Abstract
Circadian rhythms govern homeostasis and organism physiology. Nutritional cues act as time givers, contributing to the synchronization between central and peripheral clocks. Neuronal food-synchronized clocks are thought to reside in hypothalamic nuclei such as the ventromedial hypothalamus (VMH) and the dorsomedial hypothalamus or extrahypothalamic brain areas such as nucleus accumbens. Interestingly, the metabolic sensor of nicotinamide adenine dinucleotide-dependent deacetylase sirtuin-1 (SIRT1) is highly expressed in the VMH and was shown to contribute to both control of energy balance and clock function. We used mice with targeted ablation of Sirt1 in the steroidogenic factor 1 neurons of the VMH to gain insight on the role played by this deacetylase in the modulation of the central clock by nutritional inputs. By studying circadian behavior and circadian gene expression, we reveal that SIRT1 operates as a metabolic sensor connecting food intake to circadian behavior. Indeed, under food restriction and absence of light, SIRT1 in the VMH contributes to activity behavior and circadian gene expression in the suprachiasmatic nucleus. Thus, under specific physiological conditions, SIRT1 contributes to the modulation of the circadian clock by nutrients.
Synchronization of the central and peripheral clocks is essential to maintain cellular and body homeostasis. Studies in humans have shown the association between short sleep duration and feeding time and the development of metabolic unbalances (1–4). Circadian clocks are organized hierarchically. The central clock is localized in the suprachiasmatic nucleus (SCN) of the hypothalamus, whereas additional neuronal clocks are present in other hypothalamic nuclei, including the arcuate nucleus (ARC), the ventromedial hypothalamus (VMH), and the dorsomedial hypothalamus, as well as extrahypothalamic brain areas such as the hippocampus (5–7). Peripheral tissues including the liver, intestine, and pancreas also have intrinsic clocks (8, 9). Central and peripheral clocks are synchronized by environmental cues or zeitgebers. The most effective zeitgeber is light, whose signal is transmitted to the SCN via the retinohypothalamic tract. Significantly, nutritional inputs such as feeding time and diet composition can also synchronize the clock (10–13). Although the light-entrainable oscillator is located in the SCN, the exact localization of the brain food-entrainable oscillators remains a subject of debate (14). Several hypothalamic nuclei such as the VMH and dorsomedial hypothalamus and extrahypothalamic areas including the nucleus accumbens and the amygdala have been hypothesized as composing the food entrainable oscillator (14). Although some of the molecular pathways by which light synchronizes the central clock have been elucidated (15, 16), the mechanisms as well as the molecular components involved in nutrient inputs as synchronizers of the circadian clock in the brain remain mostly unexplored. In this regard, a number of metabolic sensors including sirtuin-1 (SIRT1), AMP-activated protein kinase, and mammalian target of rapamycin have been implicated in the modulation of both hypothalamic responses and circadian regulation in peripheral tissues. However, whether these molecular sensors modulate the hypothalamic clocks remains to be elucidated (17).
In the brain, steroidogenic factor 1 (Sf1)-expressing neurons are exclusively located in the VMH and are required for the regulation of energy balance and glucose metabolism (18, 19). The VMH has efferent projections to the SCN, and VMH lesions dampen feeding circadian rhythms, corticosterone release, and circadian behavioral activity (20, 21). SIRT1, a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase for both histone and nonhistone proteins, is involved in gene expression, cellular metabolism, and aging (22–24). As NAD+ levels oscillate in a circadian manner, SIRT1 enzymatic activity oscillates as well (24, 25). Furthermore, fasting induces hypothalamic Sirt1 expression and increases NAD+ levels, suggesting that SIRT1 activity fluctuates within feeding/fasting cycles (26, 27). Remarkably, SIRT1 itself controls the clock by directly deacetylating the brain and muscle Arnt-like protein-1 (BMAL1) and period-2 (PER2) (22, 28). Hence, we hypothesized that SIRT1 may contribute as a metabolic sensor for the circadian system by relaying nutritional information to the central clock. We show that mice with a targeted ablation of the Sirt1 gene in Sf1 neurons of the hypothalamus (18) display a reduction in the period length in the involuntary light entrainable activity under constant darkness and scheduled feeding. Moreover, these effects are accentuated in mice fed a high-caloric diet. Importantly, expression profiles of several core clock and clock-controlled genes revealed changes in the acrophase and amplitude in the SCN and in a lesser degree in the VMH. Taken together, our results indicate that the NAD+-dependent deacetylase SIRT1 in the Sf1 neurons modulates the endogenous clock by nutritional inputs.
Materials and Methods
Animals
Animals and protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee of the University of California, Irvine. To generate animals with Sirt1 ablation in Sf1 neurons, Sirt1loxP/loxP mice were crossed with Sf1-Cre;Sirt1loxP/loxP mice (19). To verify the genotype, tails were cut and the DNA was extracted by alkaline method, and the DNA was amplified by PCR. Six-month-old male mice were housed in individual cages and fed either standard diet (SD) or high-fat (HF) diet containing 60% kcal from fat (D12492; Research Diets), starting 15 days before the experiment and throughout the experiment. Animals were euthanized using CO2 asphyxiation.
SIRT1 immunohistochemistry on brain sections
Mice were perfused with 10% neutral buffered formalin, brains were sectioned on a microtome, and immunohistochemistry was performed on free-floating brain sections. Sections were washed in PBS (27.6 g sodium phosphate, dibasic + 160 g sodium chloride in 20 L distilled H2O at pH 7.4)-azide (0.8 g of sodium azide in 4 L of PBS) three times for 10 minutes each. Sections were then incubated in 0.3% H2O2 for 30 minutes to block the endogenous peroxidase activity and subsequently rinsed in PBS three times for 10 minutes each. All sections were then placed in blocking solution of 3% normal donkey serum in PBT-azide (2.5 mL of Triton X-100 in 1000 mL of PBS-azide) for 2 hours. Sections were then incubated overnight at 4°C into 1:1000 rabbit anti-SIRT1 primary antisera (29) in PBT-azide containing 3% (wt/vol) normal donkey serum. Sections were then rinsed with PBS six times for 10 minutes each and then transferred for 2 hours into 1:1000 biotin-conjugated donkey antirabbit secondary antisera (Jackson ImmunoResearch; 711–065-152) in PBT containing 3% (wt/vol) normal donkey serum and subsequently rinsed in PBS three times for 10 minutes each. Avidin biotin complex solution (Vectastain Elite PK-6100 ABC kit; Vector Laboratories) was prepared (1:500 in PBS), and after 30 minutes, brain sections were incubated in this solution for 1 hour and subsequently rinsed in PBS two times for 10 minutes each. Sections were then incubated in 0.04% 3,3′-diaminobenzidine and 0.01% H2O2 in PBS for 8 minutes and mounted on gelatin coated glass slides for visualization.
