Abstract
Background
Clinical and animal studies indicate that transfusions of older stored RBCs impair clinical outcomes as compared to fresh RBC transfusions. It has been suggested that this effect is due to inhibition of NO-mediated vasodilation following transfusion of older RBC units. However, to date this effect has not been identified in human transfusion recipients.
Study Design and Methods
Forty-three hospitalized patients with transfusion orders were randomized to receive either fresh (< 14 days) or older stored (> 21 days) RBC units. Prior to transfusion, and at selected time points after the start of transfusion, endothelial function was assessed using non-invasive flow-mediated dilation assays.
Results
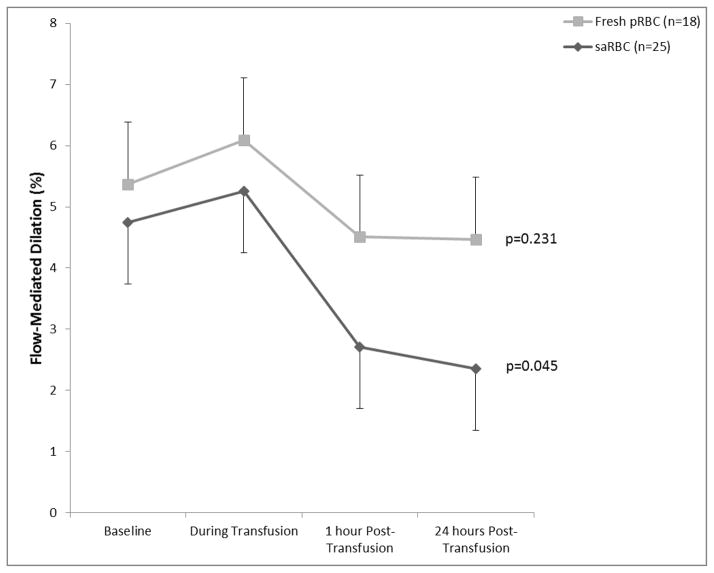
Following transfusion of older RBC units, there was a significant reduction in NO-mediated vasodilation at 24 hours after transfusion (p=0.045), while fresh RBC transfusions had no effect (p=0.231).
Conclusions
The present study suggests for the first time a significant inhibitory effect of transfused RBC units stored > 21 days on NO-mediated vasodilation in anemic hospitalized patients. This finding lends further support to the hypothesis that deranged NO signaling mediates adverse clinical effects of older RBC transfusions. Future investigations will be necessary to address possible confounding factors and confirm these results.
Keywords: Blood transfusion, RBC, storage lesion, endothelial function, FMD
Introduction
Blood transfusion is the most common therapeutic procedure in hospitalized patients, with approximately 15 million red blood cell (RBC) units transfused annually in the US to approximately 5 million patients (1). Despite undeniable therapeutic benefits, transfusions are also associated with serious adverse reactions (2). Even when the well-recognized hazards are excluded, transfusion of RBCs remains an independent contributor of morbidity and mortality; furthermore most (but not all) studies show that these effects increase with RBC storage time (2–20). A recent meta-analysis supports this association (21), as do studies in dogs where transfusion of older stored RBC units (storage-aged RBCs; saRBCs), but not fresh RBCs, increased morbidity and mortality in animals with experimental pneumonia (22, 23). While data from ongoing randomized controlled trials of fresh versus saRBC transfusion are eagerly anticipated (RECESS (24), ABLE (25), and the Red Cell Storage Duration and Outcomes in Cardiac Surgery study (26), the results may not be definitive, as mathematical modeling suggests that some adverse effects of RBC storage time on recipient outcomes may be beyond the ability of such trials to detect (27).
Several pathologic changes occur in RBCs during storage (the “RBC storage lesion”) that may contribute to adverse effects of saRBC transfusions. These changes include the depletion of adenosine triphosphate (ATP) and 2,3-diphosphoglycerate (2,3-DPG) (14, 15), reduced blood flow velocity and increased endothelial adhesion (28, 29), progressive membrane dysfunction leading to hemolysis, elaboration of lipid mediators, microparticle formation (30–35) and disruptive effects on nitric oxide (NO)-mediated vasodilation (36, 37). In particular, we and others proposed saRBC-mediated reductions in NO bioavailability and inhibition of NO-mediated vasodilation as potentially important mechanisms underlying adverse transfusion effects (22, 23, 31, 37–39). We demonstrated in rat aortic rings that compared to fresh RBCs, saRBCs that were stored for 28–42 days produced significant inhibition of methacholine-stimulated and NO-mediated vasodilation (36). However, the validity of the proposed mechanisms has not been directly explored in human subjects.
In this study we measured endothelial function using brachial artery flow-mediated dilation (FMD) (40) in hospitalized subjects randomized to receive either fresh (<14 days old) or saRBC units (>21 days old) to test the hypothesis that saRBCs adversely affect NO-mediated vasodilation.
Materials and Methods
Subjects
Eligible subjects were anemic inpatients at Emory University Hospital between the ages of 18 and 80 years, whose physician ordered a clinically indicated transfusion of one or more units of allogeneic packed RBCs. Patients were excluded if: they had received blood transfusions within a week of screening; they were due to receive directed donor, autologous, or whole blood units; they were on nitrates or vasoactive infusions; they were pregnant; they were unsuitable for FMD measurements due to anatomy or clinical conditions; or they were unable to give informed consent. Additionally patients requiring emergent transfusion, transfusion after working hours or requiring invasive procedures during the study period were excluded. Written informed consent was obtained and the study was approved by the Emory Institutional Review Board.
