Abstract
Reprogramming technology has opened the possibility of converting one cell type into another by forced expression of transgenes. Transduction of adenoviral vectors encoding 3 pancreatic transcription factors, Pdx1, Ngn3, and MafA, into mouse pancreas results in direct reprogramming of exocrine cells to insulin-producing β-like cells. We hypothesized that cultured adult pancreatic duct cells could be reprogrammed to become insulin-producing β-cells by adenoviral-mediated expression of this same combination of factors. Exocrine were isolated from adult mouse insulin 1 promoter (MIP)-green fluorescent protein (GFP) transgenic mice to allow new insulin-expressing cells to be detected by GFP fluorescence. Cultured cells were transduced by an adenoviral vector carrying a polycistronic construct Ngn3/Pdx1/MafA/mCherry (Ad-M3C) or mCherry sequence alone as a control vector. In addition, the effects of glucagon-like peptide-1 (GLP-1) receptor agonist, exendin-4 (Ex-4) on the reprogramming process were examined. GFP+ cells appeared 2 days after Ad-M3C transduction; the reprogramming efficiency was 8.6 ± 2.6% by day 4 after transduction. Ad-M3C also resulted in increased expression of β-cell markers insulin 1 and 2, with enhancement by Ex-4. Expression of other β-cell markers, neuroD and GLP-1 receptor, were also significantly up-regulated. The amount of insulin release into the media and insulin content of the cells were significantly higher in the Ad-M3C-transduced cells; this too was enhanced by Ex-4. The transduced cells did not secrete insulin in response to increased glucose, indicating incomplete differentiation to β-cells. Thus, cultured murine adult pancreatic cells with a duct phenotype can be directly reprogrammed to insulin-producing β-like cells by adenoviral delivery of 3 pancreatic transcription factors.
β-Cell replacement therapy is an attractive therapeutic approach for diabetes, because reduced β-cell mass is a fundamental component of both types 1 and 2 diabetes (1). Transplanted islet cells have been shown to function very well in diabetic subjects (2), but current approaches require cadaveric donor pancreases and immunosuppression, which greatly limit the number of subjects that can be treated. Optimism comes from the finding that human embryonic stem cells (ESCs) and induced pluripotent stem (iPS) cells can be directed to become mature β-cells (3–6). But in addition, there is considerable interest in the potential of β-cell regeneration as a path to replacement therapy (7).
The pancreatic duct cells (PDCs) are a potential cell source for new islet formation. In spite of some discrepant results, some lineage tracing experiments in rodents indicate that adult PDC can serve as multipotent progenitors that can form new exocrine and endocrine cells (8–12). We have demonstrated in vitro generation of new islets from cultured human PDC (13, 14); however, these PDC-derived islets have not been produced in sufficient numbers to be useful for clinical application.
The reprogramming success of iPS cells (15) has opened the possibility of converting one mature cell type directly into another by forced expression of transgenes. It has been previously shown that injection of adenoviral vectors encoding 3 transcription factors, Pdx1, Ngn3, and MafA, into mouse pancreas results in reprogramming of exocrine cells to insulin-producing β-cells in sufficient numbers to ameliorate diabetes (16, 17). Pdx1 (pancreatic and duodenal homeobox gene 1) is necessary for both pancreatic and β-cell development, Ngn3 (neurogenin 3) is essential for the specification of islet cells, and MafA is a key for the final stage of β-cell maturation (6). The combination of all the 3 factors proved to be absolutely required for the reprogramming of exocrine cells to the insulin-producing β-like cells (16).
Herein we report a novel approach to the in vitro direct reprogramming of cultured cells with a duct phenotype to β-like cells using a single adenoviral polycistronic vector carrying 3 pancreatic transcription factors, Pdx1, Ngn3, and MafA (17). In addition, we demonstrate the enhancing effect of glucagon-like peptide-1 (GLP-1) receptor agonist, exendin-4 (Ex-4) on this process.
Materials and Methods
Animals
Mouse insulin 1 promoter (MIP)-green fluorescent protein (GFP) male homozygous transgenic mice were backcrossed 10 times with B6 female mice; both strains were purchased from The Jackson Laboratory. These MIP-GFP transgenic mice express enhanced GFP under the control of the mouse insulin 1 promoter (18). Enhanced GFP fluorescence is detected in tissues where insulin 1 is normally expressed. The pattern of GFP fluorescence and insulin expression by immunostaining within the islet were completely identical, and GFP expression was observed only in β-cells but not in non-β-cells of the islet or in the exocrine pancreas (18). Adult 9-week-old male DBA/2 mice were also obtained from Jackson. All animal procedures were approved by the Institutional Animal Care and Use Committee of the Joslin Diabetes Center.
Cell isolation and culture
Islets and pancreatic ductal cells were isolated from MIP-GFP or DBA/2 mice, as previously described (19) with minor modifications. Mice were fasted overnight and then received ip injections of streptozocin (200 mg/kg; Sigma) 1 hour before isolation, which minimized contamination of the exocrine cell cultures with β-cells. The common bile duct was cannulated and injected with cold M199 media containing 1.5-mg/mL collagenase (Liberase RI; Roche), and the whole pancreas was resected. The pancreases were digested at 37°C for 17 minutes, and islets were separated from exocrine tissues by a density gradient using Histopaque 1077 (Sigma). After the islets were removed, the pellet containing acinar and duct cells was collected. This β-cell depleted exocrine tissue was suspended in PBS, allowed to settle under gravity at room temperature (RT) for 10 minutes, and then the supernatant was aspirated to remove low-density components including dead cells. After washing 5 times with PBS, residual tissue was centrifuged at 1000 rpm for 1 minute. To dissociate exocrine tissue into single cells, the pellet was resuspended in PBS containing 0.025% trypsin-EDTA (Invitrogen) and incubated at 37°C for 5 minutes. The trypsinized tissues were placed into CMRL medium 1066 (Gibco, Invitrogen Corp) containing 10% (vol/vol) fetal bovine serum (FBS) (Cellgro), and centrifuged at 1000 rpm for 1 minute. The pellet was resuspended in CMRL supplemented with 10% FBS, 100-U/mL penicillin and 100-μg/mL streptomycin (Invitrogen), and 0.02% soybean trypsin inhibitor (Sigma). Exocrine cells were plated at 10 × 104 cells/mL on collagen (soluble type 1)-coated 6-well culture plate (Cellmatrix I-A, at 6 μg/cm2; Nitta Gelatin). After 3 days in CMRL with 10% FBS, the media were then changed to DMEM/F12 (Gibco) supplemented with 10% FBS, 100-U/mL penicillin and 100-μg/mL streptomycin, 25mM glucose (Mediatech), 10mM nicotinamide (Sigma), and 20-ng/mL epidermal growth factor (Becton Dickinson & Co). The exocrine cells were cultured for an additional 4 days, and adherent cells formed epithelial monolayers, whereas most of the initial acinar cells were dead at this stage. Over 95% of the adherent cells expressed the ductal cell-specific marker pan Cytokeratin (pan-CK) (Figure 1). Cells were cultured at 37°C in a humidified atmosphere containing 5% CO2.