Circadian behavior
Locomotor activity was detected using optical beam motion detection (Philips Respironics). Data were collected using the Minimitter VitalView data acquisition system, version 5.0. The tau values corresponding to light entrainable behavior was calculated by obtaining the slopes at the onset of the free activity component and then computed using Clocklab software (Actimetrics) by the least-squares fits method, and by χ2 periodogram method.
SCN and VMH dissection
Brains were cut in serial coronal sections of 100 μM and 30 μM, respectively (see Supplemental Figure 1A) using a cryostat (Leyca). The 100-μM sections were stored at −80°C to be dissected afterward, and the 30-μM sections were Nissl stained and used as guides to identified and accurately dissect the SCN and VMH in each brain. The SCN and the VMH nuclei were dissected with microflat needles in a microscope in frozen conditions with dry ice. Total RNA was extracted from each sample using TRIzol (Invitrogen) following the manufacturer's instructions scaled 1:10. Total RNA was resuspended in 10 μL of ribonuclease-free water and quantified in a nano-drop. cDNA was synthesized from 200 ng of RNA using the cDNA synthesis kit i-Script (Bio-Rad Laboratories). The obtained cDNA was then diluted 1:10 and 5 μL were used as template for PCR amplification using SYBR Green (Bio-Rad Laboratories) as fluorogenic intercalating dye and the CFX96 real-time system (Bio-Rad Laboratories). We used β-actin as a housekeeping gene. The primers used for the amplification are presented (Supplemental Table 1).
Data analysis
Experimental results are expressed as means ± SEM with six animals per group and six animals per time point. An unpaired Student's t test was used to compare two groups from a different genotype or the same genotype. To compare the differences between days and genotype in animals exposed to constant darkness (DD), data were analyzed by a two-way ANOVA followed by a Bonferroni's post hoc test for multiple comparisons. For circadian analysis of behavior and gene expression, the time series data were analyzed by a one-way ANOVA followed by a Bonferroni's post hoc test for multiple comparisons. Slopes were compared by a two-tailed test. Circadian parameters were obtained using the Time Series Analysis Seriel Cosinor 6.3 (http://www.euroestech.com/). First, we determined the correct distribution (data interdependency and normal distribution) for each time series data by lag plots/Q-test and normal probability plots/K-S test (Supplemental Figure 2), and then we calculated the acrophase and amplitude by the Cosinor method using a period of 24 hours (30). The values for each animal and each gene were averaged and analyzed statistically.
Results
Sf1-Cre;Sirt1loxP/loxP mice show decreased levels of SIRT1 in Sf1 neurons
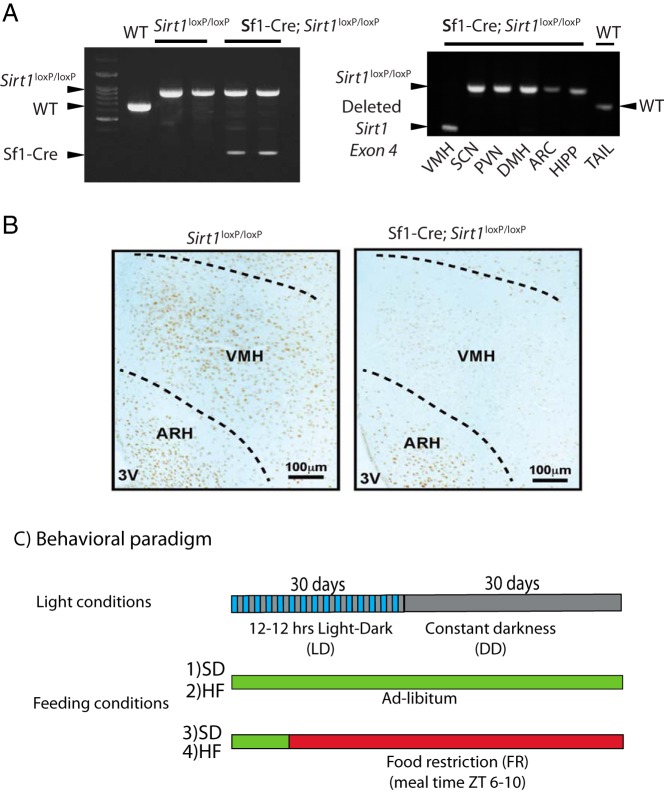
The expression of the CRE recombinase under the control of the stereoidogenic factor 1 promoter, which is specifically expressed in the Sf1 neurons in mice containing the allele loxP-flanked exon 4 of Sirt1, yields a Sirt1-null allele (Sf1-Cre;Sirt1loxP/loxP). As a consequence, these transgenic mice do not express SIRT1 in the Sf1-neurons of the VMH (18, 19). We confirmed the genotype of the Sf1-Cre;Sirt1loxP/loxP mice using primers targeting sequences upstream and downstream of the loxP flanked Sirt1 exon 4 and primers targeting the Cre gene. Sirt1loxP/loxP mice show the recombinant allele Sirt1 flanked by the loxP sequences, whereas the Sf1-Cre;Sirt1loxP/loxP show an additional band corresponding to the CRE recombinase (Figure 1A, left panel). To further validate the specific deletion of Sirt1, we extracted DNA from different hypothalamic nuclei and hippocampus and used primers flanking the loxP sequences for amplification. Ablation of exon 4 was present only in the VMH, confirming Sf1 neurons-specific deletion (Figure 1A, right panel).
Figure 1.
Sf1-Cre;Sirt1loxP/loxP mice show decreased levels of SIRT1 in Sf1 neurons. A, Left panel, Multiplex PCR genotyping showing the amplified DNA from the mouse tails using primers flanking the loxP sequences on the Sirt1 exon 4 allele and primers targeting the transgenic CRE allele. Right panel, PCR from DNA extracted from microdissected hypothalamic nuclei (see Materials and Methods and Supplemental Figure 1) using primers targeting the loxP sequences on the Sirt1 exon 4 allele. HIPP, hippocampus; WT, wild type. B, Representative micrographs of brains from Sirt1loxP/loxP and Sf1-Cre;Sirt1loxP/loxP immunohistochemistry using specific antibodies anti-SIRT1. Dark brown staining shows SIRT1-positive neurons displaying the reduction of SIRT1-positive neurons in the VMH from the Sf1-Cre;Sirt1loxP/loxP mice. C, Behavioral analysis. The first bar denotes the two consecutive periods of LD (interspersed blue/gray bar) followed by the DD period (gray bar). During these periods, Sirt1loxP/loxP and Sf1-cre;Sirt1loxP/loxP mice were fed either an SD or a HF diet in ad libitum (green bar) or food restriction (red bar).