Study Design
Consented and enrolled subjects were typed, cross-matched, and randomized to receive either fresh RBCs (<14 days) or saRBCs (>21 days). Randomization was performed by the Emory University Hospital Blood Bank using sealed envelopes opened individually for each participant. Investigators performing and analyzing study measurements were blinded to the randomization. Following hospital policy, full details of each unit were available to the patient and clinical care team. Demographics and clinical characteristics were documented. Leukoreduced CPD/AS1 RBC units were purchased from the American Red Cross Blood Services, Southern Region. RBC units were irradiated prior to transfusion if requested by the clinical team. Transfusions were performed according to standard hospital protocols. Blood tests and vascular measurements were performed at the pretransfusion baseline, 30 minutes after initiation of transfusion, 1 hour and 24 hours post-completion of transfusion. The study team did not alter any clinical care for the participants, including medication administration or diet.
Endothelial function assessment
Subjects underwent ultrasonography for determination of brachial artery FMD prior to transfusion, and at the aforementioned time points to assess the primary outcome measure of changes in FMD following fresh RBC vs. saRBC transfusions. FMD is a reliable surrogate for vascular health, and reflects endothelium and NO-mediated arterial function.(41, 42) Ultrasonography of the brachial artery was performed at the bedside using a high-resolution 10 MHz ultrasound transducer (Acuson) before and after suprasystolic inflation of a blood pressure cuff for 5 minutes in the ipsilateral upper arm. (43) Briefly, on cuff deflation reactive hyperemia produces an acute increase in shear stress; imaging of the brachial artery is performed continually for the next 120 seconds. Subsequent image analysis was performed by an investigator blinded to the timing using a validated program (Brachial Analyzer, Medical Instruments, Inc.). Brachial artery FMD was calculated as (hyperemic diameter-baseline diameter)/baseline diameter*100. Values obtained were adjusted during statistical analysis for baseline diameter using allometric scaling as recently described.(44) With regard to reproducibility, the mean difference in FMD (%) between two consecutive assessments performed in 11 subjects an average of 8 days apart was 1.26% (+/−0.76%, r=0.75) in our laboratory. The mean difference in the FMD (%) between two readings of the same 11 measurements was 0.82% (+/−0.48%, r=0.97).
Laboratory Measurements
Plasma cell-free hemoglobin quantitation
Plasma free hemoglobin was determined by spectrophotometry. Standards utilized were from Count-A-Part Cyanmethemoglobin Standard Set (Diagnostic Technology, Inc.). Drabkin’s Reagent was used to make 1:2 dilutions of the hemoglobin standards (24.0 mg/dL and 77.4 mg/dL) in order to generate a standard curve. Samples were centrifuged at 3400 RPM for 10 minutes at 4°C, and the plasma supernatant was removed to another tube. The plasma was centrifuged again under the same conditions, the supernatant was removed to a clean tube, and then diluted in Drabkin’s Reagent and concentrations were calculated in reference to the standard curve. The assay was performed at an absorbance of 540 nm with standards and samples being run simultaneously within the same 96 well plate. Drabkin’s Reagent was used as a blank in order to correct for background and subtracted from the resultant value of each well.
2,3-Diphosphoglycerate and adenosine triphosphate assays
In order to stabilize 2,3-DPG and ATP in blood samples for subsequent quantitation, perchloric acid extraction was performed. A 1 mL blood sample was added to a tube containing 2 mL of 5% perchloric acid (PCA), which was being continuously vortexed. The solution was maintained at 4°C for 20 minutes, and then centrifuged at 4°C for 10 minutes at 3400 RPM. The clear supernatant was transferred to a clean tube and centrifuged again. 2 mL of the resulting supernatant was transferred to a clean tube and, while vortexing, 300 μL of 3M K2CO3 was added. The resulting precipitate was allowed to settle for 20 minutes at 4°C. The extract was then centrifuged at 3400 RPM (4°C) for 10 minutes. The resulting clear, neutralized supernatant was transferred to a clean tube; a 0.3 mL aliquot was set aside at 4°C for 2,3-DPG quantitation and the remaining volume was capped tightly and stored frozen at −80°C in aliquots. The PCA extract was tested for 2,3-DPG and ATP content utilizing commercially available kits (Roche Diagnostics GmbH) according to the manufacturer’s instructions. 2,3-DPG content of the blood samples was assayed within 24 hours of extraction and the concentration calculated using correction factors provided by the manufacturer. ATP content was calculated using an internal standard curve.
Nitrite and nitrate levels
Blood samples were collected in distilled water-rinsed centrifuge tubes containing 100 μL of 100 mM N-ethylmaleimide and 5 μL of 0.5 mM ethylenediaminetetraacetic acid (EDTA). Blood samples were then centrifuged to obtain a plasma sample. Plasma samples were flash frozen and stored at −80°C until further analysis. At the time of measurement, plasma samples were thawed on ice and nitrite and nitrate concentrations were quantified by ion chromatography using an ENO20 Analyzer (Eicom USA, San Diego, CA, USA) as previously described.(45)
Measurement of additional plasma analytes
IL-6, IL-2, TNF-alpha and MCP-1 were quantified by Luminex assay, while C-reactive protein was quantified by ELISA.
Statistical Analysis
Normally distributed variables were reported as means ± standard deviation. In contrast, non-normal data were reported as median (IQR) since means would have been heavily affected by data skewness. Differences between groups were assessed using t-tests for continuous variables, and chi-square or Fischer exact tests for categorical variables, where appropriate. Statistical analysis of allometrically scaled FMD was performed using a linear mixed effects model to account for baseline diameter, and provided estimates of these parameters by time in both groups. The model-based means are unbiased with unbalanced and incomplete data, provided that the missing data are non-informative. An unstructured form in the repeated measurements was assumed. Comparison of 2,3-DPG, ATP, and absolute change in FMD were performed using repeated measures ANOVA. The remaining metabolites were not normally distributed. Differences between these were analyzed using Mann-Whitney U, Friedman’s two-way ANOVA and Wilcoxon Signed Rank tests when log-transformation was not possible. Two-tailed P-values <0.05 were considered statistically significant. Analyses were performed using IBM SPSS Statistics Version 21 (Armonk, NY, USA).
