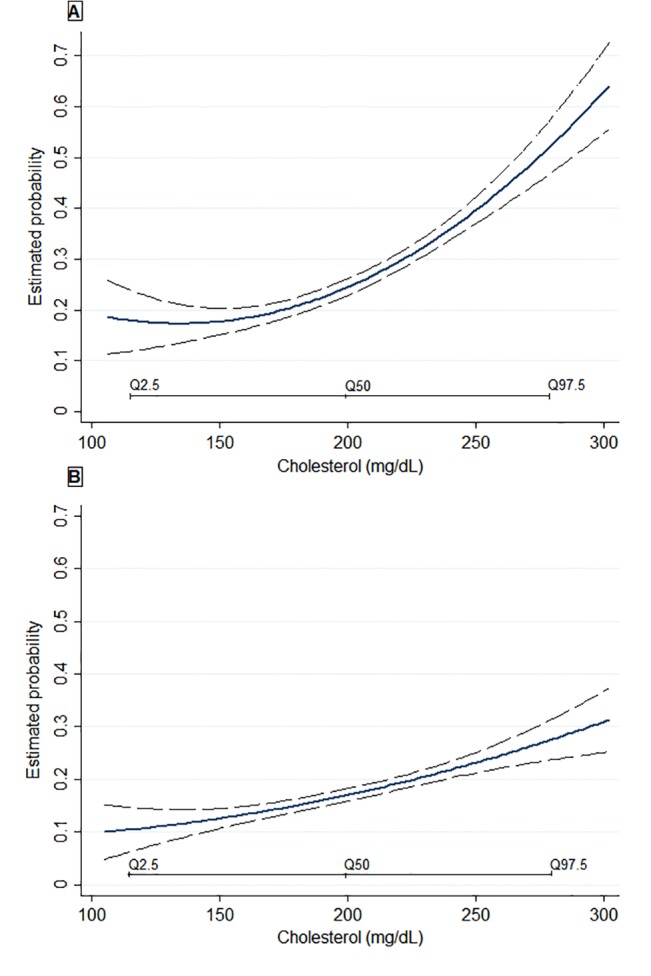
Estimated probability is based on multivariable models adjusted for maternal age, education, parity, smoking during pregnancy, BMI pre-pregnancy, gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Q2.5, Q50, Q97.5 represent the 2.5th, 50.0th, and the 97.5th percentiles of the studied population. Long-dashes represent the 95%CIs.
Table 3. Association of maternal fasting lipid profile in early pregnancy with offspring cardiometabolic traits at 4 years of age, Rhea pregnancy cohort Crete, Greece.
| Fasting TC levels in early pregnancy | Fasting LDL-C levels in early pregnancy | ||||||
|---|---|---|---|---|---|---|---|
| (per increase in 40 mg/dL) | (per increase in 15 mg/dL) | ||||||
| (n = 348) | (n = 348) | ||||||
| Cardiometabolic traits at 4 years of age | n | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Adiposity outcomes | |||||||
| RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | ||
| Overweight/obese | 64 | 1.18 (0.92, 1.50) | 1.27 (0.97, 1.66) | 1.42 (1.03, 1.95) | 1.03 (0.91, 1.17) | 1.07 (0.93, 1.22) | 1.10 (0.94, 1.29) |
| WC (cm) ≥ 90th pct | 30 | 1.07 (0.73, 1.56) | 1.07 (0.71, 1.62) | 1.24 (0.74, 2.05) | 1.02 (0.84, 1.23) | 1.07 (0.87, 1.32) | 1.12 (0.88, 1.44) |
| β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | ||
| Child BMI | 348 | 0.08 (-0.12, 0.29) | 0.04 (-0.18, 0.26) | 0.01 (-0.24, 0.26) | 0.02 (-0.08, 0.13) | 0.00 (-0.12, 0.13) | -0.01 (-0.16, 0.13) |
| WC (cm) | 348 | 0.34 (-0.26, 0.95) | 0.29 (-0.41, 1.00) | 0.40 (-0.25, 1.07) | 0.34 (-0.26, 0.95) | 0.29 (-0.41, 1.00) | 0.40 (-0.25, 1.07) |
| Sum of 4 Skinfolds (mm) | 341 | 2.53 (0.92, 4.14) | 2.76 (1.00, 4.52) | 3.30 (1.41, 5.20) | 0.77 (-0.08, 1.63) | 0.80 (-0.16, 1.77) | 1.11 (0.08, 2.13) |
| Non-fasting lipid levels | β-coef. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | |
| TC(mg/dl) | 294 | 4.14 (1.00, 7.28) | 3.43 (0.04, 6.83) | 3.25 (-0.53, 7.04) | 2.09 (0.56, 3.61) | 1.93 (0.28, 3.58) | 1.90 (-0.04, 3.85) |
| HDL-C(mg/dl) | 294 | -0.18 (-1.47, 1.11) | -0.59 (-2.06, 0.87) | -1.04 (-2.74, 0.65) | -0.35 (-0.95, 0.24) | -0.56 (-1.24, 0.10) | -0.71 (-1.49, 0.06) |
| Blood pressure levels | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | β-coef. (95%CI) | |
| SBP percentiles | 284 | -0.05 (-0.35, 0.24) | -0.07 (-0.40, 0.25) | -0.06 (-0.42, 0.29) | -0.04 (-0.19, 0.09) | -0.05 (-0.22, 0.10) | -0.06 (-0.24, 0.10) |
| DBP percentiles | 284 | -0.19 (-0.50, 0.12) | -0.19 (-0.52, 0.12) | -0.15 (-0.51, 0.21) | -0.08 (-0.23, 0.06) | -0.09 (-0.24, 0.06) | -0.09 (-0.27, 0.07) |
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; pct, percentile
Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome)
Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI
Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05
Maternal blood pressure levels in early pregnancy in association with offspring cardiometabolic traits at 4 years of age