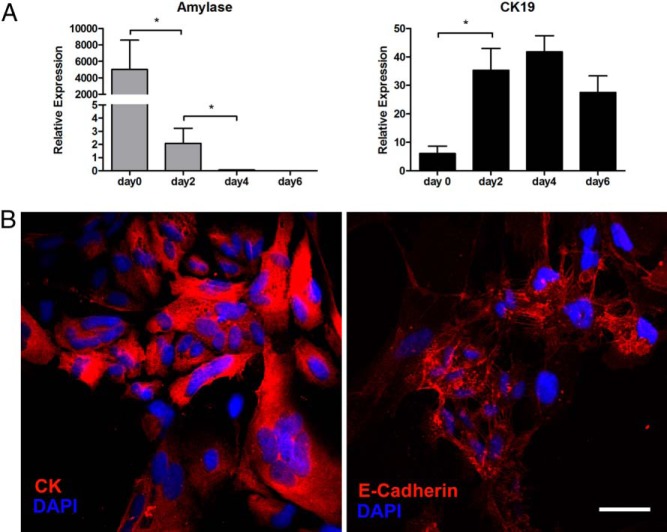
Figure 1.
Characterization of isolated exocrine cells. A, Changes in the gene expression profile of exocrine cells 0, 2, 4, and 6 days after isolation. Freshly isolated exocrine cells (d 0) had high expression of amylase, which disappeared in just 4 days. The results were obtained from the adherent cells after floating cells were removed on each day except day 0 (freshly isolated nonadherent exocrine cells). Mean ± SEM, 4 independent experiments (each with duplicates). *, P < .05. B, Seven days after isolation, the adherent cells had proliferated and formed epithelial-like monolayers with cobblestone-like morphology; immunostaining was for pan-CK (red) (left panel) and E-cadherin (red) (right panel). Blue represents nuclear staining with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar, 50 μm. Images are representative of 4 independent experiments.
Transduction of ductal cells with adenovirus
Media were changed to serum-free DMEM/F12, and the attached ductal cells were then incubated with adenoviruses at a dose of 50 multiplicity of infection for 4 hours at 37°C until being replaced with fresh culture medium. The transduced ductal cells were cultured in DMEM/F12 supplemented with 10% FBS, 100-U/mL penicillin and 100-μg/mL streptomycin, 5mM glucose, and 10mM nicotinamide, in combination with or without 50-ng/mL Ex-4 (Sigma). The media were changed every day until assessment.
Preparation of adenoviruses and vector construction
Recombinant adenoviruses containing Ngn3, Pdx1, and MafA were prepared using the ViraPower adenoviral expression system (Invitrogen) according to the manufacturer's instructions (Figure 2A). Full-length mouse Ngn3, Pdx1, and MafA cDNAs were cloned into a shuttle vector pENTR 2B containing mCherry reporter as a fragment ligated by SalI/NotI sites, and then into the pAd/CMV/V5-DEST adenoviral vector (Invitrogen) to construct a polycistronic expression vector encoding all the 3 transcription factors, called Ad-M3mCherry (Ngn3/Pdx1/MafA/mCherry [Ad-M3C]). Ad-M3C was amplified by transduction into 293A producer cell line. The adenoviral construct (pAd-M3C) consists of 4 different coding regions separated by 3 autonomous ”self-cleaving” 2A protein elements (Figure 2A). Ad-mCherry (Ad-C) was prepared as the control in the same manner. High titer virus (2–10 × 1010 plaque-forming units/mL) was obtained by purification using the Vivapure Adenopack (Sartorius). Viral tittering was performed with direct counting of Cherry+ cells and immunostaining of inserted genes (Pdx1, Mafa, and Ngn3) 2 days after transduction in HEK293A cells. In this procedure, we confirmed that all the 3 transcriptional factors could be efficiently expressed in the Cherry+-transduced cells, as previously described (17, 20). We have shown the immunostaining of HEK293 cells infected with the pAd-M3 polycistronic expression virus, which is the same construct as used in this experiment. As the results, most cherry+ cells express the M3 factors (Ngn3, Mafa, and Pdx1), indicating excellent coexpression from this construct (20).
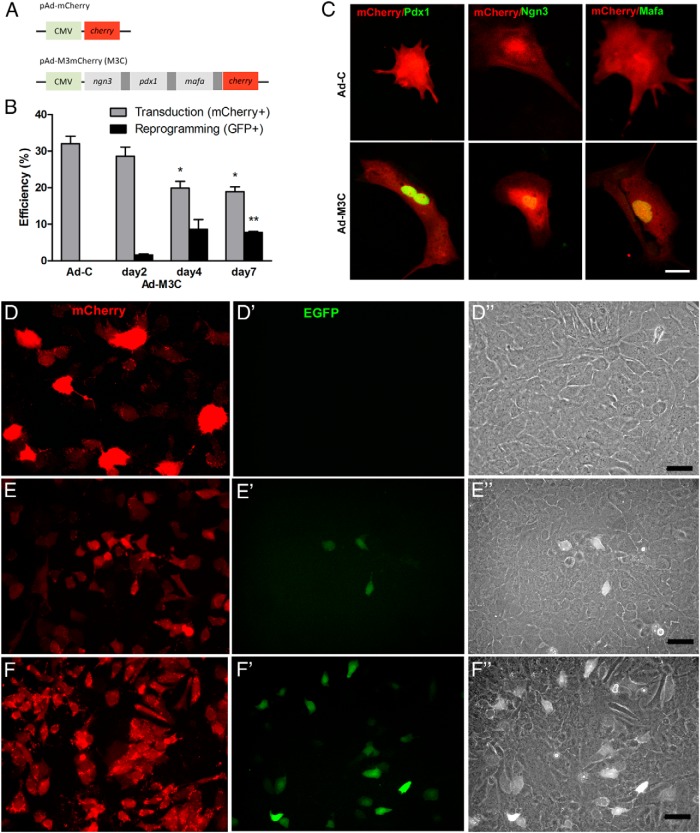
Figure 2.