Results
Subject Characteristics
Of the 142 patients screened, 98 failed screening or were excluded; 44 subjects were randomized. One subject withdrew later, and of the remaining 43 subjects, 25 were randomized to receive saRBC units and 18 to fresh RBCs (Table 1). There were no significant differences in the clinical characteristics between the groups; 57% of subjects had an underlying malignancy and 25% had received chemotherapy
Table 1.
Demographic and Clinical Characteristics
| Variables | All Patients (n=43) | Recipients of saRBCs (>21 days) (n=25) | Recipients of fresh RBCs (<14 days) (n=18) |
|---|---|---|---|
| Age, years | 59 ± 13 | 58 ± 12 | 60 ± 15 |
| Male, n (%) | 24 (55) | 13 (52) | 11 (58) |
| Race | |||
| White, n (%) | 23 (54) | 15 (63) | 8 (42) |
| Black, n (%) | 17 (40) | 7 (29) | 10 (53) |
| Blood Type | |||
| A− | 1 (2) | 1 (4) | 0 |
| A+ | 12 (27) | 5 (20) | 7 (36) |
| B− | 1 (2) | 1 (4) | 0 |
| B+ | 7 (16) | 5 (20) | 2 (11) |
| O+ | 23 (52) | 13 (54) | 10 (53) |
| Number of Units Transfused | |||
| 1 | 6 (14) | 5 (20) | 1 (5) |
| 2 | 36 (82) | 20 (80) | 16 (84) |
| 3 | 1 (2) | 0 | 1 (5) |
| 4 | 1 (2) | 0 | 1 (5) |
| Clinical Characteristics | |||
| Baseline Hemoglobin, g/dL | 7.6 ± 0.8 | 7.7 ± 0.7 | 7.4 ± 0.9 |
| Post-Transfusion Hemoglobin, g/dL | 9.6 ± 1.1 | 9.3 ± 1.0 | 9.9 ± 1.0 |
| Systolic Blood Pressure, mmHg | 126 ± 17 | 123 ± 17 | 131 ± 17 |
| Diastolic Blood Pressure, mmHg | 70 ± 10 | 69 ± 9 | 72 ± 11 |
| Coronary Artery Disease, n (%) | 11 (25) | 5 (20) | 6 (32) |
| Diabetes Mellitus, n (%) | 12 (29) | 5 (21) | 7 (39) |
| Dyslipidemia, n (%) | 9 (21) | 3 (13) | 6 (33) |
| Current Smoking, n (%) | 2 (5) | 2 (8) | 0 |
| Family History of CAD, n (%) | 2 (5) | 2 (9) | 0 |
| Known malignancy, n (%) | 25 (57) | 17 (68) | 17 (90) |
| Undergoing chemotherapy, n (%) | 11 (25) | 9 (36) | 2 (11) |
| Surgery during index admission, n (%) | 19 (43) | 9 (36) | 10 (53) |
| Medications | |||
| Statin, n (%) | 3 (8) | 1 (4) | 2 (12) |
| Aspirin, n (%) | 6 (15) | 3 (13) | 3 (18) |
| ACE-I/ARB, n (%) | 3 (8) | 1 (4) | 2 (12) |
Values are mean ± SD, or n (%). There were no statistically significant differences between groups. CAD; coronary artery disease, ACE-I; angiotensin-converting enzyme inhibitor, ARB; angiotensin receptor blocker.
All transfused units were leukoreduced CPD/AS1 packed RBC units that were crossmatch-compatible with the recipient. The majority of subjects (82%) were transfused two units of RBCs. Two subjects in the fresh group received three and four units, while the remaining six subjects received a single unit. There were no significant differences in the pre- or post-transfusion hemoglobin or the number of units transfused per patient between groups. Only two of the study participants received plasma transfusions within 2 days of the study, and those units were ABO-identical with the recipient; none of the patients were transfused with platelets in the peri-study period. No adverse transfusion reactions occurred during the study period, and no positive direct antiglobulin test results were seen. The mean storage duration of saRBC and fresh RBC units was 29.6 ± 4.9 and 9.6 ± 3.9 days, respectively (p<0.001).
Endothelial function
There was no significant difference in the baseline pre-transfusion FMD between subjects randomized to saRBC compared to fresh RBC transfusion (5.2 ± 4.4% versus 4.7 ± 4.4% respectively, p=0.7). Although the change in FMD between the two groups did not reach statistical significance (p=0.2), post-hoc analysis revealed that while FMD remained unchanged in subjects receiving fresh RBCs (p=0.231), it significantly declined in those receiving saRBCs over the 24 hours following transfusion (p=0.045) (Figure 1). Resting brachial artery diameter did not change significantly over time in either group (p=0.7 and p=0.3 for fresh RBC and saRBC) or between the groups (p=0.9), and peak-velocity at hyperemia did not change significantly post-transfusion (p=0.08 and p=0.4 for saRBC and fresh RBC). FMD analyzed without allometric adjustment produced similar results.
Figure 1.
Flow-Mediated Dilation during Transfusion of Storage-Aged and Fresh Blood Units. RBCs= packed red blood cells; saRBCs= storage-aged red blood cells. Values represent absolute change in FMD (%), adjusted for baseline brachial artery diameter using allometric scaling.