An increase of 10mmHg in diastolic blood pressure in early pregnancy was associated with 23% increased risk of offspring overweight/obesity (RR: 1.22, 95%CI: 1.03, 1.45), and greater skin fold thickness by 1.71 mm (95%CI: 0.57, 2.86) in the fully adjusted model (Table 4). We found no association between maternal blood pressure levels in early pregnancy and offspring lipids and blood pressure percentiles at 4 years of age (Table 4).
Table 4. Association of maternal blood pressure levels in early pregnancy with offspring cardiometabolic traits at 4 years of age, Rhea pregnancy cohort Crete, Greece.
| SBP in early pregnancy | DBP in early pregnancy | ||||||
|---|---|---|---|---|---|---|---|
| (per increase in 10 mm Hg) | (per increase in 10 mm Hg) | ||||||
| (n = 536) | (n = 536) | ||||||
| Cardiometabolic traits at 4 years of age | n | Model 1 | Model 2 | Model 3 | Model 1 | Model 2 | Model 3 |
| Adiposity outcomes | |||||||
| RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | RR (95%CI) | ||
| Overweight/obese | 156 | 1.18 (1.01, 1.37) | 1.11 (0.95, 1.31) | 1.15 (0.97, 1.36) | 1.23 (1.05, 1.45) | 1.19 (1.01, 1.41) | 1.22 (1.03, 1.45) |
| WC (cm) ≥ 90th pct | 60 | 1.16 (0.93, 1.44) | 1.02 0.81, 1.29) | 1.03 (0.82, 1.31) | 1.19 (0.94, 1.50) | 1.09 (0.84, 1.40) | 1.09 (0.84, 1.41) |
| β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | ||
| Child BMI | 536 | 0.15 (-0.00, 0.30) | 0.05 (-0.10, 0.21) | 0.09 (-0.06, 0.26) | 0.13 (-0.03, 0.29) | 0.06 (-0.09, 0.23) | 0.09 (-0.06, 0.25) |
| WC (cm) | 532 | 0.38 (0.01, 0.76) | 0.20 (-0.18, 0.58) | 0.22 (-0.12, 0.57) | 0.36 (-0.05, 0.79) | 0.21 (-0.19, 0.62) | 0.26 (-0.09, 0.61) |
| Sum of 4 Skinfolds (mm) | 524 | 1.59 (0.35, 2.83) | 0.98 (-0.32, 2.30) | 1.11 (-0.19, 2.42) | 1.96 (0.79, 3.13) | 1.49 (0.32, 2.66) | 1.71 (0.57, 2.86) |
| Non-fasting lipid levels | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | |
| TC(mg/dl) | 458 | -0.33 (-2.50, 1.83) | -0.81 (-3.05, 1.42) | -0.97 (-3.22, 1.27) | -0.85 (-3.50, 1.79) | -1.03 (-3.70, 1.63) | -1.12 (-3.86, 1.60) |
| HDL-C(mg/dl) | 458 | 0.64 (-0.26, 1.54) | 0.80 (-0.16, 1.77) | 0.80 (-0.20, 1.80) | -0.21 (-1.23, 0.80) | 0.04 (-1.00, 1.10) | -0.02 (-1.11, 1.05) |
| Blood pressure levels | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | β-coeff. (95%CI) | |
| SBP percentiles | 422 | 0.05 (-0.12, 0.22) | 0.04 (-0.15, 0.23) | 0.08 (-0.10, 0.27) | 0.10 (-0.10, 0.31) | 0.08 (-0.13, 0.30) | 0.07 (-0.14, 0.29) |
| DBP percentiles | 422 | 0.00 (-0.18, 0.19) | 0.04 (-0.14, 0.24) | 0.02 (-0.16, 0.22) | 0.08 (-0.12, 0.29) | 0.06 (-0.14, 0.27) | 0.07 (-0.15, 0.29) |
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; pct, percentile
Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome)
Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI
Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05
Effect modification-Sensitivity analyses