We determined the RNA expression levels of Sirt1 by microdissecting the SCN and VMH (see Materials and Methods and Supplemental Figure 1, A and B). Sirt1 expression is significantly decreased in the VMH of the Sf1-Cre;Sirt1loxP/loxP mice compared with the expression levels in the SCN (Supplemental Figure 1B). To further determine the SIRT1 levels in the VMH, immunohistochemistry analyses were carried out in brain slices from Sirt1loxP/loxP and Sf1-Cre;Sirt1loxP/loxP using SIRT1-specific antibodies (29). As previously demonstrated (19), the number of SIRT1-positive cells in the VMH of Sf1-Cre;Sirt1loxP/loxP mice is drastically reduced as compared with the Sirt1loxP/loxP. Not surprisingly, some remaining positive cells still remain (Figure 1B) because few VMH neurons do not express Sf1 (18, 19). Also, using a variety of experimental approaches, it has been demonstrated that ablation of SIRT1 is highly specific in these mice, being exclusive to the Sf1 neurons (19). Collectively these results together with previous reports (18, 19) show that SIRT1 levels in the Sf1 neurons of the VMH are extensively decreased in Sf1-Cre;Sirt1loxP/loxP mice. We have also reasoned that ablation of Sirt1 in Sf1-expressing cells could target nonneuronal tissues. These may include the pituitary gland, testis, ovary, and adrenal gland. However, extensive analysis has demonstrated that Sf1-Cre;Sirt1loxP/loxP mice display no phenotypic defects in the hypothalamic-pituitary-adrenal/gonadal axis, including fertility, gonadal weight, serum T levels, circadian fluctuations, and stress-induced levels of corticosterone (19). Hence, the findings reported here are related to a role of SIRT1 in VMH neurons.
SIRT1 in Sf1 neurons does not determine circadian behavior of mice fed ad libitum
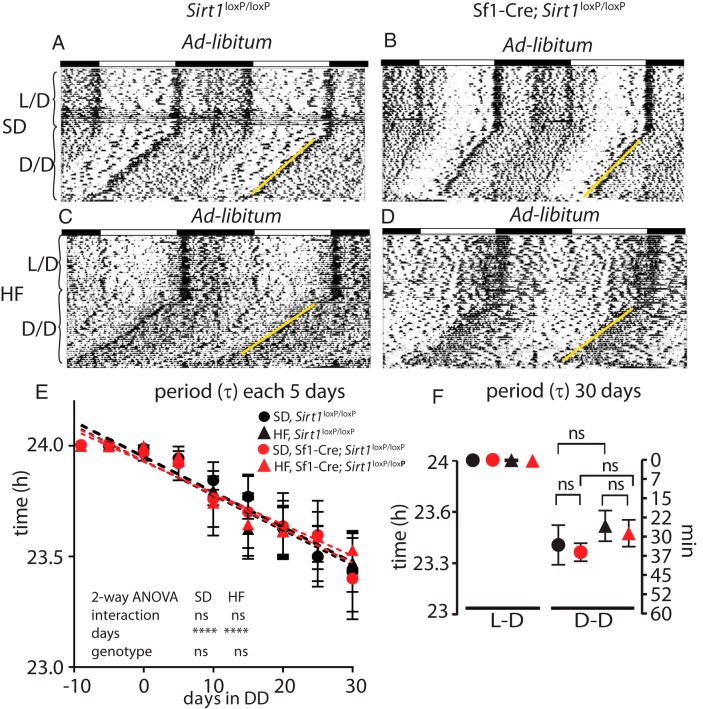
To determine whether the lack of SIRT1 in the Sf1 neurons causes an effect on circadian behavior, Sf1-Cre;Sirt1loxP/loxP mice were exposed to 12-hour light, 12-hour dark (LD) cycles followed by DD. During this experiment food was available ad libitum (Figure 1C). It has been shown that rodents show variability in the phase shift effect across the days on constant darkness (31). Hence, we calculated the endogenous period length measuring the τ-value of the light-entrainable activity (which starts at the onset of the subjective night) each 5 days, starting 10 days prior the DD, until day 30. Furthermore, because a HF diet inhibits SIRT1 expression and reprograms the endogenous clock (24, 25, 32, 33), we sought to determine how HF would influence the endogenous clock in both groups of animals. The expected period shortening was observed under DD and ad libitum feeding (Figure 2, A–D), as reflected by the decrease in (τ) value (32, 33), in both genotypes and regardless of the diet (either SD or HF) [day effect in SD (F [9, 100] = 7.719, P < .0001); HF (F [9, 100] = 12.5, P < .0001), two-way ANOVA]. No genotype effect was found (Figure 2E). Also, measuring this value at the end of the constant darkness, no differences were found between the two different genotypes (Figure 2F), indicating that the reduction in the period length triggered by the absence of light is not dependent on SIRT1 in Sf1 neurons. To further characterize the rhythmic behavioral responses, we calculated the endogenous period by using χ2 periodograms and the acrophase (defined as the time point at which the fitted cosine reaches maximum value) by using the Cosinor analysis (see Materials and Methods). These parameters confirmed the lack of significant differences between the genotypes (Supplemental Figure 3, A and B, ad libitum, and Supplemental Figure 3C).
Figure 2.
The circadian behavior of Sf1-Cre;Sirt1loxP/loxP mice is not altered under ad libitum feeding. Double-plotted actograms measuring light-entrainable activity, showing that under DD and ad libitum feeding, both, the Sirt1loxP/loxP (A and C) and Sf1-Cre;Sirt1loxP/loxP (B and D) feed, SD (A and B) or HF diet (C and D) show no differences in the onset of the light-entrainable activity (yellow lines). The plots show the progression of the light-entrainable activity measured as τ-value through 10 days before exposure on DD and 30 days thereafter. The genotype has no effect on the τ, acrophase, or period (see Supplemental Figure 3, A and B, ad lib) on mice feed ad libitum through the DD exposure (E) and during 30 days on DD (F). Black circles, Sirt1loxP/loxP fed SD; black triangles, Sirt1loxP/loxP feed HF diet; red circles, Sf1-Cre;Sirt1loxP/loxP fed SD; red triangles, Sf1-Cre;Sirt1loxP/loxP feed HF diet (n = 6 animals per group).