Adenoviral transduction of 3 transcriptional factors induced reprogramming of cells with duct phenotype to GFP+ β-like cells. A, Schematic constructs. CMV, cytomegaloviral promoter. Dark gray bar, 2A peptide, which mediates polycistronic protein expression. Cherry, monomeric cherry fluorescent protein. The construct pAd-M3m Cherry (M3C) consists of 4 different coding regions separated by 3 2A protein elements. Ad-C was prepared as the control. B, Transduction and reprogramming efficiency was assessed by quantification of the percentage of mCherry+ cells and GFP+ cells of total cells, respectively, using exocrine cells from MIP-GFP mice. Reprogrammed cells would be insulin-expressing GFP+ cells. Mean ± SEM, 3 independent experiments. *, P < .05; **, P < .001 compared with day 2. C, Expression of 3 transcription factors in Ad-C- and Ad-M3C-transduced cells 7 days after transduction of cells from DBA/2 mice. Transduced cells were marked by mCherry+ (red). Nuclear immunostaining of Pdx1, Ngn3, and MafA (green) was confirmed in cells transduced with Ad-M3C (bottom panels) but not detected in cells transduced with Ad-C (top panels). Images are representative of 3 independent experiments. Scale bar, 25 μm. D–F, The presence of mCherry and GFP was examined in the cells 2 days after transduction with Ad-C (D) or Ad-M3C (E) and then at 7 days after Ad-M3C (F) transduction. Transduced cells from MIP-GFP mice were marked by mCherry and some became GFP+ after Ad-M3C transduction. None of the mCherry+ cells in the Ad-C-transduced cells became GFP+. D”–F”, In all 3 conditions, the transduced GFP+ cells had a cobblestone-like morphology with differential interference contrast. Images are representative of 3 independent experiments. Scale bars, 25 μm (D) and 50 μm (E and F).
Real-time RT-PCR analysis
Total RNA was extracted using the PicoPure RNA Isolation kit (Applied Biosystems Arcturus) according to the manufacturer's instructions, and 250 ng of RNA were used for cDNA synthesis. First-strand cDNA was synthesized with M-MLV reverse transcriptase, random primers and dNTP mix (Promega). Quantitative real-time RT-PCRs were performed in duplicate using specific primers (Supplemental Table 1) and Power SYBR Green PCR Master Mix (Applied Biosystems) in a 7300 Real-Time or 7900HT Fast Real-Time PCR System (Applied Biosystems). Data were analyzed by the comparative Δcycle threshold (Ct) method (21) and presented relative to the housekeeping gene TATA box-binding protein instead of fold changes that can be misleading when the controls or original samples have negligible expression.
Immunostaining
Cells were fixed on collagen-coated 6-well culture plate with 4% paraformaldehyde for 20 minutes at RT. Paraformaldehyde-fixed cells were washed 3 times with PBS containing 1M glycine (Sigma) using a shaker (Orbit 1000; Labnet) at 100 rpm for 10 minutes in each wash. Cells were permeabilized with PBS containing 0.3% Triton X-100 (Sigma) for 30 minutes at RT and then washed 3 times with PBS for 10 minutes in each wash. After 1 hour of blocking with PBS containing 0.5% Triton X-100 and 2% normal donkey serum (Jackson ImmunoResearch), cells were incubated with primary antibodies overnight at 4°C, and then with biotinylated or fluorescence-conjugated secondary antibodies for 1 hour at RT. The primary and secondary antibodies are listed in the Supplemental Table 2. 4′,6-diamidino-2-phenylindole (Sigma) was used for nuclear staining. Images were acquired in confocal mode on a Zeiss LSM 410 inverted microscope.
Insulin secretion and content
The supernatants of culture media were collected and stored at −20°C until the assay of insulin secretion into the media. It should be noted that the media (2 mL/well in the 6-well plate) were changed every 24 hours until assessment, and that the amount of insulin in the media were measured at 24-hour time periods. Cells plated on collagen-coated 6-well culture plates were washed 3 times with PBS and then with DMEM/F12 containing 0.05% collagenase (Sigma) and 0.025% trypsin/EDTA for 10 minutes to dissolve the collagen gel. The cells were collected and homogenized 3 times by sonication with a Sonic Dismembrator (Fisher Scientific) for 10 seconds followed by 15 seconds on ice. The total volume of each sample was divided into 2 equal aliquots: one to determine the amount of insulin content in the cells and the other to quantify cellular DNA content. DNA content was analyzed using the CyQUANT Cell Proliferation Assay kit (Molecular Probes), according to the manufacturer's instructions. The amount of insulin secretion into the media and the insulin content of the cells were measured using an Insulin (Rat) ELISA kit (ALPCO), according to the manufacturer's instructions, and the values were normalized to the total amount of DNA content in each well.
Glucose-stimulated insulin secretion (GSIS)
Cells were incubated for 1 hour at 37°C with Krebs-Ringer Bicarbonate HEPES solution containing low glucose (2.8mM), high glucose (20.2mM), or high glucose with 1-methyl-3-isobutylxanthine (1mM); 1 mL was employed for each well of the 6-well plate to assess GSIS. The supernatants of culture media were collected at 1-hour time periods after each incubation, and the amount of insulin released in response to stimulation by glucose was measured using an Insulin (Rat) ELISA kit, according to the manufacturer's instructions. The values were normalized to the total DNA content in each well.
Statistics
Results are expressed as mean ± SEM and statistically (*, P < .05; **, P < .01; ***, P < .001) significant differences were assessed using unpaired Student's t test.
Results
Characterization of isolated exocrine cells
The exocrine tissue fraction was separated from islets by density gradient centrifugation, and dissociated single exocrine cells were plated on collagen-coated culture plates. The absence of contaminating islet cells was confirmed by the lack of GFP fluorescence and gene expression of β-cell markers. When assessed with real-time RT-PCR immediately after isolation (d 0), the expression of β-cell-specific genes, insulin 1 and 2, could not be detected, indicating the absence of β-cells in the starting population. Initially, freshly isolated exocrine cell preparations are composed of both acinar and duct cells. Expression of the acinar-cell gene, amylase, was about 100-fold higher than that of duct-cell gene, cytokeratin (CK)19 in the starting material (Figure 1A). This indicates that the freshly isolated exocrine cell fraction (d 0) is mainly composed of acinar cells, which corresponds to the largest cell population in the pancreas. The exocrine cells were allowed to settle and attach without changing media for 3 days. After the floating cells were washed off, the expression of CK19 of the attached cells was greatly increased on day 2 of culture, whereas the expression of amylase was markedly decreased and became undetectable by day 4 of culture, indicating that the attached cells are characterized by a ductal phenotype. The cells with an acinar phenotype completely disappeared by day 7 of culture. The attached cells proliferated and reached about 80% confluence by day 7, forming epithelial-like monolayers with a cobblestone-like morphology typical of epithelial cells (Figure 1B). Consistent with the mRNA analysis, immunostaining confirmed that more than 95% of the attached cells expressed the ductal cell markers, CK and E-cadherin at the end of culture period (d 7) (Figure 1B). These findings could be explained by selective survival of the attached initial duct cells coupled with the elimination of acinar cells due to the lack of adhesion and the high frequency of apoptosis during the first days of culture, or possibly by transdifferentiation of acinar cells to duct cells (22, 23). The final composition of attached cells was characterized by a ductal phenotype, although it is not possible to distinguish the acinar-derived cells from the duct cells.