In the mediation model, further adjustment for birth characteristics, child's anthropometry and life-style behaviors did not substantively alter the adjusted estimations for most childhood outcomes (Tables 2, 3 and 4). Further analyses showed evidence for an interaction between child sex and maternal pre-pregnancy BMI in response to offspring overweight/obesity and central adiposity (p for interaction <0.05), but not with skinfold thickness (Table 5). The greatest risk for these adiposity outcomes was observed for girls whose mothers were overweight/obese prior to gestation (RR-overweight/obesity: 3.54, 95%CI: 1.80, 6.98; RR-central adiposity: 5.33, 95%CI: 2.17, 13.07), whereas similar associations in boys were not significant (Table 5). We saw no evidence for a multiplicative interaction of maternal metabolic profile in early pregnancy with maternal smoking during pregnancy, gestational weight gain or child BMI (p for interaction >0.05).
Table 5. Association of pre-pregnancy BMI with offspring obesity measures at 4 years of age, stratified by child sex, Rhea pregnancy cohort, Crete, Greece.
| Offspring adiposity measures at 4 years of age | |||
|---|---|---|---|
| Overweight/obese | WC (cm) ≥ 90th percentile a | Sum of 4 skinfolds (mm) a | |
| (n = 134) | (n = 72) | (n = 601) | |
| RR (95% CI) | RR (95% CI) | β-coeff.(95% CI) | |
| Maternal pre-pregnancy BMI (kg/m 2 ) | |||
| All (n = 618) | 1.08 (1.04, 1.13) | 1.10 (1.04, 1.16) | 0.51 (0.25, 0.77) |
| Boys (n = 324) | 1.04 (0.98, 1.10) | 1.02 (0.93, 1.11) | 0.22 (-0.07, 0.52) |
| Girls (n = 294) | 1.13 (1.06, 1.20) | 1.19 (1.10, 1.29) | 0.79 (0.34, 1.25) |
| P for interaction | 0.032 | 0.004 | 0.030 |
| Overweight/Obese (≥25kg/m 2 ) prior to gestation | |||
| All (n = 209) | 1.83 (1.19, 2.81) | 1.97 (1.11, 3.49) | 5.10 (2.49, 7.71) |
| Boys (n = 116) | 1.10 (0.61, 2.01) | 0.97 (0.42, 2.21) | 3.03 (0.07, 5.99) |
| Girls (n = 93) | 3.54 (1.80, 6.98) | 5.33 (2.17, 13.07) | 7.59 (3.10, 12.08) |
| P for interaction | 0.007 | 0.007 | 0.061 |
BMI, Body Mass Index; WC, Waist Circumference
All models are adjusted for maternal age, education, parity, smoking during pregnancy, gestational weight gain, birth weight, breastfeeding duration, and
TV watching at 4 years of age (hours/day).
aAlso adjusted for child height.
Bold indicated statistically significant differences at p<0.05.
To elucidate whether gestational diabetes modified the observed results, we performed a sensitivity analysis in which we excluded all women who were diagnosed with gestational diabetes (n = 50). Results did not differ substantially from those derived from the main analysis (S4 Table, S5 Table and S6 Table). We also found no difference in the observed estimates after excluding preterm births (data not shown).
Discussion
In this prospective pregnancy cohort we showed that exposure to metabolic dysregulation in early pregnancy may predict increased risk of obesity in preschool children. To our knowledge this is the first study examining maternal metabolic profile in early pregnancy with the use of fasting serum samples in association with offspring cardiometabolic risk.