2,3-Diphosphoglycerate Levels
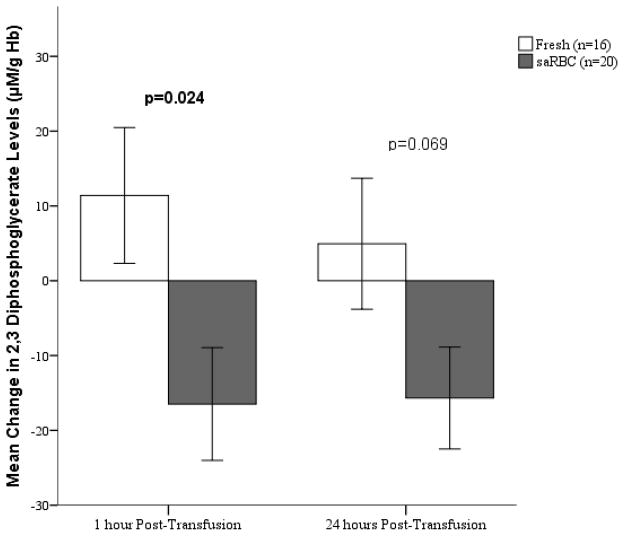
Although there were no differences in 2,3-DPG levels at baseline in recipients of saRBC and fresh RBC (55.2 ± 35.6 and 44.3 ± 21.8 μM/gHgb respectively, p=0.3), there were small but statistically significant changes during the next 24 hours between the groups (Figure 2). Specifically, at 1 hour after transfusion 2,3-DPG levels declined in recipients of saRBCs, but increased in those transfused with fresh RBCs (p=0.024). The trend at 24 hours was similar but did not reach statistical significance (Figure 2).
Figure 2.
Change in 2,3-diphosphoglycerate Levels after Transfusion of Storage-aged and Fresh Blood Units. RBCs= packed red blood cells; saRBCs= storage-aged red blood cells. Values represented are mean change in 2,3-diphosphoglycerate (DPG) levels compared to baseline. The interaction of time point and blood age was statistically significant (p=0.042). Note the significant difference in the change in 2,3 DPG at 1 hour between recipients of saRBC and fresh RBC.
Nitrite and Nitrate Levels
The changes in serum nitrite and nitrate levels were not statistically significant with either saRBC or fresh RBC transfusion, however, there was a trend towards a decrease in nitrite levels 24 hours after saRBC compared to fresh RBC transfusions (p=0.052) (Table 2).
Table 2.
Metabolites and Inflammatory Markers
| saRBC | Fresh RBC | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Baseline | 1 hour Post- Transfusion | 24 hours Post- Transfusion | Baseline | 1 hour Post- Transfusion | 24 hours Post- Transfusion | |
| Free Hgb (g/dL) | 0.111 (0.054) | 0.114 (0.050) | 0.116 (0.054) | 0.142 (0.137) | 0.125 (0.086) | 0.109 (0.174) |
| NO2 (μM) | 0.97 (1.71) | 0.66 (1.56) | 0.64 (1.23) | 1.68 (1.54) | 1.90 (3.53) | 1.47 (2.35) |
| NO3 (μM) | 99.8 (109.7) | 79.7 (90.8) | 77.4 (54.3) | 107.0 (136.8) | 96.2 (147.5) | 71.6 (78.9) |
| ATP (μM/g Hgb) | 4.90 (6.50) | 5.26 (7.91) | 4.70 (4.11) | 4.14 (4.24) | 5.82 (1.18) | 3.10 (4.26) |
| IL-6 (pg/mL) | 23.2 (29.4) | 21.0 (26.3) | 18.9 (30.8) | 20.0 (33.4) | 14.9 (33.0) | 18.0 (18.6) |
| IL-2 (pg/mL) | 0.60 (1.80) | 0.40 (2.15) | 0.52 (2.91) | 0.01 (0.48) | 0.01 (0.62) | 0.01 (0.35) |
| TNFα (pg/mL) | 6.80 (8.80) | 7.32 (10.58) | 6.98 (10.04) | 4.76 (6.63) | 4.84 (5.56) | 4.84 (4.66) |
| MCP-1 (pg/mL) | 170.1 (217.6) | 161.3 (264.4) | 227.7 (249.5) | 170.3 (187.2) | 185.8 (168.3) | 191.9 (134.9) |
| CRP (μg/mL) | 188.9 (238.7) | 185.8 (330.0) | 162.7 (189.0) | 138.7 (100.7) | 145.1 (205.0) | 187.8 (230.3) |
Values are median (IQR). NO2; nitrite, NO3; nitrate, ATP; adenosine triphosphate, IL; interleukin, TNF; tumor necrosis factor, MCP; monocyte chemotactic protein, CRP; C-reactive protein. The differences between saRBC and fresh RBC recipients were not statistically significant.
Other Metabolites
A small increase in cell-free hemoglobin levels was noted 1 hour after transfusion of saRBC (p=0.039) but not fresh RBCs. ATP levels increased 1 hour after fresh RBC transfusion (p=0.043) and decreased 24-hours after saRBC (p=0.026). The remaining metabolites and inflammatory marker levels did not change significantly (Table 2).
Discussion
NO is a crucial vasodilator that actively modulates blood flow and oxygen delivery to local tissues (46, 47). Common disorders including cardiovascular disease and its risk factors are known to reduce NO bioavailability leading to vascular dysfunction and related morbidities (48, 49). We and others have hypothesized that older stored RBCs can also reduce NO bioavailability in transfusion recipients, contributing to endothelial dysfunction, abnormalities of vasodilator tone, and ultimately to end-organ dysfunction (14, 37–39, 46, 50). In this study, we randomized patients to fresh vs. old stored RBCs, and measured their vascular reactivity with FMD, which is a specific measure of vascular NO bioavailability.(51) While there were no significant differences in the primary outcome measure of changes in FMD following fresh RBC vs. saRBC transfusions, the results showing a decrease in FMD response in recipients of saRBC (but not fresh RBC) transfusion suggest that infusion of RBCs stored > 21 days can significantly reduce NO-mediated vasodilation in stable anemic hospitalized patients. These findings will need to be confirmed in subsequent larger studies.