Prolonged DD and food restriction alters circadian behavior of Sf1-Cre;Sirt1loxP/loxP mice
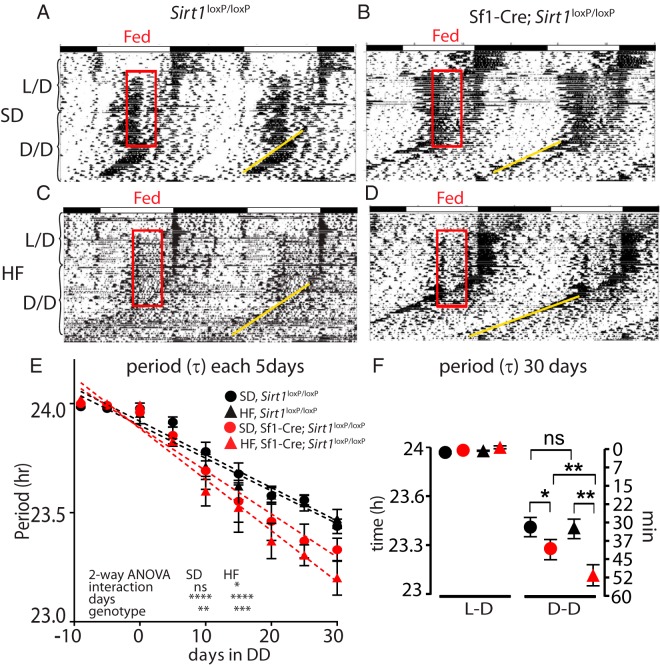
The SCN can be reset in mice subjected to food restriction (FR) (also known as feeding entrainment and scheduled feeding) or caloric restriction in DD (31, 34, 35). Thus, we reasoned that SIRT1 could play a role in synchronizing the clock in mice maintained in DD and fed for a restricted period of time. We exposed Sf1 neuron-specific SIRT1 mutant mice to a feeding-restricted schedule from zeitgeber time (ZT) 6 to ZT10, using an experimental paradigm analogous to those described above in ad libitum feeding conditions (Figure 1C). Under feeding entrainment, both Sirt1loxP/loxP and Sf1-Cre;Sirt1loxP/loxP mice developed food anticipatory activity and light-entrainable activity (13, 14, 31). However, when mice were exposed to HF, the food anticipatory activity was drastically reduced in both genotypes (Figure 3, A–D, and Supplemental Figure 4) (36). Analyzing the evolution of the (τ) value of the light entrainable activity in mice under FR and DD, we also found significant differences in the (τ) value (Figure 3E) [day effect SD (F [8, 63] = 41.18, P < .0001); HF (F [8, 90] = 49.54 P < .0001), two way ANOVA]. These were noticeable in mice fed the SD (Figure 3E) [genotype SD (F [1, 63] = 8.999 P = .0039)], although they became remarkably more pronounced in mice fed a HF diet [genotype HF (F [1, 90] = 15.82 P < .0001), two way ANOVA].
Figure 3.
Prolonged DD and FR alters the circadian behavior in the Sf1-Cre;Sirt1loxP/loxP mice. Double-plotted actograms measuring spontaneous free activity under DD and FR in the Sirt1loxP/loxP (A and C) and Sf1-Cre;Sirt1loxP/loxP (B and D) fed SD (A and B) or HF diet (C and D). Note the two behavioral components corresponding to the light-entrainable activity and the food anticipatory activity prior to the onset of subjective dark period and feeding period, respectively (see Supplemental Figure 4). The genotype shows a strong effect in the τ-value, acrophase, and period in mice under FR (E and F; see Supplemental Figure 3, A, B, D, and E). Under DD and FR, the reduction of the period (τ) is higher on the Sf1-cre;Sirt1loxP/loxP than the Sirt1loxP/loxP. This effect is enhanced on HF diet (F). *, P < .05, **, P < .001, ***, P < .001, two way-ANOVA followed by Bonferroni's post hoc test (n = 6 animals per group).
To gain insight into the effect mediated by HF, we calculated the ratio between the (τ) value at ad libitum conditions to the (τ) of mice in FR conditions. This analysis revealed strong differences between the Sirt1loxP/loxP and the Sf1-Cre;Sirt1loxP/loxP mice (SD groups, P = .00025; HF groups, P < .0001, two tailed test) (Supplemental Figure 3E). Remarkably, comparing the slopes from the mice fed either SD or HF within the same genotype, we observed a significant diet-mediated effect only in the Sf1-Cre;Sirt1loxP/loxP group (SD vs HF, P < .01, two tailed test).
Analyzing the period at the end of the experiment, without external zeitgebers (DD and ad libitum food), both Sirt1loxP/loxP and Sf1-Cre;Sirt1loxP/loxP mice show a period shortening of about 30 minutes. Under these conditions, no significant differences were revealed between the genotypes or diets (Figure 2F). When restricted feeding was administered under DD, Sirt1loxP/loxP animals maintained the same period shortening of 30 minutes, whereas the Sf1-Cre;Sirt1loxP/loxP mice showed a period shortening of 40 minutes (P < .05, two way ANOVA followed by Bonferroni's post hoc test) (Figure 3F). In HF the Sirt1loxP/loxP mice in HF maintained the same 30 minutes of period shortening, whereas the Sf1-Cre;Sirt1loxP/loxP mice decreased the period length by 51 minutes (P < .01, two way ANOVA followed by Bonferroni's post hoc test). Finally, we analyzed the diet effect between mice of the same genotype and found differences only within the Sf1-Cre;Sirt1loxP/loxP group (P < .01, two way ANOVA followed by Bonferroni's post hoc test).
Analysis of the acrophase by the Cosinor method or the period by χ2 periodogram starting at the end of the FR in DD revealed that Sf1-Cre;Sirt1loxP/loxP mice fed either a SD or a HF diet present a higher shift in both acrophase and period as compared with the Sirt1loxP/loxP mice (P < .05, t test) (Supplemental Figure 3, A and B, food restriction, and Supplemental Figure 3D). However, no differences were found between the Sf1-Cre;Sirt1loxP/loxP fed SD compared with those fed a HF diet. This apparent discrepancy is explained because the fitting line method to calculate the (τ) allows the calculation of the period from the onset of activity independently of additional behavioral components (eg, food anticipatory activity). Also, because infrared sensors measure overall activity (computed by the Cosinor and χ2 periodogram methods), it is possible that subtle behavioral effects may be masked.