Our findings are in consistence with previous epidemiological studies examining the effect of maternal pre-pregnancy BMI with child BMI measures [7, 8, 35], and fat mass [12, 14, 22, 36, 37]. The associations of maternal pre-pregnancy BMI with childhood adiposity may be explained by intrauterine mechanisms or shared environmental, life-style and genetic characteristics [9]. Animal studies suggest that epigenetic alterations induced by maternal overnutrition in pregnancy may modulate expression of genes that regulate adipogenesis, glucose homeostasis, inflammation, and/or insulin signaling, including genes encoding hormones (e.g., leptin), nuclear receptors (adipogenic and lipogenic transcription factors PPARγ and PPARα, respectively), gluconeogenic enzymes and transmembrane proteins [38]. Moreover, adverse maternal conditions such as maternal obesity, have been demonstrated in animal studies to affect placental morphology, blood flow, feto-maternal exchanges and endocrine function, which have direct consequences for fetal tissue development, and may lead to a higher offspring susceptibility to develop metabolic disorders [39]. Although adjustment for several sociodemographic and lifestyle related characteristics did not explain our findings, we cannot rule out the possibility of residual confounding mainly related to shared mother-child lifestyle. The observed effects were not mediated by pregnancy complications such as gestational diabetes, birth characteristics, and infant feeding patterns (breastfeeding), which have been associated with both maternal BMI and offspring postnatal growth. Additionally, our results remained substantially the same after adjusting for paternal BMI, implying potential intrauterine mechanisms in the observed associations.
The greatest risk for overweight/obesity and central adiposity was observed for girls whose mothers were overweight/obese prior to gestation. The long-term effects of the same environmental insult, such as maternal obesity, can have various phenotypic effects on male and female offspring [17]. There are no consistent findings from epidemiological studies on offspring sex-specific responses to maternal weight status, while sex specificity in response to maternal anthropometry has been shown in fetal growth measures [40]. Animal studies have shown that there are sex-specific differences in the regulation and expression of placental genes, proteins, steroids and structure [41, 42]. Microarray animal experiments also showed that the response to a high-fat diet during gestation triggers sex-specific epigenetic alterations throughout the genome, together with sexually dimorphic deregulation of clusters of imprinted genes: mainly cell signaling involving immune cells, and uptake and metabolism of amino acids for females, and development and function of the vascular system, and uptake and metabolism of glucose and fatty acids for males [43]. Timing of exposure is also a critical issue in sex differences in developmental programming. Female fetuses respond more than males to the mother’s preconception nutrition and metabolism, while, in contrast, male fetuses are more vulnerable to metabolic changes during gestation, especially after the first trimester of pregnancy [44, 45]. Further epidemiological studies are needed to explore the sex-specific causal variables and how females versus males respond and adapt to maternal obesity.
To our knowledge, this is the first study to investigate associations of maternal blood pressure levels at the first trimester of pregnancy with offspring cardiometabolic traits. We found that high blood pressure levels in early pregnancy were associated with increased risk of overweight/obesity and increased fat mass at 4 years of age after excluding pre-eclamptic pregnancies. The observed effects were not attenuated by pre-pregnancy BMI or weight gain during pregnancy, implying an independent role of maternal blood pressure to offspring fat distribution. Maternal hypertension in early pregnancy may disrupt the typical physiological changes in the spiral arteries of the decidua and myometrium, resulting in poor placental perfusion, early placental hypoxia and oxidative stress [46]. Therefore, it may be possible that even a small increase in blood pressure levels (10mmHg) in early pregnancy may predispose to adverse metabolic outcomes and increased fat mass later in life.
We also found that abnormal fasting cholesterol levels in early pregnancy were associated with increased risk of offspring overweight/obesity, and greater fat mass at 4 years of age. The observed associations were not attenuated by maternal BMI pre-pregnancy, gestational diabetes status, birth size, or child BMI at age of outcome assessment. Gademan et al reported recently a positive association between maternal lipid levels and offspring fat percentage and waist-to height ratio values at 5–6 years of age children [22]. There are no other studies so far on the association of maternal lipid levels in pregnancy with offspring cardiometabolic traits other than adiposity measures. Maternal dyslipidemia could increase the oxidative stress in the fetus resulting not only in damage of the vessel wall, but also in the disruption of normal placentation. Hypercholesterolemia in early pregnancy has been associated with increased offspring atherosclerotic lesions both in animal models [47], and in human tissues [48]. One potential explanation for such increased cardiometabolic risk in children of mothers with hypercholesterolemia, is the induction of a constitutional state of overexpression of “atherogenes” in the fetal vascular wall by maternal hypercholesterolemia or the resulting fatty-streak formation [48].
Strengths and limitations
The strengths of the present study include the population-based prospective design and detailed cardiometabolic measurements in early pregnancy and in childhood. Unlike previous epidemiologic studies, blood pressure, lipids, glucose, and insulin concentrations were not collected from medical records but measured during the study follow up according to validated protocols. Moreover, fasting serum samples were available in early pregnancy that is a rather complicated goal for a cohort involving pregnant women. The exclusion of mother-child pairs with multiple pregnancies, pregnancies with preeclampsia, as well as adjustment for several sociodemographic variables, reduced the likelihood of potential confounding. However, because of the observational study design, residual confounding because of other unmeasured confounders may still occur.
