Abstract
Thyroid hormones are released from thyroglobulin (Tg) in lysosomes, which are impaired in infantile/nephropathic cystinosis. Cystinosis is a lysosomal cystine storage disease due to defective cystine exporter, cystinosin. Cystinotic children develop subclinical and then overt hypothyroidism. Why hypothyroidism is the most frequent and earliest endocrine complication of cystinosis is unknown. We here defined early alterations in Ctns−/− mice thyroid and identified subcellular and molecular mechanisms. At 9 months, T4 and T3 plasma levels were normal and TSH was moderately increased (∼4-fold). By histology, hyperplasia and hypertrophy of most follicles preceded colloid exhaustion. Increased immunolabeling for thyrocyte proliferation and apoptotic shedding indicated accelerated cell turnover. Electron microscopy revealed endoplasmic reticulum (ER) dilation, apical lamellipodia indicating macropinocytic colloid uptake, and lysosomal cystine crystals. Tg accumulation in dilated ER contrasted with mRNA down-regulation. Increased expression of ER chaperones, glucose-regulated protein of 78 kDa and protein disulfide isomerase, associated with alternative X-box binding protein-1 splicing, revealed unfolded protein response (UPR) activation by ER stress. Decreased Tg mRNA and ER stress suggested reduced Tg synthesis. Coordinated increase of UPR markers, activating transcription factor-4 and C/EBP homologous protein, linked ER stress to apoptosis. Hormonogenic cathepsins were not altered, but lysosome-associated membrane protein-1 immunolabeling disclosed enlarged vesicles containing iodo-Tg and impaired lysosomal fusion. Isopycnic fractionation showed iodo-Tg accumulation in denser lysosomes, suggesting defective lysosomal processing and hormone release. In conclusion, Ctns−/− mice showed the following alterations: 1) compensated primary hypothyroidism and accelerated thyrocyte turnover; 2) impaired Tg production linked to ER stress/UPR response; and 3) altered endolysosomal trafficking and iodo-Tg processing. The Ctns−/− thyroid is useful to study disease progression and evaluate novel therapies.
The function of individual molecular events in the thyroid gland has been unraveled by the study of monogenic defects, occurring spontaneously in human or engineered in mice (for reviews see references 1 and 2). We here address the effects in mouse thyroid of genetic ablation of the lysosomal membrane cystine exporter, cystinosin, which is absent in a rare multisystemic autosomal recessive lysosomal cystine storage disease, named infantile cystinosis (in short, cystinosis) (for reviews see references 3 and 4). Cystinosis leads almost invariably to primary hypothyroidism during the first years of life, whereas other endocrine organs are later affected (4–6).
Cystinosin, a seven-transmembrane protein of 367 amino acids displaying two strong lysosomal targeting motives (7), is the only known lysosomal membrane cystine exporter, driven by coupled proton efflux (8). Cystine is an obligatory end-degradation product of disulfide-bearing proteins. Once exported out of lysosomes, cystine is rapidly reduced into cysteine by cytosolic reducing systems. Cysteine together with glutamate participates in glutathione synthesis and thus cell redox homeostasis. The accumulation of lysosomal cystine in cystinosis can be corrected by substrate depletion therapy based on oral cysteamine, but compliance is very demanding. Cysteamine rearranges in lysosomes with cystine to a mixed disulfide that egresses via the lysine transporter (9).
The earliest manifestation of cystinosis, usually during the first year of life, is a kidney Fanconi syndrome, recognized by high urinary loss of solutes including water, salts, glucose, and phosphate together with ultrafiltrated plasma proteins. Infantile cystinosis usually leads to renal failure, even under compliant cysteamine treatment. During the first decade of life, most cystinotic children further develop subclinical and then overt hypothyroidism (5, 10). Although early compliant cysteamine treatment improves body growth and can avoid thyroid hormone replacement (11), eventually about half of treated cystinotic adults require thyroid hormones. Overall, kidney and thyroid dysfunctions are the less cysteamine-preventable complications of cystinosis (12, 13). Thus, better understanding of cellular and tissular pathogenic mechanisms in kidneys and thyroid are mandatory.