Taken together, our data indicate that the endogenous clock is differentially affected in DD only under food restriction in the Sf1-Cre;Sirt1loxP/loxP as compared with the Sirt1loxP/loxP mice. It has been previously reported that Sf1-Cre;Sirt1loxP/loxP mice show metabolic disturbances when in HF (19). To exclude the effects of metabolic unbalance in our behavioral tests, we measured body weight and caloric intake throughout the experiment (Supplemental Figure 5, A and B). No significant differences between the Sf1-Cre;Sirt1loxP/loxP and the Sirt1loxP/loxP mice were found. Because Sf1-cre;Sirt1loxP/loxP mice show increased leptinemia on a HF diet, we determined whether levels of blood leptin are altered in our experimental conditions. Although all groups of mice feed a HF diet show an increase on leptinemia, no differences were found between the genotypes (Supplemental Figure 5C). This apparent discrepancy with previous reports is explained because the mice in our study were on a HF diet only for 6 weeks, a time not sufficient to reach the onset of metabolic disturbances, which are observed at 40 weeks in HF feeding (19).
Nutrient entrainment affects circadian gene expression in the SCN and VMH of Sf1-Cre;Sirt1loxP/loxP mice
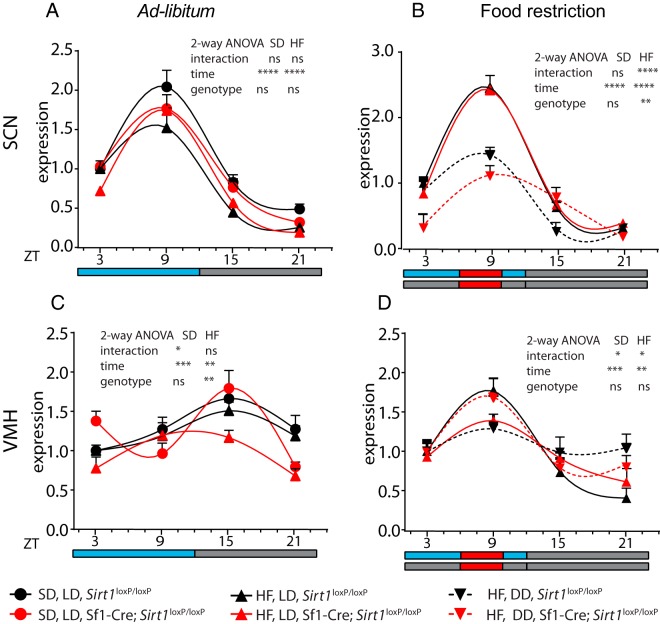
Expression of Per2 in the SCN can be shifted by light or feeding conditions (34, 37). Hence, we analyzed the expression pattern of Per2 in both SCN and VMH from Sirt1loxP/loxP and Sf1-Cre;Sirt1loxP/loxP mice during the circadian cycle. Because we analyzed the effect of food restriction as unique external zeitgeber, we used as referential zeitgeber the time corresponding to the feeding period (ZT6 to ZT 10) in all mice groups, including those killed in LD or DD (Supplemental Figure 1D) (31, 34). Importantly, in ad libitum-fed mice, Per2 expression is higher during the day in the SCN, whereas it peaks at night in the VMH as previously reported (7, 38) (Figure 4, A and C), further validating our analysis.
Figure 4.
Daily expression profile of Per2 is altered in the SCN and VMH of Sf1-cre;Sirt1loxP/loxP mice under FR. The nuclei were microdissected from the brain of the mice (see Supplemental Figure 1, A and D) killed every 6 hours and fed ad libitum (A and C) or FR (B and D). Black symbols, Sirt1loxP/loxP mice; red symbols, Sf1-cre;Sirt1loxP/loxP; circles and solid lines, SD and LD; triangles and solid lines, HF diet and LD; inverted triangles and dotted lines, HF diet, DD conditions. Significance between genotypes at specific ZT and experimental condition is indicated by the symbols: ψ, SD, LD; HF diet, LD. *, HF diet, DD; (one symbol, P < .05; two symbols, P < .01; three symbols, P < .001; two-way ANOVA followed by Bonferroni's test; n = 6 mice per group).
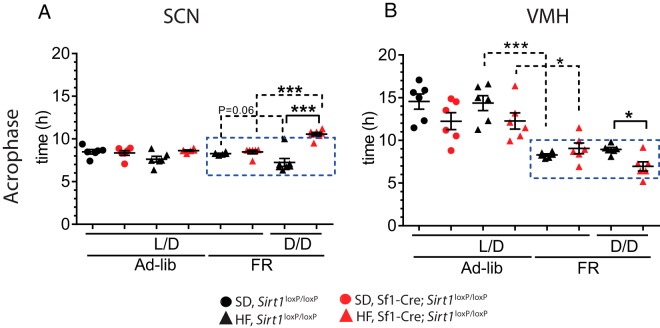
Gene expression features were determined using one-way ANOVA (Supplemental Table 2). The acrophase was then calculated using the Cosinor method (Supplemental Table 3). Because light is the dominant zeitgeber in the SCN, no differences were observed in the acrophase when comparing the Sirt1loxP/loxP vs Sf1-Cre;Sirt1loxP/loxP mice under LD conditions (Figure 5A). However, we observed a very significant difference of about 4 hours in the acrophase in the Sf1-Cre;Sirt1loxP/loxP mice with respect to Sirt1loxP/loxP mice (P < .0001, t test), when mice are in DD (Figure 5A and Supplemental Table 3). Per2 expression in the VMH is similarly affected in the acrophase under DD, amounting to a difference of 1.9 hours (P < .05) (Figure 5B and Supplemental Table 3). Then we compared the acrophase of mice in LD and DD for each genotype separately. Although we found that the acrophase in the SCN of the Sirt1loxP/loxP mice have a strong tendency to differ (P = .06, t test), the Sf1-Cre;Sirt1loxP/loxP mice show a highly significant difference (P < .0001, t test) (Figure 5A).
Figure 5.
The acrophase of Per2 expression is differentially affected in the SCN and the VMH of Sf1-cre;Sirt1loxP/loxP mice by food restriction in constant darkness. Black symbols, Sirt1loxP/loxP; red symbols, Sf1-Cre;Sirt1loxP/loxP; circles, SD; triangles, HF diet. Blue rectangle denotes the meal period. A, In the SCN, the acrophase of Per2 shows a phase delay in the Sf1-Cre;Sirt1loxP/loxP compared with the Sirt1loxP/loxP mice under DD, food restriction, and HF diet. Comparing the effects by experimental condition, the DD affects the Sf1-Cre;Sirt1loxP/loxP mice more than Sirt1loxP/loxP mice. B, In the VMH, the same conditions provoke a phase advance in the acrophase in the Sf1-Cre;Sirt1loxP/loxP mice. Food restriction slightly affects the Sf1-Cre;Sirt1loxP/loxP mice, whereas Sirt1loxP/loxP mice are strongly affected by this condition. *, P < .05, **, P < .01, ***, P < .001, t test (n = 6 animals per group).