The levels of attrition in the Rhea cohort is similar to those found in other birth cohort studies. Offspring of more educated women, and of older women were more likely to attend follow-up clinical assessment. Assuming that mothers and children with a higher BMI are less likely to participate in a detailed obesity follow-up, our estimates may be underestimated. A selection bias could be theoretically generated by the possibility that we included only women receiving an early ultrasound. However, all pregnant women in Greece have to attend several compulsory prenatal visits, one of which take place around 12 weeks of gestation, which is the time of our enrolment phase. Information on maternal pre-pregnancy weight was self-reported, which might have led to misclassification of maternal BMI pre-pregnancy. However, we have performed a validation study comparing self-reported pre-pregnancy weight with clinically measured weight in the first prenatal visit, which was available in our cohort, showing high correlation (r 0.93) and a fairly good agreement between self-reported and objectively measured BMI (Bland-Altman plots, data not shown). We were not able to measure fasting lipid levels to children at 4 years follow up, as expected at this age of follow up. It has been shown that children fasting serum lipids levels have small differences with non-fasting levels[49].
Conclusions
The results of the present study indicate that metabolic dysregulation in early pregnancy may determine offspring obesity at preschool age. The complex underlying mechanisms that explain these findings require additional study. Further follow-up of this cohort will allow to determine whether maternal metabolic profile in early pregnancy is associated with a broader range of offspring cardiometabolic disorders at later ages.
Supporting Information
a Statistically significant differences (p<0.05), based on Mann-Whitney U test for two independent samples and Pearson's χ2 test for independence.
(PDF)
a Statistically significant differences (p<0.05), based on Mann-Whitney U test for two independent samples and Pearson's χ2 test for independence.
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure. All models were adjusted for child sex (except models using offspring systolic and diastolic blood pressure percentiles as an outcome) maternal age, education level, parity, smoking during pregnancy, gestational weight gain, birth weight, breastfeeding duration, TV watching at 4 years of age (hours/day) and paternal BMI. Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05.
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome). Model 2: model 1 further adjusted for maternal age, education level, parity and smoking during pregnancy Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05.
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome) Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI. Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure;. Model 1: adjusted for child sex (except models using offspring systolic and diastolic blood pressure percentiles as an outcome). Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI. Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences p<0.05.
(PDF)
Acknowledgments
The authors would particularly like to thank all the cohort participants for their generous collaboration.
Data Availability
Ethical restrictions prevent public sharing of data from the Rhea pregnancy cohort study, as imposed by the Research Ethics Committee of the Rhea Cohort Study. Data can be made available to all interested researchers upon request by contacting the Research Committee at rhea@med.uoc.gr or the Principal Investigators of the study (Prof. M. Kogevinas, kogevinas@creal.cat; Dr. L. Chatzi, lchatzi@med.uoc.gr).
Funding Statement
Rhea project was financially supported by European projects (EU FP6-2003-Food-3-NewGeneris, EU FP6. STREP Hiwate, EU FP7 ENV.2007.1.2.2.2. Project No 211250 Escape, EU FP7-2008-ENV-1.2.1.4 Envirogenomarkers, EU FP7-HEALTH-2009- single stage CHICOS, EU FP7 ENV.2008.1.2.1.6. Proposal No 226285 ENRIECO, EU- FP7- HEALTH-2012 Proposal No 308333 HELIX) and the Greek Ministry of Health (Program of Prevention of obesity and neurodevelopmental disorders in preschool children, in Heraklion district, Crete, Greece: 2011-2014; “Rhea Plus”: Primary Prevention Program of Environmental Risk Factors for Reproductive Health, and Child Health: 2012-15). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes (Lond). 2011. July;35(7):891–8. 10.1038/ijo.2010.222 [DOI] [PubMed] [Google Scholar]
- 2. Cunningham SA, Kramer MR, Narayan KM. Incidence of childhood obesity in the United States. N Engl J Med. 2014. January 30;370(5):403–11. 10.1056/NEJMoa1309753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.OESD website. Available: http://www.oecd.org/health/obesity-update.htm. Assesed 2015 April 17.
