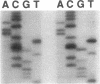
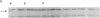Abstract
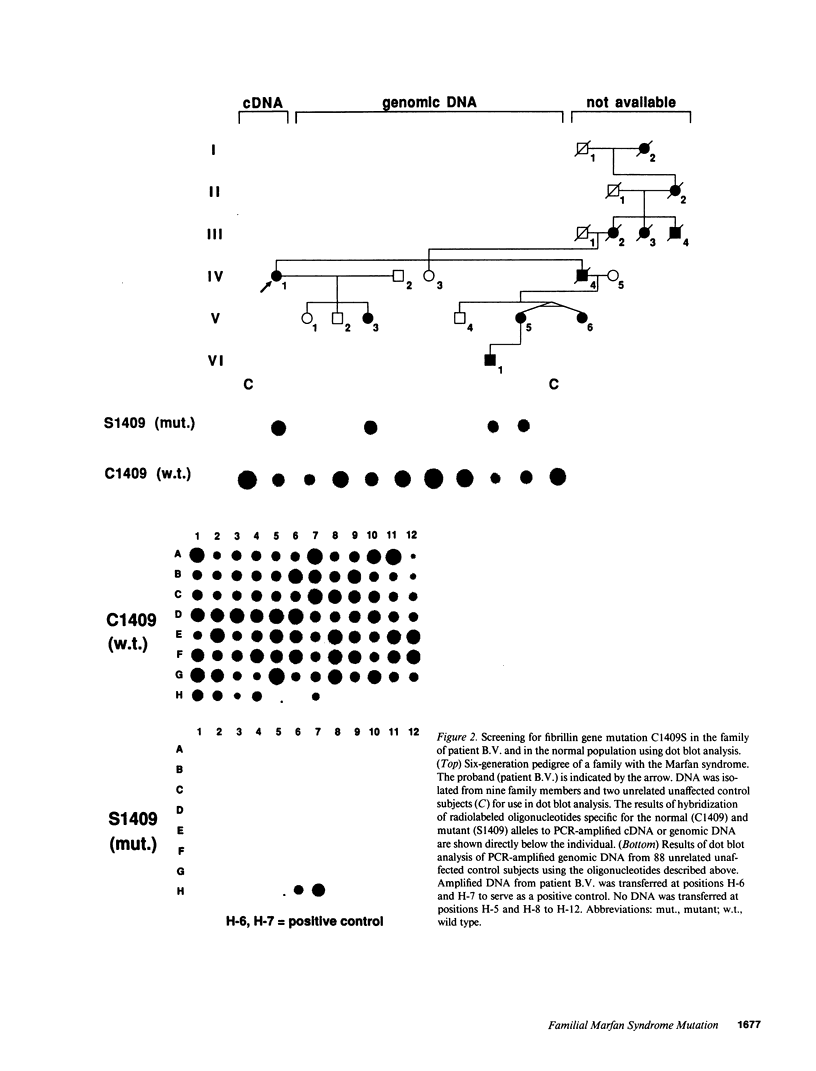
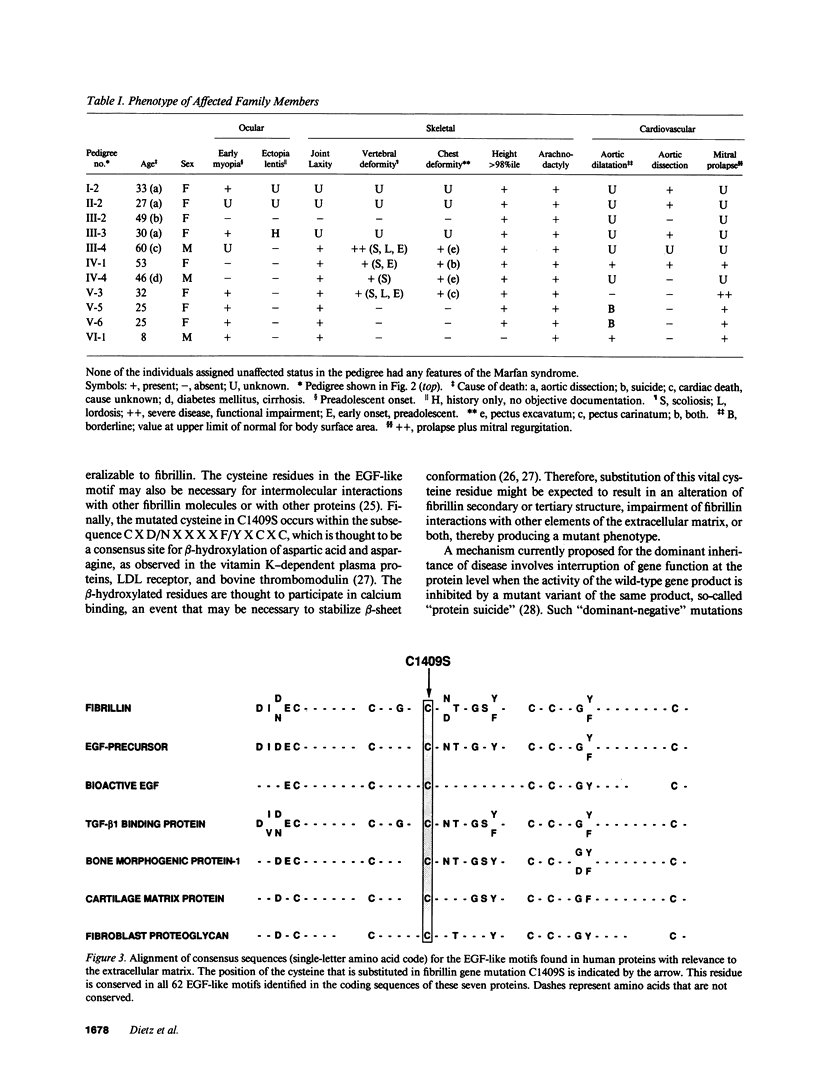
To examine the associations among fibrillin gene mutations, protein function, and Marfan syndrome phenotype, we screened for alterations in the fibrillin coding sequence in patients with a range of manifestations and clinical severity. A cysteine to serine substitution at codon 1409 (C1409S) was identified in an epidermal growth factor (EGF)-like motif from one fibrillin allele which segregates with the disease phenotype through three generations of a family affected with the Marfan syndrome. This alteration was not observed in 60 probands from other families or in 88 unrelated normal individuals. The altered cysteine is completely conserved in all EGF-like motifs identified in fibrillin, and in all proteins that contain this motif. These observations strongly indicate that C1409S is the disease-producing mutation in this family. The phenotype of individuals carrying C1409S varied widely with respect to onset of disease, organ-system involvement, and clinical severity; certain affected adults were unaware of their status before being diagnosed through this investigation. We conclude that fibrillin gene defects cause familial Marfan syndrome, that mutations in the EGF-like motif of the fibrillin gene are not uniformly associated with severe disease, and that fibrillin genotype is not the sole determinant of Marfan phenotype.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beighton P., de Paepe A., Danks D., Finidori G., Gedde-Dahl T., Goodman R., Hall J. G., Hollister D. W., Horton W., McKusick V. A. International Nosology of Heritable Disorders of Connective Tissue, Berlin, 1986. Am J Med Genet. 1988 Mar;29(3):581–594. doi: 10.1002/ajmg.1320290316. [DOI] [PubMed] [Google Scholar]
- Cooke R. M., Wilkinson A. J., Baron M., Pastore A., Tappin M. J., Campbell I. D., Gregory H., Sheard B. The solution structure of human epidermal growth factor. 1987 May 28-Jun 3Nature. 327(6120):339–341. doi: 10.1038/327339a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Cutting G. R., Pyeritz R. E., Maslen C. L., Sakai L. Y., Corson G. M., Puffenberger E. G., Hamosh A., Nanthakumar E. J., Curristin S. M. Marfan syndrome caused by a recurrent de novo missense mutation in the fibrillin gene. Nature. 1991 Jul 25;352(6333):337–339. doi: 10.1038/352337a0. [DOI] [PubMed] [Google Scholar]