The exact causative link between cystinosis and hypothyroidism remains unexplained. As for the kidneys, defective thyroid function was originally attributed to atrophy with pathognomonic cystine crystals (5). However, the pathogenic role of crystals is questioned, and early impairment of proteolysis in cystinotic lysosomes has been evidenced and attributed to lysosomal redox imbalance (14). Thyroid hormones (THs) are released in lysosomes by proteolytic cleavage of engulfed thyroglobulin (Tg), although proteolysis may be initiated in the follicular lumen (15, 16). Tg is an oligomer of 330-kDa monomers, which assume a compact globular form stabilized by a huge number of disulfide bonds (>100/monomer) (17). Tg is dimerized in the endoplasmic reticulum (ER) and then undergoes compaction in the follicular lumen by intermolecular disulfide cross-linking to form insoluble thyroid globules for maximal storage (18, 19). Luminal compaction is attributed to extrinsic [secreted protein disulfide isomerase (PDI)] and intrinsic disulfide bond exchange mechanisms [via well preserved thioredoxin (CXXC) motives] (20). The extent of luminal Tg cross-linking varies among species and is related to age and follicle activation state (18, 19, 21). Tg unfolding via disulfide bond reduction by lysosomal reducing equivalents thus appears necessary to expose cryptic peptides targeted by lysosomal proteases (22). Stepwise Tg proteolytic processing depends on synergistic endo- and then exopeptidases, including the aspartyl protease, cathepsin D, and cysteine proteases, eg, cathepsin B (23–27). Cysteine proteases also require a reducing environment. These requirements would predict that Tg unfolding and cysteine protease attack are impaired when lysosomal cystine accumulation causes redox imbalance.
At low TSH, basal TH production is supported by endocytosis of Tg from the colloid via small endocytic pits (ie, micropinocytosis, reviewed in reference 28). This is regulated by expression and activation of tandem rate-limiting GTPase catalysts, Rab5 and Rab7, driving together vesicular transfer to lysosomes (29, 30). In some species such as mice, acute stimulation with high TSH dose triggers micrometric colloid uptake by protrusion of actin-dependent lamellipodia followed by macropinocytosis [also named phagocytosis; (31)], which brings large amounts of Tg to lysosomes in the form of colloid droplets. How released THs cross the lysosomal membrane remains unknown. This step could involve a similar transporter as monocarboxylate transporter-8 (Mct-8) (32) operating at the basolateral membrane for secretion into blood capillaries. However, a Mct-8 defect is unlikely in a monogenic disorder such as cystinosis.
We and others recently reported on the early kidney lesions and adaptations (33, 34) in a cystinosin-knockout mice strain of congenic C57BL/6 background (Ctns−/− mice), which mimics human cystinosis (35, 36). After a 3-month lag phase without detectable lesion, proximal tubular cells (PTCs) of Ctns−/− mice showed defective endolysosomal trafficking and lysosomal proteolysis, resulting into amorphous lysosomal inclusions and then cystine crystals. At the lesional stage, apoptosis and proportional proliferation revealed accelerated PTC turnover (33). We here extended our study of Ctns−/− mice to the thyroid gland, on the premise of shared high apical endocytic activity of disulfide-rich proteins and early defects in cystinotic children.