- 4. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med. 2008. July 3;359(1):61–73. 10.1056/NEJMra0708473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009. Sep;27(5):358–68. 10.1055/s-0029-1237424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Poston L. Maternal obesity, gestational weight gain and diet as determinants of offspring long term health. Best Pract Res Clin Endocrinol Metab. 2012. October;26(5):627–39. 10.1016/j.beem.2012.03.010 [DOI] [PubMed] [Google Scholar]
- 7. Yu Z, Han S, Zhu J, Sun X, Ji C, Guo X. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627 10.1371/journal.pone.0061627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Patro B, Liber A, Zalewski B, Poston L, Szajewska H, Koletzko B. Maternal and paternal body mass index and offspring obesity: a systematic review. Ann Nutr Metab. 2013;63(1–2):32–41. [DOI] [PubMed] [Google Scholar]
- 9. Lawlor DA, Relton C, Sattar N, Nelson SM. Maternal adiposity—a determinant of perinatal and offspring outcomes? Nat Rev Endocrinol. 2012. Nov;8(11):679–88. 10.1038/nrendo.2012.176 [DOI] [PubMed] [Google Scholar]
- 10. Kyle UG, Pichard C. The Dutch Famine of 1944–1945: a pathophysiological model of long-term consequences of wasting disease. Curr Opin Clin Nutr Metab Care. 2006 July;9(4):388–94. [DOI] [PubMed] [Google Scholar]
- 11. Bekkers MB, Brunekreef B, Smit HA, Kerkhof M, Koppelman GH, Oldenwening M, et al. Early-life determinants of total and HDL cholesterol concentrations in 8-year-old children; the PIAMA birth cohort study. PLoS One. 2011;6(9):e25533 10.1371/journal.pone.0025533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gaillard R, Steegers EA, Duijts L, Felix JF, Hofman A, Franco OH, et al. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the generation R study. Hypertension. 2014. April;63(4):683–91. 10.1161/HYPERTENSIONAHA.113.02671 [DOI] [PubMed] [Google Scholar]
- 13.Oostvogels AJ, Stronks K, Roseboom TJ, van der Post JA, van Eijsden M, Vrijkotte TG. Maternal pre-pregnancy BMI, offspring's early postnatal growth and metabolic profile at age 5–6 years: the ABCD-study. J Clin Endocrinol Metab. 2014 Jun 23:jc20141561. [DOI] [PubMed]
- 14.Perng W, Gillman MW, Mantzoros CS, Oken E. A prospective study of maternal prenatal weight and offspring cardiometabolic health in midchildhood. Ann Epidemiol. 2014 Sep 3. [DOI] [PMC free article] [PubMed]
- 15.Derraik JG, Ayyavoo A, Hofman PL, Biggs JB, Cutfield WS. Increasing maternal prepregnancy body mass index is associated with reduced insulin sensitivity and increased blood pressure in their children. Clin Endocrinol (Oxf). 2014 Nov 11. [DOI] [PubMed]
- 16. Gabory A, Roseboom TJ, Moore T, Moore LG, Junien C. Placental contribution to the origins of sexual dimorphism in health and diseases: sex chromosomes and epigenetics. Biol Sex Differ. 2013;4(1):5 10.1186/2042-6410-4-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Aiken CE, Ozanne SE. Sex differences in developmental programming models. Reproduction. 2013. January;145(1):R1–13. 10.1530/REP-11-0489 [DOI] [PubMed] [Google Scholar]
- 18. Reynolds SA, Roberts JM, Bodnar LM, Haggerty CL, Youk AO, Catov JM. Newborns of preeclamptic women show evidence of sex-specific disparity in fetal growth. Gend Med. 2012. Dec;9(6):424–35. 10.1016/j.genm.2012.10.013 [DOI] [PubMed] [Google Scholar]
- 19. Behl M, Rao D, Aagaard K, Davidson TL, Levin ED, Slotkin TA, et al. Evaluation of the association between maternal smoking, childhood obesity, and metabolic disorders: a national toxicology program workshop review. Environ Health Perspect. 2013. February;121(2):170–80. 10.1289/ehp.1205404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Poston L. Gestational weight gain: influences on the long-term health of the child. Curr Opin Clin Nutr Metab Care. 2012. May;15(3):252–7. 10.1097/MCO.0b013e3283527cf2 [DOI] [PubMed] [Google Scholar]
- 21. Davis EF, Lazdam M, Lewandowski AJ, Worton SA, Kelly B, Kenworthy Y, et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: a systematic review. Pediatrics. 2012. June;129(6):e1552–61. 10.1542/peds.2011-3093 [DOI] [PubMed] [Google Scholar]
- 22. Gademan MG, Vermeulen M, Oostvogels AJ, Roseboom TJ, Visscher TL, van Eijsden M, et al. Maternal prepregancy BMI and lipid profile during early pregnancy are independently associated with offspring's body composition at age 5–6 years: the ABCD study. PLoS One. 2014;9(4):e94594 10.1371/journal.pone.0094594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seed PT, Chappell LC, Black MA, Poppe KK, Hwang YC, Kasabov N, et al. Prediction of preeclampsia and delivery of small for gestational age babies based on a combination of clinical risk factors in high-risk women. Hypertens Pregnancy. 2011;30(1):58–73. 10.3109/10641955.2010.486460 [DOI] [PubMed] [Google Scholar]