Efficiency of transduction and reprogramming
PDCs isolated from MIP-GFP mice, which allow insulin-expressing cells to be detected by GFP fluorescence, were transduced with an adenoviral vector carrying a polycistronic construct Ad-M3C or Ad-C as a control vector. The efficiencies of transduction and reprogramming were assessed by counting the numbers of mCherry+ and GFP+ cells, respectively. The mCherry+ cells appeared 24 hours after transduction in both Ad-C- and Ad-M3C-transduced cells. The transduction efficiency (as assessed by the percentage of mCherry+ cells of total number of cells) was 32.0 ± 2.0% and 28.6 ± 2.4% 2 days after transduction of Ad-C and Ad-M3C, respectively (Figure 2, B and D–F). After exocrine cells isolated from DBA/2 mice were transduced with Ad-M3C or Ad-C, nuclear expression of the 3 transcription factors, Pdx1, Ngn3, and MafA, was confirmed with immunostaining in the Ad-M3C-transduced cells 7 days after transduction but not in the controls (Figure 2C). GFP+ cells were detected 2 days after transduction in the Ad-M3C-transduced cells, whereas none of the mCherry+ cells became GFP+ in the Ad-C-transduced cells (Figure 2, D–F). The reprogramming efficiency (as assessed by the percentage of GFP+ cells of total cells) was 1.6 ± 0.2% at day 2 of Ad-M3C transduction (Figure 2B). By differential interference contrast (DIC) imaging of all of the transduced cells had a cobblestone-like morphology (Figure 2, D”–F”). By day 7 of Ad-M3C transduction, the reprogramming efficiency reached 8.6 ± 2.6% (d 4) and 7.7 ± 0.3% (d 7), although the transduction efficiency had fallen to 19.8 ± 1.9% (d 4) and 18.9 ± 1.3% (d 7) (Figure 2B). Interestingly, GFP+-reprogrammed cells were observed in some but not all Ad-M3C-transduced mCherry+ cells. The percentage of GFP+-reprogrammed cells of total mCherry+-transduced cells was 5.7 ± 1.6%, 44.8 ± 26.1%, and 41.4 ± 5.7% on days 2, 4, and 7 of Ad-M3C transduction, respectively.
GFP and insulin expression in Ad-M3C-transduced cells
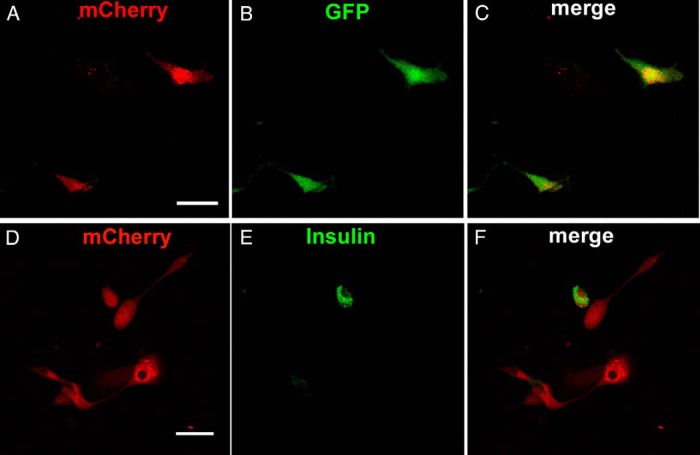
There was coexpression of mCherry and GFP in cells from MIP-GFP mice fixed 7 days after Ad-M3C transduction and examined with confocal microscopy (Figure 3, A–C). Some mCherry+ (red)-transduced cells also expressed GFP fluorescence, indicating activity of the insulin promoter. Ad-M3C-treated mCherry+ (red) cells from DBA/2 mice were immunostained for insulin (green) 7 days after transduction (Figure 3, D–F). Merged images (yellow) showed coexpression of mCherry and insulin in some but not all Ad-M3C-transduced cells.
Figure 3.
GFP and insulin expression in Ad-M3C-transduced cells 7 days after transduction. A–C, Coexpression of mCherry and GFP in cells from MIP-GFP mice fixed 7 days after Ad-M3C transduction and examined with confocal microscopy. Some mCherry+ (red)-transduced cells also expressed GFP fluorescence (green), indicating activity of the insulin promoter. Merged image (yellow). (D-F) Ad-M3C-treated mCherry+ (red) cells from DBA/2 mice were immunostained for insulin (green) 7 days after transduction. Merged images (yellow) showed coexpression of mCherry and insulin in some but not all Ad-M3C-transduced cells. Images are representative of 3 independent experiments. Scale bars, 50 μm.