Despite some of the limitations of the present study, as described below, previous investigations using in vitro aortic ring systems (36, 37) as well as rat (31) and dog (22, 23) transfusion models support our findings. However, there is currently limited clinical data on the vascular effects of saRBC transfusions in human recipients to either confirm or refute our results. Berra et al reported that RBC storage time (3 days versus 40 days) had no effect on the reactive hyperemia index measured by pulsatile arterial tomography in healthy volunteers receiving a single unit of autologous blood, although older blood was associated with increased markers of hemolysis and plasma nitrite (52). Likewise, Roberson and colleagues failed to demonstrate a differential effect of 7 versus 42 day aged autologous transfusion on microcirculatory flow in healthy subjects (53). As compared to the present findings, the lack of an effect of blood storage duration in these previous clinical studies may have been due to the differing characteristics of the study populations as well as the use of pulsatile tonometry which is not a direct measure of vascular NO activity. (54) Interestingly, in a study of 93 transfused trauma patients, increased storage age of RBC units was associated with decreased perfused capillary density and thenar eminence tissue oxygen saturation (55). Furthermore, transfusion of a single unit of RBC stored over 26 days significantly decreased whole blood nitrite levels (37). However, as compared to the trauma cohort studied in the aforementioned investigations (37, 55), we studied older subjects (mean age 60±15 versus 46+20 years) with predominantly medical versus traumatic/surgical etiologies of anemia, and we used FMD which is a specific measure of conductance vessel NO-bioavailability. Our subjects also had relatively lower average baseline FMD (5.1%), which reflects their age and degree of illness.
The approximate 2 percentage-point FMD decrease in the saRBC group (from 4.7% pre-transfusion to 2.4% at 24 hours post-transfusion) is comparable to, or larger than, other FMD effects described in the literature. For example, a 12 week course of statin therapy in patients with metabolic syndrome significantly improved FMD from 5.0% to 6.1% (p=0.02)(56). In patients with known coronary artery disease (CAD), coexisting diabetes mellitus type 2 was associated with a reduction in FMD to 2.5%(57). Furthermore, for every 1% decrease in FMD, the risk of cardiovascular events increases by 13% (58). Thus, the magnitude of FMD observed in recipients of saRBC units are meaningful and may have clinical implications.
While the observed FMD changes may be due to the effects of saRBC transfusions, it is important to rule out other causes. For example, several factors such as smoking, diabetes, food, medications, hypertension and body mass index (BMI) can affect FMD. However, our study population was randomized and patient characteristics, including risk factor distribution and baseline FMD were not different between the groups. While 2 of 25 patients randomized to the saRBC arm were smokers, compared to none of the 18 recipients of fresh RBCs, the difference between groups in smoking prevalence is not statistically significant (p=0.5). Additionally, it is unlikely that the cardiovascular risk factor profile changed during the 24-hour time interval of our study.
While these data suggest that saRBC transfusions reduce NO-mediated vasodilation, the present data do not allow us to identify potential mechanisms. The vasoinhibitory effects may be due to decreased NO synthesis, increased NO scavenging, reduced smooth muscle vasodilatory response to NO, and/or other causes. Although we found elevated plasma hemoglobin levels after saRBC but not fresh RBC transfusions, which would be consistent with previous experimental data suggesting that the NO scavenging effects of free hemoglobin may be partly responsible (22, 23, 31), the effect was small and the reduction in plasma hemoglobin in recipients of fresh RBC units was unexpected. Given these unexpected results, we cannot draw firm conclusions on the effects of plasma free hemoglobin on FMD in this study Recent studies demonstrating the protective effects of haptoglobin and hemopexin lend support to the finding that the FMD vasoinhibitory effects may potentially be due to free hemoglobin (or its metabolites, such as hemin).(59)
The changes observed in 2,3-DPG were unexpected. Heaton previously demonstrated in healthy recipients that AS-1 RBCs stored for 35 days prior to transfusion regenerated 2,3-DPG slowly over 24 hours, with 25% restoration within 1 hour.(60) However, Correra showed that some anemic patients have inappropriately low 2,3-DPG levels (when compared to Hb),(61) possibly because in their diseased states they don’t support adequate RBC 2,3-DPG synthesis. Since our transfusion recipients were ill and anemic, they may not be able to support normal rates of 2,3-DPG synthesis in transfused RBCs, particularly saRBCs, leading to the observed differences in 2,3-DPG levels in recipients of saRBCs vs. fresh RBCs. This possibility will require further investigations.
Strengths of our study include the large sample size, detailed study of vascular NO responsiveness, and investigation of potential mediators of vascular dysfunction in anemic adults receiving allogeneic blood transfusions for clinical reasons, a population in whom the increased risks of blood transfusion has been observed.
There are also important limitations of our study. First, the primary outcome measure of changes in FMD following fresh RBC vs. saRBC transfusions showed no statistically significant differences between recipients of fresh vs saRBC transfusions, although a post-hoc analysis showed that the latter participants experienced a significant decrease in FMD 24 hours after older RBC transfusions. Second, due to the lead time required for our study, only patients receiving routine transfusion were included in the study, thus excluding many critically ill and actively bleeding patients requiring emergent transfusion. Third, medications and meals were not held to accommodate study procedures and neither was the time of day fixed which may have confounded our results because of circadian and food related effects. Fourth, there was significant heterogeneity in the etiology of anemia among subjects although there were no differences between the randomized groups. Fifth, we also observed changes in plasma hemoglobin, 2,3-DPG and ATP which were in some cases unexpected, and we did not observe any significant changes in inflammatory markers, although our subjects had multiple co-morbidities and thus already had elevated baseline levels of inflammatory markers that may have influenced our findings. Thus, while our data provides compelling suggestions that saRBC transfusions adversely affect vascular function in ill, hospitalized transfusion recipients, the current results should be interpreted with caution. We are now beginning a follow-up study to further investigate the vascular effects of saRBC transfusions using a cross-over trial design and other modifications to control for recipient variability and address other drawbacks of the present investigations.