- 24. McCowan L, Horgan RP. Risk factors for small for gestational age infants. Best Pract Res Clin Obstet Gynaecol. 2009. December;23(6):779–93. 10.1016/j.bpobgyn.2009.06.003 [DOI] [PubMed] [Google Scholar]
- 25. Chatzi L, Plana E, Daraki V, Karakosta P, Alegkakis D, Tsatsanis C, et al. Metabolic syndrome in early pregnancy and risk of preterm birth. Am J Epidemiol. 2009. October 1;170(7):829–36. 10.1093/aje/kwp211 [DOI] [PubMed] [Google Scholar]
- 26. Jellinger PS, Smith DA, Mehta AE, Ganda O, Handelsman Y, Rodbard HW, et al. American Association of Clinical Endocrinologists' Guidelines for Management of Dyslipidemia and Prevention of Atherosclerosis: executive summary. Endocr Pract. 2012. Mar-Apr;18(2):269–93. [DOI] [PubMed] [Google Scholar]
- 27. Metzger BE, Gabbe SG, Persson B, Buchanan TA, Catalano PA, Damm P, et al. International association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care. 2010. March;33(3):676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cole TJ, Bellizzi MC, Flegal KM, Dietz WH. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000. May 6;320(7244):1240–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Linardakis M, Sarri K, Bertsias G, Papadaki A, Kafatos A. Waist circumference and waist-to-height percentiles for the youth of Crete, Greece. International Journal of Chld Health and Human Development. 2011;3:329–42. [Google Scholar]
- 30. Steinberger J, Daniels SR, Eckel RH, Hayman L, Lustig RH, McCrindle B, et al. Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the American Heart Association Atherosclerosis, Hypertension, and Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young; Council on Cardiovascular Nursing; and Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2009. February 3;119(4):628–47. 10.1161/CIRCULATIONAHA.108.191394 [DOI] [PubMed] [Google Scholar]
- 31.Karachaliou M, Georgiou V, Roumeliotaki T, Chalkiadaki G, Daraki V, Koinaki S, et al. Association of trimester-specific gestational weight gain with fetal growth, offspring obesity, and cardiometabolic traits in early childhood. Am J Obstet Gynecol. 2014 Dec 31. [DOI] [PMC free article] [PubMed]
- 32.Rasmussen KM, & Yaktine, A. L.,. Institute of medicine (committee to reexamine iom pregnancy weight guidelines, food and nutritions board and board on children, youth, and families) weight gain during pregnancy: Reexamining the guidelines. Washington D.C.2009.
- 33. Leventakou V, Georgiou V, Chatzi L, Sarri K. Relative validity of an FFQ for pre-school children in the mother-child 'Rhea' birth cohort in Crete, Greece. Public Health Nutr. 2015. Feb;18(3):421–7. 10.1017/S1368980014000445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. Am J Epidemiol. 2003. May 15;157(10):940–3. [DOI] [PubMed] [Google Scholar]
- 35. Drake AJ, Reynolds RM. Impact of maternal obesity on offspring obesity and cardiometabolic disease risk. Reproduction. 2010. September;140(3):387–98. 10.1530/REP-10-0077 [DOI] [PubMed] [Google Scholar]
- 36. Gale CR, Javaid MK, Robinson SM, Law CM, Godfrey KM, Cooper C. Maternal size in pregnancy and body composition in children. J Clin Endocrinol Metab. 2007. October;92(10):3904–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Blair NJ, Thompson JM, Black PN, Becroft DM, Clark PM, Han DY, et al. Risk factors for obesity in 7-year-old European children: the Auckland Birthweight Collaborative Study. Arch Dis Child. 2007. October;92(10):866–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Desai M, Jellyman JK, Ross MG. Epigenomics, gestational programming and risk of metabolic syndrome. Int J Obes (Lond). 2015 Feb 2. [DOI] [PubMed]
- 39. Tarrade A, Panchenko P, Junien C, Gabory A. Placental contribution to nutritional programming of health and diseases: epigenetics and sexual dimorphism. J Exp Biol. 2015. January 1;218(Pt 1):50–8. [DOI] [PubMed] [Google Scholar]
- 40. Lampl M, Gotsch F, Kusanovic JP, Gomez R, Nien JK, Frongillo EA, et al. Sex differences in fetal growth responses to maternal height and weight. Am J Hum Biol. 2010. Jul-Aug;22(4):431–43. 10.1002/ajhb.21014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clifton VL. Review: Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010. March;31 Suppl:S33–9. 10.1016/j.placenta.2009.11.010 [DOI] [PubMed] [Google Scholar]