Gene expression profile after Ad-M3C transduction
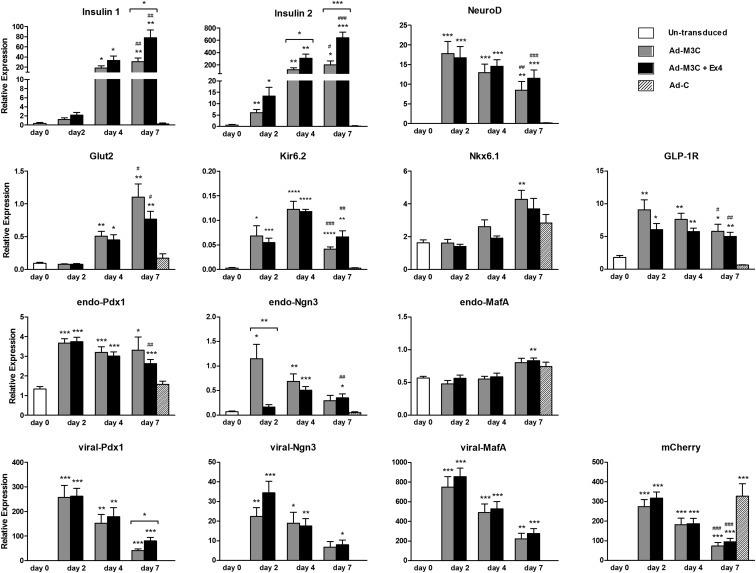
To assess conversion to a β-cell phenotype, gene expression of β-cell markers was evaluated by quantitative real-time RT-PCR (Figure 4). In addition, we asked whether the GLP-1 receptor agonist, Ex-4, could increase the efficiency of reprogramming. With Ad-M3C and with Ad-M3C plus Ex-4 (Ad-M3C+Ex4), insulin1 and insulin2 mRNA levels were detected 2 days after transduction and markedly increased 4 and 7 days after transduction. Moreover, Ex-4 enhanced the effect of Ad-M3C on insulin 2 mRNA expression at day 4, and insulin 1 and 2 mRNA expressions at day 7 of transduction. NeuroD and GLP-1R mRNA were also significantly up-regulated just 2 days after transduction with Ad-M3C and maintained at similar levels through day 7. Nkx6.1mRNA levels were gradually up-regulated 4 days after transduction and significantly increased 7 days after Ad-M3C transduction. Glut2 mRNA expression was significantly up-regulated 4 days after Ad-M3C transduction and subsequently increased at day 7, whereas the gene for ATP-sensitive potassium channel Kir6.2 mRNA were significantly up-regulated 2 days after Ad-M3C transduction, and peaked at day 4. However, Ex-4 did not affect the expression of β-cell markers other than insulin 1 and 2 through 7 days after transduction.
Figure 4.
Gene expression of phenotypic duct cells after Ad-M3C transduction. Changes in gene expression by Ad-M3C-transduction with and without Ex-4 were determined: untransduced cells as day 0, Ad-M3C-treated cells and Ad-M3C-treated plus Ex-4 (Ad-M3C+Ex4) cells on days 2, 4, and 7 of transduction, and Ad-C-treated cells on day 7. The 3 viral transgenes are referred to as viral-Pdx1, viral-Ngn3, and viral-MafA, whereas the 3 endogenous transcription factors are referred as endo-Pdx1, endo-Ngn3, and endo-MafA for encoding untranslated regions (UTRs). Mean ± SEM, 12 independent experiments (each with duplicates). *, P < .05; **, P < .01; ***, P < .001 compared with day 0; #, P < .05; ##, P < .01; ###, P < .001 compared with Ad-C on day 7. Brackets show the comparison between Ad-M3C and Ad-M3C+Ex4, *, P < .05; **, P < .01; ***, P < .001.
The 3 viral transgenes, viral-Pdx1, viral-Ngn3, and viral-MafA, encode the vector-derived sequence of the coding region, a self-cleaving 2A peptide. Thus, the endogenous 3 transcription factors encoding untranslated regions, endo-Pdx1, endo-Ngn3, and endo-MafA, can be distinguished from the 3 input genes. As expected, the 3 viral transgenes were not expressed in either untransduced cells or Ad-C-transduced cells but were markedly up-regulated 2 days after transduction with Ad-M3C; however, their expression then declined over the next 5 days (ending at day 7). The expressions of endogenous Pdx1, Ngn3, and MafA were all somewhat different. There was baseline expression of endo-Pdx1 mRNA, which increased after Ad-M3C transduction and was maintained through day 7. The expression of endo-Ngn3 mRNA in untransduced cells was very low but increased over 10-fold after Ad-M3C transduction; however, their expression then declined over the next 5 days. In contrast, endo-Ngn3 mRNA expression peaked at day 4 of Ad-M3C plus Ex-4. The Ad-M3C transduction led to modest changes in endo-MafA expression, although its significant increase was barely detected at day 4 of Ad-M3C plus Ex-4. Ex-4 had no influence on expression of any of the transcription factors except for apparent impressive inhibition of Ngn3 expression on day 2.
Expression of mCherry mRNA, a marker of transduction, was up-regulated 2 days after Ad-M3C transduction but had decreased by day 7. In contrast, mCherry gene expression in Ad-C-transduced cells was maintained at a high level on day 7 after transduction, suggesting that the decrease in mCherry expression in Ad-M3C was not consequence of toxicity by virus infection itself but due to some other factors. Similar mechanisms may be responsible for the decline in the expression of viral transgenes.
Insulin secretion and cellular content
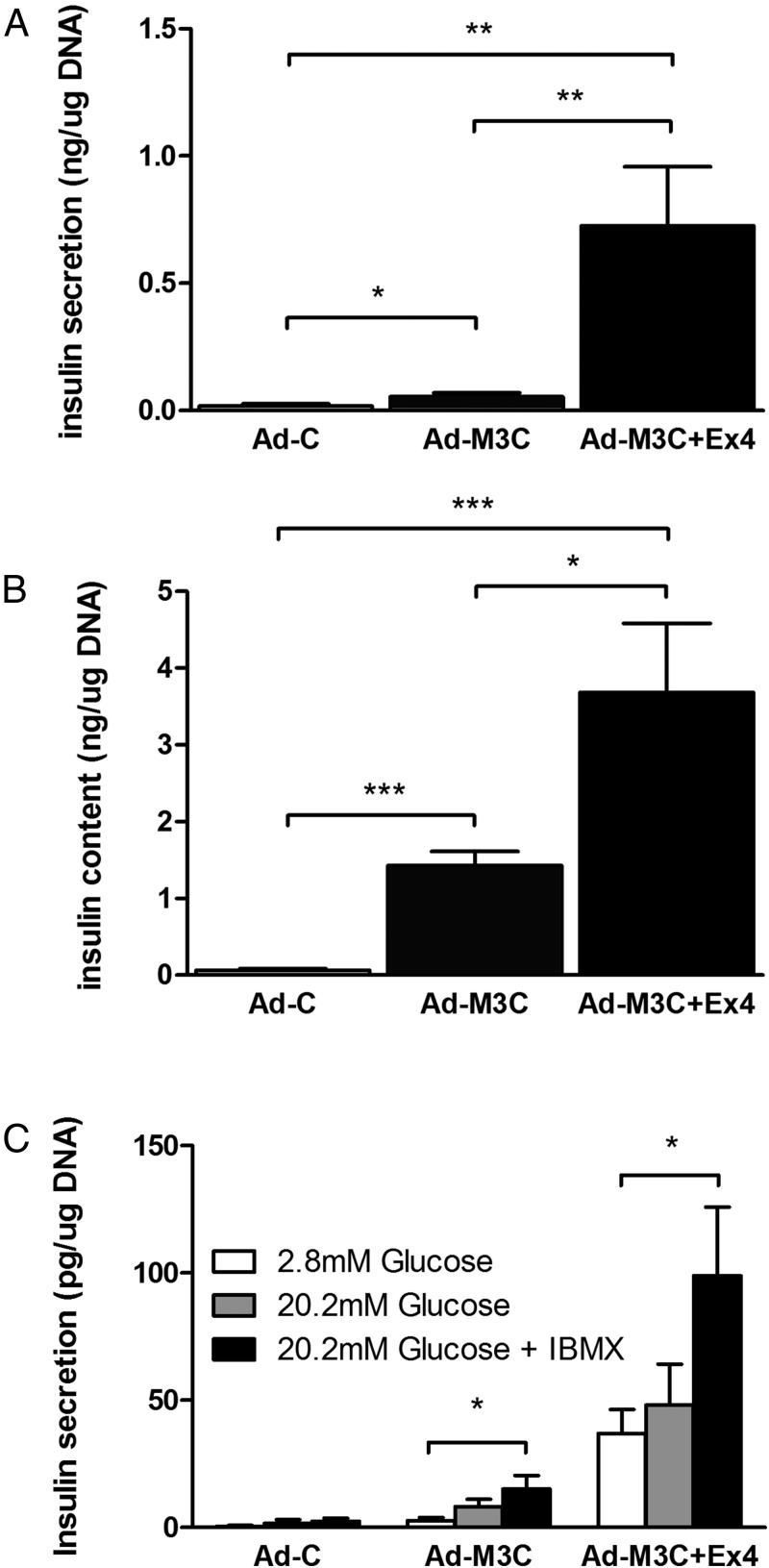
Insulin was released into the media over 24 hours from the Ad-M3C-transduced cells and this release was markedly enhanced by Ex-4 (Figure 5A). The insulin content of these cells mirrored the release pattern although the effect of Ex-4 was more impressive for release than cellular content (Figure 5B). In static incubations of 60 minutes to assess GSIS, no significant response to glucose was found (Figure 5C), but Ex-4 was able to increase secretion in the presence of glucose low (2.8mM) and high (20.2mM) glucose concentrations. The lack of GSIS might be attributed to incomplete reprogramming or immaturity.
Figure 5.