- Dietz H. C., Pyeritz R. E., Hall B. D., Cadle R. G., Hamosh A., Schwartz J., Meyers D. A., Francomano C. A. The Marfan syndrome locus: confirmation of assignment to chromosome 15 and identification of tightly linked markers at 15q15-q21.3. Genomics. 1991 Feb;9(2):355–361. doi: 10.1016/0888-7543(91)90264-f. [DOI] [PubMed] [Google Scholar]
- Gibson M. A., Kumaratilake J. S., Cleary E. G. The protein components of the 12-nanometer microfibrils of elastic and nonelastic tissues. J Biol Chem. 1989 Mar 15;264(8):4590–4598. [PubMed] [Google Scholar]
- Goldfischer S., Coltoff-Schiller B., Goldfischer M. Microfibrils, elastic anchoring components of the extracellular matrix, are associated with fibronectin in the zonule of Zinn and aorta. Tissue Cell. 1985;17(4):441–450. doi: 10.1016/0040-8166(85)90023-0. [DOI] [PubMed] [Google Scholar]
- Hall J. R., Pyeritz R. E., Dudgeon D. L., Haller J. A., Jr Pneumothorax in the Marfan syndrome: prevalence and therapy. Ann Thorac Surg. 1984 Jun;37(6):500–504. doi: 10.1016/s0003-4975(10)61142-3. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hollister D. W., Godfrey M., Sakai L. Y., Pyeritz R. E. Immunohistologic abnormalities of the microfibrillar-fiber system in the Marfan syndrome. N Engl J Med. 1990 Jul 19;323(3):152–159. doi: 10.1056/NEJM199007193230303. [DOI] [PubMed] [Google Scholar]
- Jenkins R. N., Osborne-Lawrence S. L., Sinclair A. K., Eddy R. L., Jr, Byers M. G., Shows T. B., Duby A. D. Structure and chromosomal location of the human gene encoding cartilage matrix protein. J Biol Chem. 1990 Nov 15;265(32):19624–19631. [PubMed] [Google Scholar]
- Kainulainen K., Pulkkinen L., Savolainen A., Kaitila I., Peltonen L. Location on chromosome 15 of the gene defect causing Marfan syndrome. N Engl J Med. 1990 Oct 4;323(14):935–939. doi: 10.1056/NEJM199010043231402. [DOI] [PubMed] [Google Scholar]