- 42. Mao J, Zhang X, Sieli PT, Falduto MT, Torres KE, Rosenfeld CS. Contrasting effects of different maternal diets on sexually dimorphic gene expression in the murine placenta. Proc Natl Acad Sci U S A. 2010. March 23;107(12):5557–62. 10.1073/pnas.1000440107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gabory A, Ferry L, Fajardy I, Jouneau L, Gothie JD, Vige A, et al. Maternal diets trigger sex-specific divergent trajectories of gene expression and epigenetic systems in mouse placenta. PLoS One. 2012;7(11):e47986 10.1371/journal.pone.0047986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJ. Boys live dangerously in the womb. Am J Hum Biol. 2010. May-Jun;22(3):330–5. 10.1002/ajhb.20995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Regnault N, Gillman MW, Rifas-Shiman SL, Eggleston E, Oken E. Sex-specific associations of gestational glucose tolerance with childhood body composition. Diabetes Care. 2013. October;36(10):3045–53. 10.2337/dc13-0333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett S, Bourgeois C, Cassidy A, Cloutier-Holtz K, Corbin A, Darling E, et al. Clinical Practice Guideline No.15 Hypertensive Disorders of Pregnancy. Association of Ontario Midwives. 2012.
- 47. Napoli C, de Nigris F, Welch JS, Calara FB, Stuart RO, Glass CK, et al. Maternal hypercholesterolemia during pregnancy promotes early atherogenesis in LDL receptor-deficient mice and alters aortic gene expression determined by microarray. Circulation. 2002. March 19;105(11):1360–7. [DOI] [PubMed] [Google Scholar]
- 48. Napoli C, Glass CK, Witztum JL, Deutsch R, D'Armiento FP, Palinski W. Influence of maternal hypercholesterolaemia during pregnancy on progression of early atherosclerotic lesions in childhood: Fate of Early Lesions in Children (FELIC) study. Lancet. 1999. October 9;354(9186):1234–41. [DOI] [PubMed] [Google Scholar]
- 49. Steiner MJ, Skinner AC, Perrin EM. Fasting might not be necessary before lipid screening: a nationally representative cross-sectional study. Pediatrics. 2011. September;128(3):463–70. 10.1542/peds.2011-0844 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
a Statistically significant differences (p<0.05), based on Mann-Whitney U test for two independent samples and Pearson's χ2 test for independence.
(PDF)
a Statistically significant differences (p<0.05), based on Mann-Whitney U test for two independent samples and Pearson's χ2 test for independence.
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure. All models were adjusted for child sex (except models using offspring systolic and diastolic blood pressure percentiles as an outcome) maternal age, education level, parity, smoking during pregnancy, gestational weight gain, birth weight, breastfeeding duration, TV watching at 4 years of age (hours/day) and paternal BMI. Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05.
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome). Model 2: model 1 further adjusted for maternal age, education level, parity and smoking during pregnancy Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05.
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure; Model 1: adjusted for child sex. (except models using offspring systolic and diastolic blood pressure percentiles as an outcome) Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI. Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences at p<0.05
(PDF)
BMI, Body Mass Index; WC, Waist Circumference; TC, Total Cholesterol; LDL-C, Low Density Lipoprotein Cholesterol; HDL-C, High Density Lipoprotein Cholesterol; SBP, Systolic Blood Pressure; DBP, Diastolic Blood Pressure;. Model 1: adjusted for child sex (except models using offspring systolic and diastolic blood pressure percentiles as an outcome). Model 2: model 1 further adjusted for maternal age, education level, parity, smoking during pregnancy and pre-pregnancy BMI. Model 3: model 2 additionally adjusted for gestational weight gain, birth weight, breastfeeding duration, and TV watching at 4 years of age (hours/day). Models using offspring WC and sum of skinfolds as an outcome variable were also adjusted for child height, while those using offspring non-fasting lipid levels as an outcome were also adjusted for child BMI. Bold indicated statistically significant differences p<0.05.
(PDF)
Data Availability Statement
Ethical restrictions prevent public sharing of data from the Rhea pregnancy cohort study, as imposed by the Research Ethics Committee of the Rhea Cohort Study. Data can be made available to all interested researchers upon request by contacting the Research Committee at rhea@med.uoc.gr or the Principal Investigators of the study (Prof. M. Kogevinas, kogevinas@creal.cat; Dr. L. Chatzi, lchatzi@med.uoc.gr).