We first hypothesized that cystine accumulation in lysosomes of Ctns−/− thyrocytes would primarily affect thyroid function by delaying TH generation due to impaired Tg transfer to lysosomes, combined with defective unfolding and cysteine protease activity. As an additional hypothesis, nonmutually exclusive upstream mechanism, cystinosis causes ER stress (37) to which thyrocytes are particularly prone (38) so that ER stress would impair Tg synthesis and its supply to lysosomes. ER stress triggers the complex adaptive unfolded protein response (UPR) (for review see reference 39). UPR is initiated by transmembrane ER sensors/receptors, inositol-requiring kinase-1, protein kinase RNA-like endoplasmic reticulum kinase, and/or activating transcription factor (ATF)-6. Inositol-requiring kinase-1 activation results from high substrate competition, causing a dissociation of ER resident chaperones such as glucose-regulated protein of 78 kDa (GRP78). Downstream in the UPR pathway, activation of X-box binding protein-1 (XBP-1) by alternative mRNA splicing results in multiple structural and molecular adaptive mechanisms. These include the following: 1) expansion of the ER membrane and thus ER dilatation to accommodate protein overload; 2) increased transcription of ER chaperones (GRP78) and foldases (eg, PDI) and thus protein maturation capacity; and 3) decreased translation of secreted proteins (here Tg), which together attenuate ER stress. If stress persists or adaptive response fails, cell death is triggered via transcriptional activation of proapoptotic C/EBP homologous protein (CHOP) in response to protein kinase RNA-like endoplasmic reticulum kinase-ATF-4 axis activation (for reviews, see references 39 and 40).
We found that, after a lag phase of approximately 6 months, all Ctns−/− mice developed subclinical hypothyroidism with increased TSH, thyrocyte hyperplasia/hypertrophy, and accelerated turnover as well as angioproliferative response. Relative TSH refractoriness and colloid exhaustion could be explained by the combination of impaired Tg production due to UPR response with defective endolysosomal trafficking and Tg processing. UPR response to ER stress likely links TSH stimulation to thyrocyte apoptosis and accelerated turnover.
Materials and Methods
Mice
Congenic C57BL/6 Ctns−/− mice have been described (36). Mice were treated according to the National Institutes of Health Guide for Care and Use of Laboratory Animals. Mice were fed ad libitum with pellets containing 4.30 mg/kg iodine (Carfil Quality).
TSH, T4, and T3 plasma concentrations
Plasma TSH concentrations were measured by a sensitive, heterologous, disequilibrium, double-antibody precipitation RIA as described (41). T4 and T3 concentrations were measured by coated-tube RIA (Siemens Medical Solution Diagnostics).
Histology, multiplex immunofluorescence, and morphometry
Mice thyroids were fixed in situ by whole-body perfusion-fixation as described (33). Thyroids were dissected, postfixed overnight with 4% neutral-buffered formaldehyde, and processed for paraffin embedding. Four-micrometer-thick sections were stained with hematoxylin/eosin. Immunofluorescence was performed after antigen retrieval as described (33). Appropriate combinations of the following primary antibodies were used (Table 1): mouse anti-E-cadherin (0.25 μg/mL, 610182; DB Bioscience), -ezrin (2 μg/mL, MS-661-P1; Thermo Scientific), -Ki-67 (2 μg/mL, 556003; DB Pharmingen), -Tg (1:200, M0781; Dako), and -iodo-Tg (1:100; kindly donated by Dr Ris-Stalpers, Laboratory of Pediatric Endocrinology, Academic Medical Center, Amsterdam, The Netherlands); rat anti-platelet endothelial cell adhesion molecule-1 (PECAM-1; 1:20, DIA310; Dianova), -lysosome-associated membrane protein-1 (LAMP-1; 1:100, 1D4B; Hybridoma Bank), and -KDEL (1:300, ab50601; Abcam); and rabbit anti-active caspase-3 (1:200, 9661; Cell Signaling). Immunolabeled sections were imaged with a spinning disk confocal microscope using EC Plan-NeoFluar ×40/1.3 or Plan Apochromat ×100/1.4 oil differential interference contrast objectives (cell observer spinning disk; Zeiss). Morphometric analyses were performed using Axiovision 4.8.2. software (Zeiss). Binary mask were prepared using fixed interactive thresholding. Thyrocytes, colloid, and interstitium were filled and areas were measured.
Table 1.