Remarkably, differences in the acrophase were observed when comparing the VMH of mice fed ad libitum vs food restricted (Figure 5B). Interestingly, the strongest effect of food restriction was observed in the Sirt1loxP/loxP mice (P < .0001, t test), compared with the Sf1-Cre;Sirt1loxP/loxP mice (P < .05, t test). In support of these observations, the effect on gene expression by diet, food restriction, and light at different ZTs reveals a prominent effect of DD in the SCN of the Sf1-Cre;Sirt1loxP/loxP mice and by food restriction in the VMH of Sf1-Cre mice (Supplemental Table 4). Thus, we conclude the following: 1) The endogenous clock in the SCN is predominantly altered by light conditions; 2) the Sf1-Cre;Sirt1loxP/loxP mice are more sensitive to DD; 3) the clock in the VMH is mainly affected by scheduled feeding; and 4) the scheduled feeding has weaker effects on the Sf1-Cre;Sirt1loxP/loxP mice. These data indicate that SIRT1 is detecting the feeding input as reflected by the response in the acrophase in the VMH. As a result, the VMH partially contributes to the synchronization of the SCN rhythms in DD. In contrast, the Sf1-Cre;Sirt1loxP/loxP mice appear to be less efficient in detecting scheduled feeding. This decreased capacity of the VMH appears to be associated to a reduction in the SCN synchronization to scheduled feeding.
We further analyzed the expression in the SCN and VMH of additional clock genes including Bmal1, Cry1, and Reverbα, and the SCN-enriched genes Avp, Prok2, and Grp (Supplemental Figure 6). In keeping with the profile of Per2 oscillation, the acrophase of these genes is affected in Sf1-Cre;Sirt1loxP/loxP mainly in DD and food restriction (Supplemental Table 3). We have also found significant differences in the VMH between the Sf1-Cre;Sirt1loxP/loxP and Sirt1loxP/loxP mice under these conditions (Supplemental Table 3).
Discussion
Growing epidemiological evidences and experimental data in humans have shown an association between the disruption of circadian rhythms and the predisposition to develop metabolic disorders. These perturbations are caused by modern lifestyle rhythms, also known as social zeitgebers, and include work and social demands, forcing individuals to adapt to specific type of food consumption, feeding, and sleep schedules (1–4, 17). This is particularly evident in night and shift workers, which suffer increased predisposition to develop obesity, type 2 diabetes, and cardiovascular diseases (2, 39, 40). This has been explained in part by the misalignment between endogenous circadian rhythms and daily behaviors that in turn might desynchronize central and peripheral circadian clocks (3, 24). In animal models, it has been established that the SCN is affected by nutrients when scheduled feeding is accompanied by constant darkness and/or caloric restriction (31, 34, 35, 37).
Here we have presented evidence supporting the role of the VMH on monitoring metabolic signals through the nutrient the sensor SIRT1 (19, 41). The VMH, in addition to responding to nutrient and metabolic signals by the control of behavioral and metabolic responses (42, 43), might also respond to nutrient stimuli, sending nutrient-time information to the SCN. In this context, it has been established that a diet with high caloric content modifies the endogenous clock (33, 44). Although rodents fed a high-caloric diet have been shown to present alterations in the central and peripheral clocks (33, 45), these experiments were carried out in ad libitum nutritional regimens. Thus, the question of whether high-caloric diet has particular or specific properties as external zeitgeber compared with the standard, low, or normocaloric diets remained open.
We wanted to determine whether scheduled feeding and caloric content as sole zeitgeber operate through SIRT1 as nutrient-sensor and clock-modulator in Sf1 neurons. To do so, we have measured the two main behavioral components considered as circadian outputs, the food anticipatory activity and the light-entrainable activity, and cyclic gene expression in the SCN and the VMH. Our results show a larger period length shortening in the light-entrainable activity component (from the 24 h daily synchronization) in the Sf1-Cre;Sirt1loxP/loxP mice only under food restriction but not in ad libitum feeding in DD (Figures 2 and 3). This effect may reflect the importance of the periodic activation of SIRT1 by scheduled feeding, which may enhance the role of SIRT1 as an epigenetic modulator of the circadian clock. This notion is supported by previous data showing that the NAD+/NADH ratio not only fluctuates in a circadian manner as a consequence of the circadian expression of the enzyme nicotinamide phosphoribosyltransferase, a key enzyme in the salvage pathway for the synthesis of NAD+ (25), but also because changes in feeding modulate the enzymatic activity of SIRT1 (6, 27). Importantly, under scheduled HF diet feeding, we found an increased period shortening from the normal 24-hour rhythms (Figure 3 and Supplemental Figure 3E). One possible explanation is that the HF diet could inhibit the activity and/or expression (32, 46) of the remaining SIRT1 residing in the non-Sf1 neurons of the VMH (18) or to globally inhibit SIRT1 in other hypothalamic nuclei or even extrahypothalamic areas (47, 48). Remarkably, these effects seem to be independent of the food anticipatory activity (Supplemental Figure 4). This can be explained because our model consists of single gene ablation in a small group of neurons in the hypothalamus, which might not be enough to alter food anticipatory activity. Furthermore, the food-anticipatory activity likely operates independently of the SCN or the core clock, involving motivational processes stimulated by the reward system residing in extrahypothalamic areas such as the nucleus accumbens (47, 49). Conversely, the ambulatory activity entrained by light is involuntary (50) and is directly controlled by the central clock (51).
Furthermore, we found stronger effects on the peak phase of Per2 expression in the SCN vs the VMH (Figure 5 and Supplemental Table 4), which might be explained because the SCN is directly synchronized by the photic inputs, reflecting a higher degree of desynchronization under DD compared with the VMH, and whose behavioral and molecular changes are only perceptible in DD. Hence, our results support the notion that the circadian clock uses nutrient entrainment and caloric content as external zeitgebers and that metabolic signals associated with deficient calories might be more efficient zeitgebers. Strikingly, SIRT1 appears to contribute to central clock control through efferent signals from the VMH to the SCN (21, 52) (Figure 6). Moreover, nutritional and genetic approaches have demonstrated that the deregulation of the circadian clock provokes disturbances in the control of the energy balance (24, 25). Also, mice in which Sirt1 has been ablated in most brain neurons display a deregulated central clock (23). Interestingly, the Sf1-Cre;Sirt1loxP/loxP mice present metabolic disturbances and increased susceptibility to develop type 2 diabetes (19, 37). In this context, our results suggest that SIRT1 in the VMH contributes to the synchronization and/or adaptation of the endogenous clock to feeding cues, a function that would in part explain some of the metabolic unbalances in these mice. Furthermore, the robustness of the central and peripheral clocks is correlated with improved metabolic balance (3, 12, 24). Indeed, the metabolic benefits of caloric restriction or food has been associated with control of the circadian rhythms (12, 53). Our results further suggest that under a positive energy balance (eg, high caloric intake), the alteration of the endogenous clock under weak external zeitgebers might precede the development of metabolic unbalances. This possibility is supported by the notion that Sf1-cre;Sirt1loxP/loxP mice show increased body weight, food intake, and leptinemia levels only after 40 weeks on a HF diet (Supplemental Figure 5) (19). Therefore, alteration of the endogenous clock may contribute as early event before the Sf1-cre;Sirt1loxP/loxP mice become metabolically unbalanced under a HF diet. Indeed, previous observations have established that in the absence of zeitgebers or functional clock, the organism are prone to develop metabolic unbalances (54–57). Hence, the hypothalamic mechanisms controlling energy balance seem to be tightly linked to the circadian control (58).