Clinical Implications
This study suggests a significant negative effect of transfused RBC units stored > 21 days on NO-mediated vasodilation in anemic hospitalized patients. This finding lends additional support to the hypothesis that deranged NO signaling mediates adverse clinical effects of older RBC transfusions, and should be further investigated in larger clinical studies.
Acknowledgments
Funding Sources: Support for these studies was provided by NHLBI (R01 HL095479-01, and administrative supplement to JDR), NIH grant UL1 RR025008 and the National Blood Foundation (to AR).
Footnotes
Conflict of Interest Disclosures:
None of the authors have conflicts of interests to disclose.
ClinicalTrials.gov IDENTIFIER: NCT00838331
References
- 1.Wang R, Mouliswar M, Denman S, Kleban M. Mortality of the institutionalized old-old hospitalized with congestive heart failure. Archives of Internal Medicine. 1998;158:2464–8. doi: 10.1001/archinte.158.22.2464. [DOI] [PubMed] [Google Scholar]
- 2.Tinmouth A, Fergusson D, Yee IC, Hebert PC. Clinical consequences of red cell storage in the critically ill. Transfusion. 2006;46(11):2014–27. doi: 10.1111/j.1537-2995.2006.01026.x. [DOI] [PubMed] [Google Scholar]
- 3.van de Watering L, Lorinser J, Versteegh M, Westendord R, Brand A. Effects of storage time of red blood cell transfusions on the prognosis of coronary artery bypass graft patients. Transfusion. 2006;46(10):1712–8. doi: 10.1111/j.1537-2995.2006.00958.x. [DOI] [PubMed] [Google Scholar]
- 4.Mou SS, Giroir BP, Molitor-Kirsch EA, Leonard SR, Nikaidoh H, Nizzi F, et al. Fresh whole blood versus reconstituted blood for pump priming in heart surgery in infants. N Engl J Med. 2004;351(16):1635–44. doi: 10.1056/NEJMoa041065. [DOI] [PubMed] [Google Scholar]
- 5.Weiskopf RB, Feiner J, Hopf H, Lieberman J, Finlay HE, Quah C, et al. Fresh blood and aged stored blood are equally efficacious in immediately reversing anemia-induced brain oxygenation deficits in humans. Anesthesiology. 2006;104(5):911–20. doi: 10.1097/00000542-200605000-00005. [DOI] [PubMed] [Google Scholar]
- 6.Garcia MG, Duenas E, Sola MC, Hutson AD, Theriaque D, Christensen RD. Epidemiologic and outcome studies of patients who received platelet transfusions in the neonatal intensive care unit. J Perinatol. 2001;21(7):415–20. doi: 10.1038/sj.jp.7210566. [DOI] [PubMed] [Google Scholar]
- 7.Del Vecchio A, Sola MC, Theriaque DW, Hutson AD, Kao KJ, Wright D, et al. Platelet transfusions in the neonatal intensive care unit:factors predicting which patients will require multiple transfusions. Transfusion. 2001;41(6):803–8. doi: 10.1046/j.1537-2995.2001.41060803.x. [DOI] [PubMed] [Google Scholar]
- 8.Moore FA, Moore EE, Sauaia A. Blood transfusion. An independent risk factor for postinjury multiple organ failure. Arch Surg. 1997;132(6):620–4. discussion 4–5. [PubMed] [Google Scholar]
- 9.Gong MN, Thompson BT, Williams P, Pothier L, Boyce PD, Christiani DC. Clinical predictors of and mortality in acute respiratory distress syndrome: potential role of red cell transfusion. Crit Care Med. 2005;33(6):1191–8. doi: 10.1097/01.ccm.0000165566.82925.14. [DOI] [PubMed] [Google Scholar]
- 10.Smith MJ, Le Roux PD, Elliott JP, Winn HR. Blood transfusion and increased risk for vasospasm and poor outcome after subarachnoid hemorrhage. J Neurosurg. 2004;101(1):1–7. doi: 10.3171/jns.2004.101.1.0001. [DOI] [PubMed] [Google Scholar]
- 11.Hebert PC, Wells G, Blajchman MA, Marshall J, Martin C, Pagliarello G, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 12.Lacroix J, Hebert PC, Hutchison JS, Hume HA, Tucci M, Ducruet T, et al. Transfusion strategies for patients in pediatric intensive care units. N Engl J Med. 2007;356(16):1609–19. doi: 10.1056/NEJMoa066240. [DOI] [PubMed] [Google Scholar]
- 13.Kneyber MC, Hersi MI, Twisk JW, Markhorst DG, Plotz FB. Red blood cell transfusion in critically ill children is independently associated with increased mortality. Intensive Care Med. 2007;33(8):1414–22. doi: 10.1007/s00134-007-0741-9. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds JD, Ahearn GS, Angelo M, Zhang J, Cobb F, Stamler JS. S-nitrosohemoglobin deficiency: a mechanism for loss of physiological activity in banked blood. Proc Natl Acad Sci U S A. 2007;104(43):17058–62. doi: 10.1073/pnas.0707958104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bennett-Guerrero E, Veldman TH, Doctor A, Telen MJ, Ortel TL, Reid TS, et al. Evolution of adverse changes in stored RBCs. Proc Natl Acad Sci U S A. 2007;104(43):17063–8. doi: 10.1073/pnas.0708160104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghio M, Contini P, Mazzei C, Brenci S, Barberis G, Filaci G, et al. Soluble HLA class I, HLA class II, and Fas ligand in blood components: a possible key to explain the immunomodulatory effects of allogeneic blood transfusions. Blood. 1999;93(5):1770–7. [PubMed] [Google Scholar]
- 17.Corwin HL, Carson JL. Blood transfusion--when is more really less? N Engl J Med. 2007;356(16):1667–9. doi: 10.1056/NEJMe078019. [DOI] [PubMed] [Google Scholar]
- 18.Spiess BD. Transfusion of blood products affects outcome in cardiac surgery. Semin Cardiothorac Vasc Anesth. 2004;8(4):267–81. doi: 10.1177/108925320400800402. [DOI] [PubMed] [Google Scholar]
- 19.Koch CG, Li L, Sessler DI, Figueroa P, Hoeltge GA, Mihaljevic T, et al. Duration of red-cell storage and complications after cardiac surgery. N Engl J Med. 2008;358(12):1229–39. doi: 10.1056/NEJMoa070403. [DOI] [PubMed] [Google Scholar]