List of Antibodies
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used (IF;WB) |
|---|---|---|---|---|---|
| E-cadherin | — | Purified mouse anti-E-cadherin | DB Bioscience, number 610182 | Mouse monoclonal (clone 36) | 0.25 μg/mL |
| Ki-67 | — | Purified mouse anti-human Ki-67 | DB Pharmingen, number 556003 | Mouse monoclonal (clone B56) | 2 μg/mL |
| Active-caspase 3 | — | Cleaved caspase-3 (Asp175) antibody | Cell Signaling, number 9661 | Rabbit polyclonal | 1:200 |
| PECAM-1 | — | Rat monoclonal anti-mouse endothelial cell marker CD31 (PECAM-1) | Dianova, number DIA310 | Rat monoclonal (clone SZ31) | 1:20 |
| Ezrin | aa 362–585 | Ezrin/p81/80K/cytovillin Ab-1, mouse monoclonal antibody | Thermo Scientific, number MS-661-P1 | Mouse monoclonal (clone 3C12) | 2 μg/mL |
| LAMP-1 | — | Anti-LAMP-1 1D4B antibody | Hybridoma Bank, number 1D4B | Rat monoclonal (clone 1D4B) | 1:100 |
| KDEL | — | Anti-KDEL [MAC256] antibody | Abcam, number ab50601 | Rat monoclonal (clone MAC256) | 1:300 |
| Tg | — | Monoclonal mouse anti-human thyroglobulin | Dako, number M0781 | Mouse monoclonal (clone DAK-Tg6) | 1:200; 1:1000 |
| Iodo-Tg | — | Anti-iodo-thyroglobulin antibody | Provided by Dr Ris-Stalpers | Mouse monoclonal | 1:100; 1:1000 |
| GRP78 | CT (643)GEEDTSEKDEL (654) | GRP78/BiP antibody | Thermo Scientific, number PAI-014A | Rabbit polyclonal | 2 μg/mL |
| Cathepsin D | — | Cathepsin D antibody | Santa Cruz Biotechnology, number sc-6486 | Goat polyclonal | 0.2 μg/mL |
| GAPDH | GAPDH antibody | Ambion, number AM4300 | Mouse monoclonal (clone 6C5) | 0.5 μg/mL |
Electron microscopy
Thyroids were perfusion-fixed in situ with 4% neutral-buffered formaldehyde supplemented by 0.1% glutaraldehyde and then immersion fixed in 1.5% (vol/vol) glutaraldehyde overnight, postfixed with 1% (wt/vol) OsO4 in 0.1 M cacodylate buffer for 1 hour, rinsed in veronal buffer (4 × 5 min), and stained overnight en bloc in 1% neutral uranyl acetate, all at 4°C. After extensive washing in veronal, blocks were dehydrated in graded ethanol and embedded in Spurr. Ultrathin sections were obtained (Reichert ultramicrotome), collected on 400-mesh rhodanium grids, and contrasted with 3% uranyl acetate and then lead citrate, 10 minutes each. Grids were washed with water, dried, and examined in a FEI CM12 electron microscope operating at 80 kV.
In situ hybridization
Vegf-a antisense RNA probes spanning nucleotides 94–429 of the mouse coding sequence for Vegf-a (42) were produced by RT-PCR followed by in vitro transcription with T7 RNA polymerase in the presence of digoxigenin-labeled uridine 5-triphosphate (Roche). In situ hybridization was performed on 8-μm sections as described (33).
PCR and real-time quantitative PCR (RT-qPCR)
Total RNA was extracted (TRIzol reagent; Invitrogen) and 150 ng RNA was reverse transcribed by Moloney murine leukemia virus reverse transcriptase (Invitrogen) using random hexamers. Primer sequences are described in Supplemental Table 1. PCR was performed under standard conditions with GoTaq DNA polymerase (Promega). RT-qPCR was performed as described (33) in the presence of 250 nM specific primers with Kappa SYBR Fast qPCR master mix (Kapa Biosystems) on a CFX96 touch real-time PCR detection system (Bio-Rad Laboratories). Results are presented as difference of cycle threshold (ΔCt) values normalized to actin, used as internal standard.