- Kainulainen K., Steinmann B., Collins F., Dietz H. C., Francomano C. A., Child A., Kilpatrick M. W., Brock D. J., Keston M., Pyeritz R. E. Marfan syndrome: no evidence for heterogeneity in different populations, and more precise mapping of the gene. Am J Hum Genet. 1991 Sep;49(3):662–667. [PMC free article] [PubMed] [Google Scholar]
- Kerem B., Rommens J. M., Buchanan J. A., Markiewicz D., Cox T. K., Chakravarti A., Buchwald M., Tsui L. C. Identification of the cystic fibrosis gene: genetic analysis. Science. 1989 Sep 8;245(4922):1073–1080. doi: 10.1126/science.2570460. [DOI] [PubMed] [Google Scholar]
- Kiss I., Deák F., Holloway R. G., Jr, Delius H., Mebust K. A., Frimberger E., Argraves W. S., Tsonis P. A., Winterbottom N., Goetinck P. F. Structure of the gene for cartilage matrix protein, a modular protein of the extracellular matrix. Exon/intron organization, unusual splice sites, and relation to alpha chains of beta 2 integrins, von Willebrand factor, complement factors B and C2, and epidermal growth factor. J Biol Chem. 1989 May 15;264(14):8126–8134. [PubMed] [Google Scholar]
- Krusius T., Gehlsen K. R., Ruoslahti E. A fibroblast chondroitin sulfate proteoglycan core protein contains lectin-like and growth factor-like sequences. J Biol Chem. 1987 Sep 25;262(27):13120–13125. [PubMed] [Google Scholar]
- Lee B., Godfrey M., Vitale E., Hori H., Mattei M. G., Sarfarazi M., Tsipouras P., Ramirez F., Hollister D. W. Linkage of Marfan syndrome and a phenotypically related disorder to two different fibrillin genes. Nature. 1991 Jul 25;352(6333):330–334. doi: 10.1038/352330a0. [DOI] [PubMed] [Google Scholar]
- Magenis R. E., Maslen C. L., Smith L., Allen L., Sakai L. Y. Localization of the fibrillin (FBN) gene to chromosome 15, band q21.1. Genomics. 1991 Oct;11(2):346–351. doi: 10.1016/0888-7543(91)90142-2. [DOI] [PubMed] [Google Scholar]
- Maslen C. L., Corson G. M., Maddox B. K., Glanville R. W., Sakai L. Y. Partial sequence of a candidate gene for the Marfan syndrome. Nature. 1991 Jul 25;352(6333):334–337. doi: 10.1038/352334a0. [DOI] [PubMed] [Google Scholar]
- Pratt B. M., Madri J. A. Immunolocalization of type IV collagen and laminin in nonbasement membrane structures of murine corneal stroma. A light and electron microscopic study. Lab Invest. 1985 Jun;52(6):650–656. [PubMed] [Google Scholar]