Ex-4 enhanced both insulin secretion and content of cells transduced with Ad-M3C. Ad-C-transduced cells, Ad-M3C-transduced cells, and Ad-M3C-transduced cells cultured with Ex-4 for 7 days, then the amount of insulin secreted into the media (A) and the insulin content of the cells (B) were measured by ELISA. Media were changed every 24 hours, and insulin levels were measured just before media change. Values were normalized to the total amount of DNA content in each well. Mean ± SEM, 8 independent experiments (each with duplicates); *, P < .05; **, P < .01; ***, P < .001 compared with Ad-C. C, GSIS in static incubation of Ad-C-treated PDCs, Ad-M3C-treated PDCs, and Ad-M3C-treated PDCs in combination with Ex-4 at day 7 of transduction. Cells were incubated for 1 hour either with low glucose (2.8mM), high glucose (20.2mM), or high glucose with 1-methyl-3-isobutylxanthine (IBMX) (1mM). Mean ± SEM, 4 independent experiments (each with duplicates). *, P < .05; **, P < .01.
Discussion
Our results provide evidence that cultured adult pancreatic cells with a duct phenotype can be reprogrammed in vitro into cells sharing some characteristics of pancreatic β-cells by delivering 3 transcription factors, Pdx1, Ngn3, and MafA, in a single polycistronic adenoviral vector. It had previously been shown that injection of separate adenoviral monocistronic vectors, each encoding one of these 3 transcription factors, into mouse pancreas can reprogram exocrine cells to insulin-producing β-cells in sufficient numbers to ameliorate diabetes (16). These 3 factors are known to play important roles in the development of pancreas and β-cells during embryogenesis (6, 24). In this in vivo study, it was thought that only acinar cells were reprogrammed, but our in vitro results raise questions about whether duct cells were also reprogrammed.
In the present study, we employed a new polycistronic adenoviral vector encoding all 3 pancreatic transcription factors (Ngn3/Pdx1/MafA) (17) and found that these 3 transcription factors can reprogram cultured cells with a duct phenol type to β-like cells. Our results indicate that transient expression of exogenous transgenes from a nonintegrating adenoviral vector is sufficient to induce partial reprogramming to β-cells in vitro. In the present study, more than 40% of mCherry+ cells became GFP+ cells 4 days after Ad-M3C transduction. We observed faster and higher frequency of reprogramming than our previous experiments using separate monocistronic vectors (up to 20% at d 10) (16). This may be due to differences in vector efficiency and between in vitro and in vivo reprogramming. It has been reported that mature exocrine cells can turn on endocrine programs when dissociated and cultured in vitro, and that dissociation itself could enhance cell plasticity to initiate endocrine programs (13, 14, 25, 26). Also, better efficiency from a single polycistronic adenoviral vector probably results from the 3 different transcription factors being simultaneously expressed at similar levels within individual cells, rather than the heterogeneous transduction that must occur with separate vectors. In addition, there could be a decrease in viral toxicity because a lower dose of vectors may be sufficiently efficient.
Approximately 40%, but not all of the Ad-M3C-transduced mCherry+ cells, became GFP+ insulin-producing cells, which raises interesting questions about variability in reprogramming efficiency among different cells. In this experiment, we used the primary duct cells isolated from adult mice pancreas as a potential cell source to generate new β-cells. During development, both pancreatic exocrine and endocrine cells are derived from progenitor cells that bud from the gut endoderm (6, 24). Our previous reports provided evidence that adult duct cells act as multipotent progenitors that can form new exocrine and endocrine cells (8, 9, 27), and that new islets can be generated from human duct cells under an optimal condition in vitro (13, 14). Here, we showed that GFP+ insulin-producing cells appeared 2 days after in vitro Ad-M3C transduction. This finding is consistent with previous work showing that new insulin+ cells were detected 3 days after in vivo injection of the 3 separate adenoviral vectors (16). This reprogramming seems more efficient than what is required to convert fibroblasts to iPS cells, which has recently improved to approach 10% (28), but we have not obtained a full mature β-cell phenotype. In a preliminary study, we failed to induce in vitro reprogramming of MIP-GFP mice-derived skin fibroblasts to GFP+ insulin-producing β-cells using our polycistronic adenoviral vector (data not shown). This improved efficiency may be due to the common origin of pancreatic duct and acinar cells that may lead to their retaining similar epigenetic memory from pancreatic development. The insulin content of the overall population of treated cells was less than 1% of what is found in normal β-cells (about 4 pg of insulin per 1-pg DNA) (29), but some of the individual reprogrammed cells no doubt had a higher content.