Western and lectin blotting
Thyroid glands were dissected and homogenized in Western blot lysis buffer (150 mM NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate, 50 mM Tris, pH 8.0) or in subcellular fractionation buffer 250 mM sucrose, 3 mM imidazole, and 1 mM EDTA (pH 7.0) buffer supplemented with Complete protease inhibitors (Roche) and phosphatase inhibitors (sodium orthovanadate, pyrophosphate, and fluoride, all 2 mM). Loading was normalized to protein concentration, measured by bicinchoninic acid method (Sigma-Aldrich). Samples were reduced or not, as indicated, with 50 or 100 mM dithiothreitol (DTT) for 10 minutes and denatured by boiling for 5 minutes. Western blotting was performed as described (43) using mouse anti-Tg (1:1000, M0781; Dako), or -glyceraldehyde-3 phosphate dehydrogenase (GAPDH; 0.5 μg/mL, AM4300; Ambion); rat anti-KDEL (2.5 μg/mL, ab50601; Abcam); rabbit anti-GRP78 (2 μg/mL, PA1–014A; Thermo Scientific); or goat anti-cathespin D (0.2 μg/mL, sc-6486; Santa Cruz Biotechnology). Lectin blotting was performed with wheat germ agglutinin lectin (10 μg/mL; Vector Biolabs) after electrophoresis under reducing conditions as described (44). Specificity of lectin signal was demonstrated by neuraminidase digestion at 37°C for 18 hours, following the manufacturer's instructions (New England BioLabs).
Cathepsin B assay
Thyroid glands were dissected and homogenized in 250 mM sucrose, 3 mM imidazole, and 1 mM EDTA (pH 7.0). Cathepsin B activity was measured as total minus 100 μM CA-074-resistant fraction (Sigma-Aldrich) in a fluorimetric assay using benzyloxycarbonyl-L-phenylalanyl-L-arginine 4-methylcoumaryl-7-amide (45), or Nα-benzoyl-DL-arginine-β-naphthylamide hydrochloride, with undistinguishable results. Activity was normalized to protein concentration measured by the bicinchoninic acid method (Sigma-Aldrich).
Analytical subcellular fractionation
Excised thyroid glands from three to four wild-type (WT) or Ctns−/− mice, aged 9–11 months, were pooled in 250 mM sucrose, 3 mM imidazole, and 1 mM EDTA (pH 7.0), supplemented with Complete protease inhibitors (Roche), and homogenized therein with a Polytron (3 × 5 sec, nominal 8500 speed). Homogenates were cleared through 40 μm BD falcon filters and first resolved by crude differential sedimentation to isolate cell debris and nuclei (1.5 104 g × min), postnuclear particles (6.3 106 g × min), and a final supernatant. Postnuclear particles were washed once by resuspension and resedimentation to minimize colloid contamination and then equilibrated by sedimentation into 1.10–1.30 (grams per milliliter) linear sucrose gradients in a SW55Ti rotor (57 106 g × min). Ten fractions were collected from the bottom and assayed for density (weight) and β-hexosaminidase activity as described (33). Aliquots of equal volume were analyzed by Western blotting for iodo-Tg (1:1000). Compared blots were transferred and revealed in the same membrane and then quantified using ImageJ software (National Institutes of Health, Bethesda, Maryland).
Statistical analyses
Statistical significance was tested using a Mann-Whitney U test (see Figures 1A, 3A and B, 5A and B, 6C, and 7B and C; and Supplemental Figure 4), Student's t test (Figures 1D and 2B) or χ2 (Figure 6B). Differences were considered significant for P < .05. Except for specific thyroid weight, there was no significant difference between males and females in each group and for each comparison; thus, genders were not discriminated on scatter plots.