- 20.Edgren G, Kamper-Jorgensen M, Eloranta S, Rostgaard K, Custer B, Ullum H, et al. Duration of red blood cell storage and survival of transfused patients (CME) Transfusion. 50(6):1185–95. doi: 10.1111/j.1537-2995.2010.02583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Sun J, Solomon SB, Klein HG, Natanson C. Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion. 2012;52(6):1184–95. doi: 10.1111/j.1537-2995.2011.03466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cortes-Puch I, Wang D, Sun J, Solomon SB, Remy KE, Fernandez M, et al. Washing older blood units before transfusion reduces plasma iron and improves outcomes in experimental canine pneumonia. Blood. 2013 doi: 10.1182/blood-2013-11-539353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Solomon SB, Wang D, Sun J, Kanias T, Feng J, Helms CC, et al. Mortality increases after massive exchange transfusion with older stored blood in canines with experimental pneumonia. Blood. 2013;121(9):1663–72. doi: 10.1182/blood-2012-10-462945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiner ME, Assmann SF, Levy JH, Marshall J, Pulkrabek S, Sloan SR, et al. Addressing the question of the effect of RBC storage on clinical outcomes: the Red Cell Storage Duration Study (RECESS) (Section 7) Transfus Apher Sci. 43(1):107–16. doi: 10.1016/j.transci.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lacroix J, Hebert P, Fergusson D, Tinmouth A, Blajchman MA, Callum J, et al. The Age of Blood Evaluation (ABLE) Randomized Controlled Trial: Study Design. Transfus Med Rev. doi: 10.1016/j.tmrv.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 26.Koch CG. The Red Cell Storage Duration and Outcomes in Cardic Surgery study. Clinical Trialsgov. ( http://clinicaltrialsgov/ct2/show/NCT00458783) Cited August 17, 2012.
- 27.Pereira A. Will clinical studies elucidate the connection between the length of storage of transfused red blood cells and clinical outcomes? An analysis based on the simulation of randomized controlled trials. Transfusion. 2013;53(1):34–40. doi: 10.1111/j.1537-2995.2012.03656.x. [DOI] [PubMed] [Google Scholar]
- 28.Yalcin O, Ortiz D, Tsai AG, Johnson PC, Cabrales P. Microhemodynamic aberrations created by transfusion of stored blood. Transfusion. 2013 doi: 10.1111/trf.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tsai M, Kita A, Leach J, Rounsevell R, Huang JN, Moake J, et al. In vitro modeling of the microvascular occlusion and thrombosis that occur in hematologic diseases using microfluidic technology. J Clin Invest. 2012;122(1):408–18. doi: 10.1172/JCI58753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zimring JC, Smith N, Stowell SR, Johnsen JM, Bell LN, Francis RO, et al. Strain-specific red blood cell storage, metabolism, and eicosanoid generation in a mouse model. Transfusion. 2013 doi: 10.1111/trf.12264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donadee C, Raat NJ, Kanias T, Tejero J, Lee JS, Kelley EE, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. 2011;124(4):465–76. doi: 10.1161/CIRCULATIONAHA.110.008698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Silliman CC, Moore EE, Kelher MR, Khan SY, Gellar L, Elzi DJ. Identification of lipids that accumulate during the routine storage of prestorage leukoreduced red blood cells and cause acute lung injury. Transfusion. doi: 10.1111/j.1537-2995.2011.03186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tissot JD, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol. 2010;17(6):571–7. doi: 10.1097/moh.0b013e32833ec217. [DOI] [PubMed] [Google Scholar]
- 34.Hod EA, Cadwell CM, Liepkalns JS, Zimring JC, Sokol SA, Schirmer DA, et al. Cytokine storm in a mouse model of IgG-mediated hemolytic transfusion reactions. Blood. 2008;112(3):891–4. doi: 10.1182/blood-2008-01-132092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hod EA, Zhang N, Sokol SA, Wojczyk BS, Francis RO, Ansaldi D, et al. Transfusion of red blood cells after prolonged storage produces harmful effects that are mediated by iron and inflammation. Blood. 2010;115(21):4284–92. doi: 10.1182/blood-2009-10-245001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alexander JT, El-Ali AM, Newman JL, Karatela S, Predmore BL, Lefer DJ, et al. Red blood cells stored for increasing periods produce progressive impairments in nitric oxide-mediated vasodilation. Transfusion. 2013;53(11):2619–28. doi: 10.1111/trf.12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stapley R, Owusu BY, Brandon A, Cusick M, Rodriguez C, Marques MB, et al. Erythrocyte storage increases rates of NO and nitrite scavenging: implications for transfusion-related toxicity. Biochem J. 2012;446(3):499–508. doi: 10.1042/BJ20120675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kanias T, Gladwin MT. Nitric oxide, hemolysis, and the red blood cell storage lesion: interactions between transfusion, donor, and recipient. Transfusion. 2012;52(7):1388–92. doi: 10.1111/j.1537-2995.2012.03748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roback JD. Vascular effects of the red cell storage lesion. Hematology. 2011:475–9. doi: 10.1182/asheducation-2011.1.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halcox JP, Nour KR, Zalos G, Quyyumi AA. Endogenous endothelin in human coronary vascular function: differential contribution of endothelin receptor types A and B. Hypertension. 2007;49(5):1134–41. doi: 10.1161/HYPERTENSIONAHA.106.083303. [DOI] [PubMed] [Google Scholar]