There was a recent report of primary mouse gall bladder epithelial cells being reprogrammed ex vivo to β-like cells using adenoviral-mediated expression of Neurogenin-3 (NEUROG3), Pdx1, and MafA (30). Other reports have described in vitro reprogramming of AR42J cells to β-like cells using pancreatic transcription factors (31, 32). AR42J cells are a rat pancreatic exocrine cell line, originally derived from a chemically induced pancreatic tumor (33). Pancreatic transcription factors, including Ngn3, Pdx1, and MafA, were sufficient to produce insulin-producing β-cells but not differentiated enough for GSIS. About 70% of the transduced AR42J cells were reported as insulin positive using a polycistronic adenoviral vector with 3 factors, which complements our findings with cultured duct cells; it must be remembered, however, that a cell line is being compared with very different primary duct cells. Progress has also been made using dedifferentiated cells obtained originally from enzyme digested human pancreases. These cells were then adenovirally transduced with 4 transcription factors, Pdx1, Ngn3, MafA, and Pax4, and then differentiation towards a β-cell phenotype was enhanced by inhibition of epithelial-mesenchymal transition (EMT) and TGF-β signaling (34). Conversion of human cells with duct like characteristics to a β-cell-like phenotype has also been accomplished with a cocktail of adenoviruses expressing Pdx1, Ngn3, MafA, and Pax6 instead of Pax4 (35).
An important issue is whether the transduced cells are of duct origin or whether they are transdifferentiated acinar cells that have assumed a duct-like phenotype having lost amylase expression and gained that of CK. The transdifferentiation question has received a great deal of attention (36, 37). Our conclusion is that both types of cells are probably being transduced, and that the original duct cells are likely in the majority. We base this on the remarkable die-off of acinar cells (22, 38) and the well-documented ability of primary duct cells to survive in tissue culture (14). Using lineage tracing with human pancreatic tissue, Houbracken et al concluded that after 7 days in culture that only 18% of the cells with a ductal phenotype were derived from acinar cells (22). Yet in our study, 45% of the Cherry+ cells were also positive for GFP. With our overall transduction efficiency of 20%, it seems highly likely that cells derived from acinar cells could only account for a small proportion of the 8.6% reprogramming found for the entire preparation.
GLP-1 can enhance insulin gene expression and GSIS, induce proliferation and maturation in the pancreas, and also promote β-cell survival (39). Other studies have reported that GLP-1 can enhance β-cell differentiation from rodent PDCs and ES cells (40). In our study, GLP-1 receptor expression was up-regulated in the reprogrammed cells and administration of the GLP-1 receptor agonist, Ex-4, resulted in increased insulin message, secretion, and content but not GSIS. One must wonder whether further maturation of these cells could be enhanced by residence in an in vivo environment, as has been shown with human embryonic stem cells (3, 4). This possibility could be examined in the future by transplanting these cells into syngeneic mice. Gastric inhibitory polypeptide (GIP) is another incretin secreted from gastrointestinal tract, which stimulates insulin secretion from islets. In future studies it will be of interest to assess the expression of GIP receptor in reprogrammed β-like cells.
In summary, cultured adult PDCs can be directly reprogrammed into insulin-producing cells with β-cell characteristics by adenoviral delivery of 3 pancreatic transcription factors, Ngn3, Pdx1, and MafA. Moreover, Ex-4 could enhance this process. Although this reprogramming approach was unable to generate true β-cells, the prospect of eventual success raises the possibility that patient-specific exocrine cells could someday be expanded and reprogrammed in vitro to provide new β-cells that can be transplanted. The prospects for expanding duct cells in culture seem much better than for acinar cells, which fare very poorly in culture (13). This study provides novel insights into the reprogramming of cultured cells with a duct phenotype, which could become a new approach to β-cell replacement therapy for diabetes.
Acknowledgments
We thank Jennifer Hollister-Lock, Vaja Tchipashvili, Brooke Morris Sullivan, and Chris Cahill (Joslin Diabetes Center) for their expert technical assistance. We also thank Dr Christopher Wright (Vanderbilt University) for the kind gift of Pdx-1 antibody.
This work was supported by grants from the Juvenile Diabetes Research Foundation and the Hahnemann Hospital Foundation (G.C.W.), by National Institutes of Health Grants R01 DK066056 and DK093909 (to S.B.-W.) and P30 DK036836 (Joslin Diabetes Research Center and its Advanced Microscopy Core), and by the Diabetes Research and Wellness Foundation.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- Ad-C
- Ad-mCherry
- Ad-M3C
- Ngn3/Pdx1/MafA/mCherry
- CK
- cytokeratin
- ESC
- embryonic stem cell
- Ex-4
- exendin-4
- FBS
- fetal bovine serum
- GFP
- green fluorescent protein
- GIP
- gastric inhibitory polypeptide
- GLP-1
- glucagon-like peptide-1
- GSIS
- glucose-stimulated insulin secretion
- iPS
- induced pluripotent stem
- MIP
- mouse insulin 1 promoter
- Ngn3
- neurogenin 3
- PDC
- pancreatic duct cell
- Pdx1
- pancreatic and duodenal homeobox gene 1
- RT
- room temperature.