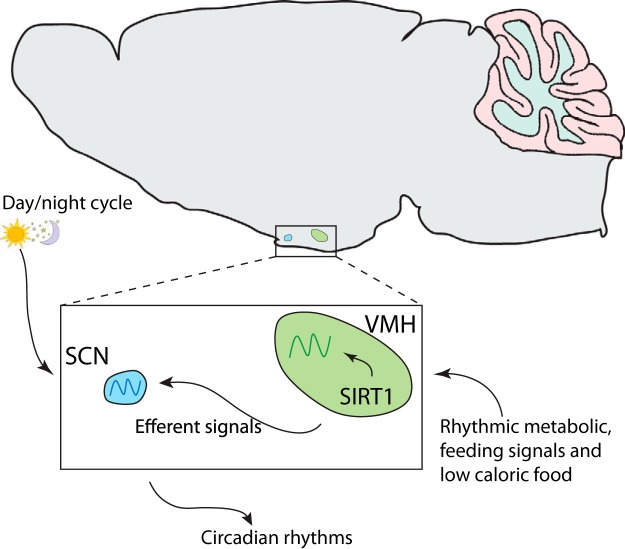
Figure 6.
Model depicting the action of scheduled feeding on the modulation of the central clock through SIRT1 within Sf1 neurons. Feeding signals can act as zeitgebers, depending on timed conditions and caloric content, which in turn might modulate the cyclic activation of SIRT1 in the Sf1 neurons and thus controlling the endogenous clock in the VMH and SCN through efferent signals.
We have shown that SIRT1 in the Sf1 neurons participates in the synchronization of the endogenous clock by nutrient inputs, possibly contributing to the adaptive mechanisms triggered by the central clock to optimize the energetic resources of the organism. Further studies will determine whether SIRT1 in the Sf1 neurons is implicated in healthy aging via circadian clock modulation.
Acknowledgments
We thank all the members of the Sassone-Corsi and Borrelli laboratories for discussions and insights.
This work was supported in part by the unrestricted support of the Louis-Jeantet Foundation. Work in the Center for Epigenetics and Metabolism is supported by the National Institute of Health, the Merieux Fondation, and INSERM (Institut National de la Sante et Recherche Medicale, France). R.O.-S. is supported by a fellowship from the Government of Mexico and by the Della Martin Foundation. R.C. is supported by the Louis-Jeantet Foundation.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 1936
- ARC
- arcuate nucleus
- DD
- constant darkness
- FR
- food restriction
- HF
- high-fat
- LD
- 12-hour light, 12-hour dark
- NAD+
- nicotinamide adenine dinucleotide
- PER2
- period-2
- SCN
- suprachiasmatic nucleus
- SD
- standard diet
- Sf1
- steroidogenic factor 1
- SIRT1
- sirtuin-1
- VMH
- ventromedial hypothalamus
- ZT
- zeitgeber time.
References
- 1. Möller-Levet CS, Archer SN, Bucca G, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci USA. 2013;110(12):E1132–E1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA. 2013;110(14):5695–5700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Buxton OM, Cain SW, O'Connor SP, et al. Adverse metabolic consequences in humans of prolonged sleep restriction combined with circadian disruption. Sci Transl Med. 2012;4(129):129ra143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake, and meal timing in healthy adults. Sleep. 2013;36(7):981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rath M, Rovsing L, Møller M. Circadian oscillators in the mouse brain: molecular clock components in the neocortex and cerebellar cortex. Cell Tissue Res. 2014:1–13. [DOI] [PubMed] [Google Scholar]
- 6. Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–549. [DOI] [PubMed] [Google Scholar]
- 7. Feillet CA, Mendoza J, Albrecht U, Pevet P, Challet E. Forebrain oscillators ticking with different clock hands. Mol Cell Neurosci. 2008;37(2):209–221. [DOI] [PubMed] [Google Scholar]
- 8. Cermakian N, Sassone-Corsi P. Environmental stimulus perception and control of circadian clocks. Curr Opin Neurobiol. 2002;12(4):359–365. [DOI] [PubMed] [Google Scholar]
- 9. Eckel-Mahan KL. Circadian oscillations within the hippocampus support memory formation and persistence. Front Mol Neurosci. 2012;5:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14(23):2950–2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. [DOI] [PubMed] [Google Scholar]
- 12. Hatori M, Vollmers C, Zarrinpar A, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stephan FK. The. “Other” circadian system: food as a zeitgeber. J Biol Rhythms. 2002;17(4):284–292. [DOI] [PubMed] [Google Scholar]
- 14. Bechtold DA, Loudon AS. Hypothalamic clocks and rhythms in feeding behaviour. Trends Neurosci. 2013;36(2):74–82. [DOI] [PubMed] [Google Scholar]
- 15. Nam Hye J, Boo K, Kim D, et al. Phosphorylation of LSD1 by PKCα is crucial for circadian rhythmicity and phase resetting. Mol Cell. 2014;53(5):791–805. [DOI] [PubMed] [Google Scholar]
- 16. Crosio C, Cermakian N, Allis CD, Sassone-Corsi P. Light induces chromatin modification in cells of the mammalian circadian clock. Nat Neurosci. 2000;3(12):1241–1247. [DOI] [PubMed] [Google Scholar]
- 17. Orozco-Solis R, Sassone-Corsi P. Epigenetic control and the circadian clock: linking metabolism to neuronal responses. Neuroscience. 2014;264(0):76–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dhillon H, Zigman JM, Ye C, et al. Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron. 2006;49(2):191–203. [DOI] [PubMed] [Google Scholar]
- 19. Ramadori G, Fujikawa T, Anderson J, et al. SIRT1 deacetylase in SF1 neurons protects against metabolic imbalance. Cell Metab. 2011;14(3):301–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Egawa M, Inoue S, Satoh S, Takamura Y, Nagai K, Nakagawa H. Acute and chronic effects of VMH lesions on circadian rhythms in food intake and metabolites. Brain Res Bull. 1993;31(3–4):293–299. [DOI] [PubMed] [Google Scholar]
- 21. Moga MM, Moore RY. Organization of neural inputs to the suprachiasmatic nucleus in the rat. J Comp Neurol. 1997;389(3):508–534. [DOI] [PubMed] [Google Scholar]
- 22. Nakahata Y, Kaluzova M, Grimaldi B, et al. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell. 2008;134(2):329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chang H-C, Guarente L. SIRT1 mediates central circadian control in the SCN by a mechanism that decays with aging. Cell. 2013;153(7):1448–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bellet MM, Orozco-Solis R, Sahar S, Eckel-Mahan K, Sassone-Corsi P. The time of metabolism: NAD+, SIRT1, and the circadian clock. Cold Spring Harb Symp Quant Biol. 2011;76:31–38. [DOI] [PubMed] [Google Scholar]