- 41.Green DJ, Dawson EA, Groenewoud HMM, Jones H, Thijssen DHJ. Is flow-mediated dilation nitric oxide mediated?: A meta-analysis. Hypertension. 2014;63(2):376–82. doi: 10.1161/HYPERTENSIONAHA.113.02044. [DOI] [PubMed] [Google Scholar]
- 42.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–85. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Al Mheid I, Patel R, Murrow J, Morris A, Rahman A, Fike L, et al. Vitamin D status is associated with arterial stiffness and vascular dysfunction in healthy humans. Journal of the American College of Cardiology. 2011;58(2):186–92. doi: 10.1016/j.jacc.2011.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Atkinson G, Batterham AM. The percentage flow-mediated dilation index: a large-sample investigation of its appropriateness, potential for bias and causal nexus in vascular medicine. Vascular medicine. 2013;18(6):354–65. doi: 10.1177/1358863X13508446. [DOI] [PubMed] [Google Scholar]
- 45.Bryan NS, Calvert JW, Gundewar S, Lefer DJ. Dietary nitrite restores NO homeostasis and is cardioprotective in endothelial nitric oxide synthase-deficient mice. Free Radic Biol Med. 2008;45(4):468–74. doi: 10.1016/j.freeradbiomed.2008.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Singel DJ, Stamler JS. Chemical physiology of blood flow regulation by red blood cells: the role of nitric oxide and S-nitrosohemoglobin. Annu Rev Physiol. 2005;67:99–145. doi: 10.1146/annurev.physiol.67.060603.090918. [DOI] [PubMed] [Google Scholar]
- 47.Suematsu M, Suganuma K, Kashiwagi S. Mechanistic probing of gaseous signal transduction in microcirculation. Antioxid Redox Signal. 2003;5(4):485–92. doi: 10.1089/152308603768295230. [DOI] [PubMed] [Google Scholar]
- 48.Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, et al. Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. Journal of Clinical Investigation. 1995;95(4):1747–55. doi: 10.1172/JCI117852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, et al. Prognostic Value of Coronary Vascular Endothelial Dysfunction. Circulation. 2002;106:653–8. doi: 10.1161/01.cir.0000025404.78001.d8. [DOI] [PubMed] [Google Scholar]
- 50.Roback JD, Neuman RB, Quyyumi A, Sutliff R. Insufficient nitric oxide bioavailability: a hypothesis to explain adverse effects of red blood cell transfusion. Transfusion. 2011;51(4):859–66. doi: 10.1111/j.1537-2995.2011.03094.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Joannides R, Haefeli WE, Linder L, Richard V, Bakkali EH, Thuillez C, et al. Nitric oxide is responsible for flow-dependent dilatation of human peripheral conduit arteries in vivo. Circulation. 1995;91(5):1314–9. doi: 10.1161/01.cir.91.5.1314. [DOI] [PubMed] [Google Scholar]
- 52.Berra L, Coppadoro A, Yu B, Lei C, Spagnolli E, Steinbicker AU, et al. Transfusion of stored autologous blood does not alter reactive hyperemia index in healthy volunteers. Anesthesiology. 2012;117(1):56–63. doi: 10.1097/ALN.0b013e31825575e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberson RS, Lockhart E, Shapiro NI, Bandarenko N, McMahon TJ, Massey MJ, et al. Impact of transfusion of autologous 7- versus 42-day-old AS-3 red blood cells on tissue oxygenation and the microcirculation in healthy volunteers. Transfusion (Paris) 2012;52(11):2459–64. doi: 10.1111/j.1537-2995.2012.03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stewart AD, Millasseau SC, Kearney MT, Ritter JM, Chowienczyk PJ. Effects of inhibition of basal nitric oxide synthesis on carotid-femoral pulse wave velocity and augmentation index in humans. Hypertension. 2003;42(5):915–8. doi: 10.1161/01.HYP.0000092882.65699.19. [DOI] [PubMed] [Google Scholar]
- 55.Weinberg JA, MacLennan PA, Vandromme-Cusick MJ, Magnotti LJ, Kerby JD, Rue LW, 3rd, et al. The deleterious effect of red blood cell storage on microvascular response to transfusion. The journal of trauma and acute care surgery. 2013;75(5):807–12. doi: 10.1097/TA.0b013e3182a74a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Murrow JR, Sher S, Ali S, Uphoff I, Patel R, Porkert M, et al. The differential effect of statins on oxidative stress and endothelial function: atorvastatin versus pravastatin. Journal of clinical lipidology. 2012;6(1):42–9. doi: 10.1016/j.jacl.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 57.Keymel S, Heinen Y, Balzer J, Rassaf T, Kelm M, Lauer T, et al. Characterization of macro-and microvascular function and structure in patients with type 2 diabetes mellitus. American journal of cardiovascular disease. 2011;1(1):68–75. [PMC free article] [PubMed] [Google Scholar]
- 58.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 26(6):631–40. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 59.Schaer DJ, Buehler PW, Alayash AI, Belcher JD, Vercellotti GM. Hemolysis and free hemoglobin revisited: exploring hemoglobin and hemin scavengers as a novel class of therapeutic proteins. Blood. 2013;121(8):1276–84. doi: 10.1182/blood-2012-11-451229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heaton A, Keegan T, Holme S. In vivo regeneration of red cell 2,3-diphosphoglycerate following transfusion of DPG-depleted AS-1, AS-3 and CPDA-1 red cells. British journal of haematology. 1989;71(1):131–6. doi: 10.1111/j.1365-2141.1989.tb06286.x. [DOI] [PubMed] [Google Scholar]
- 61.Correra A, Graziano JH, Seaman C, Piomelli S. Inappropriately low red cell 2,3-diphosphoglycerate and p50 in transfused beta-thalassemia. Blood. 1984;63(4):803–6. [PubMed] [Google Scholar]