References
- 1. Weir GC, Bonner-Weir S. Islet β cell mass in diabetes and how it relates to function, birth, and death. Ann NY Acad Sci. 2013;1281:92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Alejandro R, Barton FB, Hering BJ, Wease S. 2008 update from the Collaborative Islet Transplant Registry. Transplantation. 2008;86(12):1783–1788. [DOI] [PubMed] [Google Scholar]
- 3. Kroon E, Martinson LA, Kadoya K, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26(4):443–452. [DOI] [PubMed] [Google Scholar]
- 4. Rezania A, Bruin JE, Riedel MJ, et al. Maturation of human embryonic stem cell-derived pancreatic progenitors into functional islets capable of treating pre-existing diabetes in mice. Diabetes. 2012;61(8):2016–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alipio Z, Liao W, Roemer EJ, et al. Reversal of hyperglycemia in diabetic mouse models using induced-pluripotent stem (iPS)-derived pancreatic β-like cells. Proc Natl Acad Sci USA. 2010;107(30):13426–13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140(12):2472–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Halban PA, German MS, Kahn SE, Weir GC. Current status of islet cell replacement and regeneration therapy. J Clin Endocrinol Metab. 2010;95(3):1034–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Inada A, Nienaber C, Katsuta H, et al. Carbonic anhydrase II-positive pancreatic cells are progenitors for both endocrine and exocrine pancreas after birth. Proc Natl Acad Sci USA. 2008;105(50):19915–19919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guo L, Inada A, Aguayo-Mazzucato C, et al. PDX1 in ducts is not required for postnatal formation of β-cells but is necessary for their subsequent maturation. Diabetes. 2013;62(10):3459–3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rovira M, Scott SG, Liss AS, Jensen J, Thayer SP, Leach SD. Isolation and characterization of centroacinar/terminal ductal progenitor cells in adult mouse pancreas. Proc Natl Acad Sci USA. 2010;107(1):75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Furuyama K, Kawaguchi Y, Akiyama H, et al. Continuous cell supply from a Sox9-expressing progenitor zone in adult liver, exocrine pancreas and intestine. Nat Genet. 2011;43(1):34–41. [DOI] [PubMed] [Google Scholar]
- 12. Al-Hasani K, Pfeifer A, Courtney M, et al. Adult duct-lining cells can reprogram into β-like cells able to counter repeated cycles of toxin-induced diabetes. Dev Cell. 2013;26(1):86–100. [DOI] [PubMed] [Google Scholar]
- 13. Bonner-Weir S, Taneja M, Weir GC, et al. In vitro cultivation of human islets from expanded ductal tissue. Proc Natl Acad Sci USA. 2000;97(14):7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yatoh S, Dodge R, Akashi T, et al. Differentiation of affinity-purified human pancreatic duct cells to β-cells. Diabetes. 2007;56(7):1802–1809. [DOI] [PubMed] [Google Scholar]
- 15. Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 16. Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455(7213):627–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li W, Cavelti-Weder C, Zhang Y, et al. Long-term persistence and development of induced pancreatic β cells generated by lineage conversion of acinar cells. Nat Biotechnol. 2014;32(12):1223–1230. [DOI] [PubMed] [Google Scholar]
- 18. Hara M, Wang X, Kawamura T, et al. Transgenic mice with green fluorescent protein-labeled pancreatic β-cells. Am J Physiol Endocrinol Metab. 2003;284(1):E177–E183. [DOI] [PubMed] [Google Scholar]
- 19. Gotoh M, Maki T, Kiyoizumi T, Satomi S, Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation. 1985;40:437–438. [DOI] [PubMed] [Google Scholar]
- 20. Li W, Nakanishi M, Zumsteg A, et al. In vivo reprogramming of pancreatic acinar cells to three islet endocrine subtypes. Elife. 2014;3:e01846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29(9):e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Houbracken I, de Waele E, Lardon J, et al. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011;141(2):731–741, 741.e731–734. [DOI] [PubMed] [Google Scholar]
- 23. Minami K, Okuno M, Miyawaki K, et al. Lineage tracing and characterization of insulin-secreting cells generated from adult pancreatic acinar cells. Proc Natl Acad Sci USA. 2005;102(42):15116–15121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240(3):530–565. [DOI] [PubMed] [Google Scholar]
- 25. Baeyens L, De Breuck S, Lardon J, Mfopou JK, Rooman I, Bouwens L. In vitro generation of insulin-producing β cells from adult exocrine pancreatic cells. Diabetologia. 2005;48(1):49–57. [DOI] [PubMed] [Google Scholar]
- 26. Stanger BZ, Hebrok M. Control of cell identity in pancreas development and regeneration. Gastroenterology. 2013;144(6):1170–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li WC, Rukstalis JM, Nishimura W, et al. Activation of pancreatic-duct-derived progenitor cells during pancreas regeneration in adult rats. J Cell Sci. 2010;123(pt 16):2792–2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Anokye-Danso F, Trivedi CM, Juhr D, et al. Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell stem cell. 2011;8(4):376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Weir GC, Halban PA, Meda P, Wollheim CB, Orci L, Renold AE. Dispersed adult rat pancreatic islet cells in culture: A, B, and D cell function. Metabolism. 1984;33(5):447–453. [DOI] [PubMed] [Google Scholar]
- 30. Hickey RD, Galivo F, Schug J, et al. Generation of islet-like cells from mouse gall bladder by direct ex vivo reprogramming. Stem Cell Res. 2013;11(1):503–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Akinci E, Banga A, Greder LV, Dutton JR, Slack JM. Reprogramming of pancreatic exocrine cells towards a β (β) cell character using Pdx1, Ngn3 and MafA. Biochem J. 2012;442(3):539–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lima MJ, Docherty HM, Chen Y, Docherty K. Efficient differentiation of AR42J cells towards insulin-producing cells using pancreatic transcription factors in combination with growth factors. Mol Cell Endocrinol. 2012;358(1):69–80. [DOI] [PubMed] [Google Scholar]
- 33. Longnecker DS, Lilja HS, French J, Kuhlmann E, Noll W. Transplantation of azaserine-induced carcinomas of pancreas in rats. Cancer Lett. 1979;7(4):197–202. [DOI] [PubMed] [Google Scholar]
- 34. Lima MJ, Muir KR, Docherty HM, et al. Suppression of epithelial-to-mesenchymal transitioning enhances ex vivo reprogramming of human exocrine pancreatic tissue toward functional insulin-producing β-like cells. Diabetes. 2013;62(8):2821–2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lee J, Sugiyama T, Liu Y, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife (Cambridge). 2013;2:e00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lemper M, Leuckx G, Heremans Y, et al. Reprogramming of human pancreatic exocrine cells to β-like cells [published online December 5, 2014]. Cell Death Differ. doi:10.1038/cdd.2014.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pinho AV, Rooman I, Reichert M, et al. Adult pancreatic acinar cells dedifferentiate to an embryonic progenitor phenotype with concomitant activation of a senescence programme that is present in chronic pancreatitis. Gut. 2011;60(7):958–966. [DOI] [PubMed] [Google Scholar]
- 38. Korbutt GS, Elliott JF, Ao Z, Smith DK, Warnock GL, Rajotte RV. Large scale isolation, growth, and function of porcine neonatal islet cells. J Clin Invest. 1996;97:2119–2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. [DOI] [PubMed] [Google Scholar]
- 40. Xu G, Kaneto H, Lopez-Avalos MD, Weir GC, Bonner-Weir S. GLP-1/Ex-4 facilitates β-cell neogenesis in rat and human pancreatic ducts. Diabetes Res Clin Pract. 2006;73(1):107–110. [DOI] [PubMed] [Google Scholar]