- Pyeritz R. E., McKusick V. A. The Marfan syndrome: diagnosis and management. N Engl J Med. 1979 Apr 5;300(14):772–777. doi: 10.1056/NEJM197904053001406. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986 Dec;103(6 Pt 1):2499–2509. doi: 10.1083/jcb.103.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakai L. Y., Keene D. R., Glanville R. W., Bächinger H. P. Purification and partial characterization of fibrillin, a cysteine-rich structural component of connective tissue microfibrils. J Biol Chem. 1991 Aug 5;266(22):14763–14770. [PubMed] [Google Scholar]
- Savage C. R., Jr, Inagami T., Cohen S. The primary structure of epidermal growth factor. J Biol Chem. 1972 Dec 10;247(23):7612–7621. [PubMed] [Google Scholar]
- Schwartz E., Goldfischer S., Coltoff-Schiller B., Blumenfeld O. O. Extracellular matrix microfibrils are composed of core proteins coated with fibronectin. J Histochem Cytochem. 1985 Apr;33(4):268–274. doi: 10.1177/33.4.3980980. [DOI] [PubMed] [Google Scholar]
- Stenflo J., Ohlin A. K., Owen W. G., Schneider W. J. beta-Hydroxyaspartic acid or beta-hydroxyasparagine in bovine low density lipoprotein receptor and in bovine thrombomodulin. J Biol Chem. 1988 Jan 5;263(1):21–24. [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Schwartz M. F., Byers P. H. Substitution of arginine for glycine at position 847 in the triple-helical domain of the alpha 1 (I) chain of type I collagen produces lethal osteogenesis imperfecta. Molecules that contain one or two abnormal chains differ in stability and secretion. J Biol Chem. 1990 Oct 25;265(30):18628–18633. [PubMed] [Google Scholar]
- Wharton K. A., Johansen K. M., Xu T., Artavanis-Tsakonas S. Nucleotide sequence from the neurogenic locus notch implies a gene product that shares homology with proteins containing EGF-like repeats. Cell. 1985 Dec;43(3 Pt 2):567–581. doi: 10.1016/0092-8674(85)90229-6. [DOI] [PubMed] [Google Scholar]
- Wong C., Dowling C. E., Saiki R. K., Higuchi R. G., Erlich H. A., Kazazian H. H., Jr Characterization of beta-thalassaemia mutations using direct genomic sequencing of amplified single copy DNA. 1987 Nov 26-Dec 2Nature. 330(6146):384–386. doi: 10.1038/330384a0. [DOI] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]