- 26. Liu Y, Dentin R, Chen D, et al. A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature. 2008;456(7219):269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 2009;4(12):e8322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Asher G, Gatfield D, Stratmann M, et al. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell. 2008;134(2):317–328. [DOI] [PubMed] [Google Scholar]
- 29. Ramadori G, Fujikawa T, Fukuda M, et al. SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab. 2010;12(1):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6(4):305–323. [PubMed] [Google Scholar]
- 31. Castillo MR, Hochstetler KJ, Tavernier RJ, Jr, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol. 2004;287(3):R551–R555. [DOI] [PubMed] [Google Scholar]
- 32. Deng XQ, Chen LL, Li NX. The expression of SIRT1 in nonalcoholic fatty liver disease induced by high-fat diet in rats. Liver Int. 2007;27(5):708–715. [DOI] [PubMed] [Google Scholar]
- 33. Kohsaka A, Laposky AD, Ramsey KM, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–421. [DOI] [PubMed] [Google Scholar]
- 34. Abe H, Honma S, Honma K. Daily restricted feeding resets the circadian clock in the suprachiasmatic nucleus of CS mice. Am J Physiol Regul Integr Comp Physiol. 2007;292(1):R607–R615. [DOI] [PubMed] [Google Scholar]
- 35. Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25(6):1514–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Persons JE, Stephan FK, Bays ME. Diet-induced obesity attenuates anticipation of food access in rats. Physiol Behav. 1993;54(1):55–64. [DOI] [PubMed] [Google Scholar]
- 37. Froy O. Circadian rhythms, aging, and life span in mammals. Physiology. 2011;26(4):225–235. [DOI] [PubMed] [Google Scholar]
- 38. Shearman LP, Zylka MJ, Weaver DR, Kolakowski LF, Jr, Reppert SM. Two period homologs: circadian expression and photic regulation in the suprachiasmatic nuclei. Neuron. 1997;19(6):1261–1269. [DOI] [PubMed] [Google Scholar]
- 39. Froy O. Circadian rhythms and obesity in mammals. ISRN Obesity. 2012;2012:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Knutson KL, Van Cauter E. Associations between sleep loss and increased risk of obesity and diabetes. Ann NY Acad Sci. 2008;1129:287–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCrimmon RJ, Fan X, Ding Y, Zhu W, Jacob RJ, Sherwin RS. Potential role for AMP-activated protein kinase in hypoglycemia sensing in the ventromedial hypothalamus. Diabetes. 2004;53(8):1953–1958. [DOI] [PubMed] [Google Scholar]
- 42. Choi Y-H, Fujikawa T, Lee J, Reuter A, Kim KW. Revisiting the ventral medial nucleus of the hypothalamus: the roles of SF-1 neurons in energy homeostasis. Front Neurosci. 2013;7:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. King BM. The rise, fall, and resurrection of the ventromedial hypothalamus in the regulation of feeding behavior and body weight. Physiol Behav. 2006;87(2):221–244. [DOI] [PubMed] [Google Scholar]
- 44. Eckel-Mahan KL, Patel VR, de Mateo S, et al. Reprogramming of the circadian clock by nutritional challenge. Cell. 2013;155(7):1464–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mendoza J, Pévet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008;586(24):5901–5910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chalkiadaki A, Guarente L. High-fat diet triggers inflammation-induced cleavage of SIRT1 in adipose tissue to promote metabolic dysfunction. Cell Metab. 2012;16(2):180–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104(4):535–545. [DOI] [PubMed] [Google Scholar]
- 48. Ramadori G, Lee CE, Bookout AL, et al. Brain SIRT1: anatomical distribution and regulation by energy availability. J Neurosci. 2008;28(40):9989–9996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Storch K-F, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA. 2009;106(16):6808–6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ahabrach H, Piedrafita B, Ayad A, et al. Chronic hyperammonemia alters the circadian rhythms of corticosteroid hormone levels and of motor activity in rats. J Neurosci Res. 2010;88(7):1605–1614. [DOI] [PubMed] [Google Scholar]
- 51. Challet E, Caldelas I, Graff C, Pevet P. Synchronization of the molecular clockwork by light- and food-related cues in mammals. Biol Chem. 2003;384(5):711–719. [DOI] [PubMed] [Google Scholar]
- 52. Ribeiro AC, Sawa E, Carren-LeSauter I, LeSauter J, Silver R, Pfaff DW. Two forces for arousal: pitting hunger versus circadian influences and identifying neurons responsible for changes in behavioral arousal. Proc Natl Acad Sci USA. 2007;104(50):20078–20083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chaix A, Zarrinpar A, Miu P, Panda S. Time-restricted feeding is a preventative and therapeutic intervention against diverse nutritional challenges. Cell Metab.20(6):991–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Coomans CP, van den Berg SAA, Houben T, et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2013;27(4):1721–1732. [DOI] [PubMed] [Google Scholar]
- 55. Shi S-Q, Ansari TS, McGuinness Owen P, Wasserman David H, Johnson Carl H. Circadian disruption leads to insulin resistance and obesity. Curr Biol. 2013;23(5):372–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu Z, Huang M, Wu X, et al. PER1 phosphorylation specifies feeding rhythm in mice. Cell Rep.7(5):1509–1520. [DOI] [PubMed] [Google Scholar]
- 57. Turek FW, Joshu C, Kohsaka A, et al. Obesity and metabolic syndrome in circadian Clock mutant mice. Science. 2005;308(5724):1043–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Sutton GM, Begriche K, Kumar KG, et al. Central nervous system melanocortin-3 receptors are required for synchronizing metabolism during entrainment to restricted feeding during the light cycle. FASEB J. 2010;24(3):862–872. [DOI] [PMC free article] [PubMed] [Google Scholar]