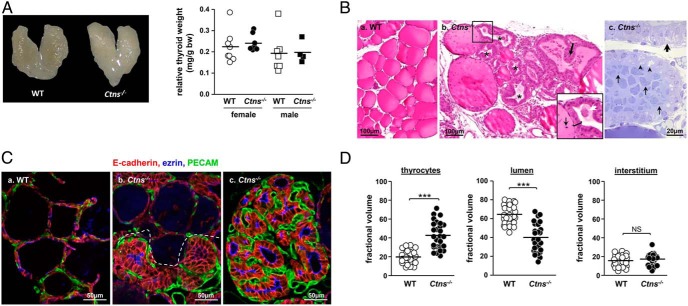
Figure 1.
Ctns−/− mice develop thyroid hyperplasia and hypertrophy associated with colloid exhaustion. A, Anatomy. Left panel, Thyroid gland from control (WT) and Ctns−/− female mice at 11 months. Right panel, Thyroid glands weight normalized to body weight in WT and Ctns−/− females and males at 9–12 months. Cystinotic mice do not develop goiter. B, Histology. Paraffin sections with hematoxylin-eosin staining (a and b) and semithin plastic section with toluidine blue staining (c), all at 9 months, are shown. a, WT thyroid is made of uniform follicles filled with homogenous colloid and delimited by flat thyrocytes. b, In Ctns−/− mice, most follicles show exhausted colloid (asterisks) surrounded by thyrocytes that are both hypertrophic (insert bracket) and hyperplastic, frequently projecting into papillae (thick arrow). Boxed area at panel b is enlarged below to emphasize the contrast between the few resting follicles (flat epithelium) with colloid bearing several cell remnants (thin arrow) and an adjacent hypertrophic/hyperplastic follicle (bracket) with almost vanished colloid (white arrow suggests dissolution of a thyroid globule). c, In the plastic section of Ctns−/− thyroid, the two upper follicles with hypertrophic thyrocytes and exhausted colloid contrast with a resting follicle below with dense colloid and flat thyrocytes. Arrowheads point to apical thyrocyte vacuolation, suggesting macropinocytosis/phagocytosis; the thick arrow points to irregular basal thyrocyte clarifications aligned along the basoapical axis, suggesting dilation of endoplasmic reticulum. In the central follicle, shed cell remnants almost fill the follicular lumen (thin arrows indicate variety of shapes). Scale bars, a and b, 100 μm; c, 20 μm. For histological time course, see Supplemental Figure 1. C and D, Immunofluorescence: multifocal activation of the angiofollicular system in Ctns−/− mice thyroids and colloid exhaustion. C, Follicular hyperplasia and hypertrophy are coupled to expansion of associated blood capillary basket. Multiplex immunofluorescence for E-cadherin (red, thyrocyte basolateral membrane), ezrin (blue, thyrocyte apical membrane), and PECAM (green, blood capillaries) in the thyroid of WT (a) and Ctns−/− (b and c) mice at 9 months, performed strictly in parallel. a, WT follicles are surrounded by a blood capillary network weakly discernible by PECAM immunolabeling. b, In this intermediate Ctns−/− pattern, notice abrupt boundary (dotted line) between resting follicles (top panel) and hyperplastic follicles (bottom panel) showing microvasculature expansion associated with much stronger PECAM signal. c, In most remodeled Ctns−/− hyperplastic foci, lumina have almost vanished and microvasculature is greatly expanded. All scale bars, 50 μm. For in situ hybridization of Vegf-a, see Supplemental Figure 2. D, Follicular hyperplasia/hypertrophy is associated with colloid exhaustion. Morphometric assessment of the fractional volume of thyrocytes, colloid, and interstitial compartment (mesenchyme and blood capillaries) in WT and Ctns−/− mice (n = 3; each analyzed in nine random fields spanning the entire thyroid section, cumulative area of 3.4 105 μm2 for each mouse). Note doubling of Ctns−/− thyrocyte fractional volume, at the expense of the lumen, but no significant change of interstitial fractional volume. ***, P < .001. NS, not significant.
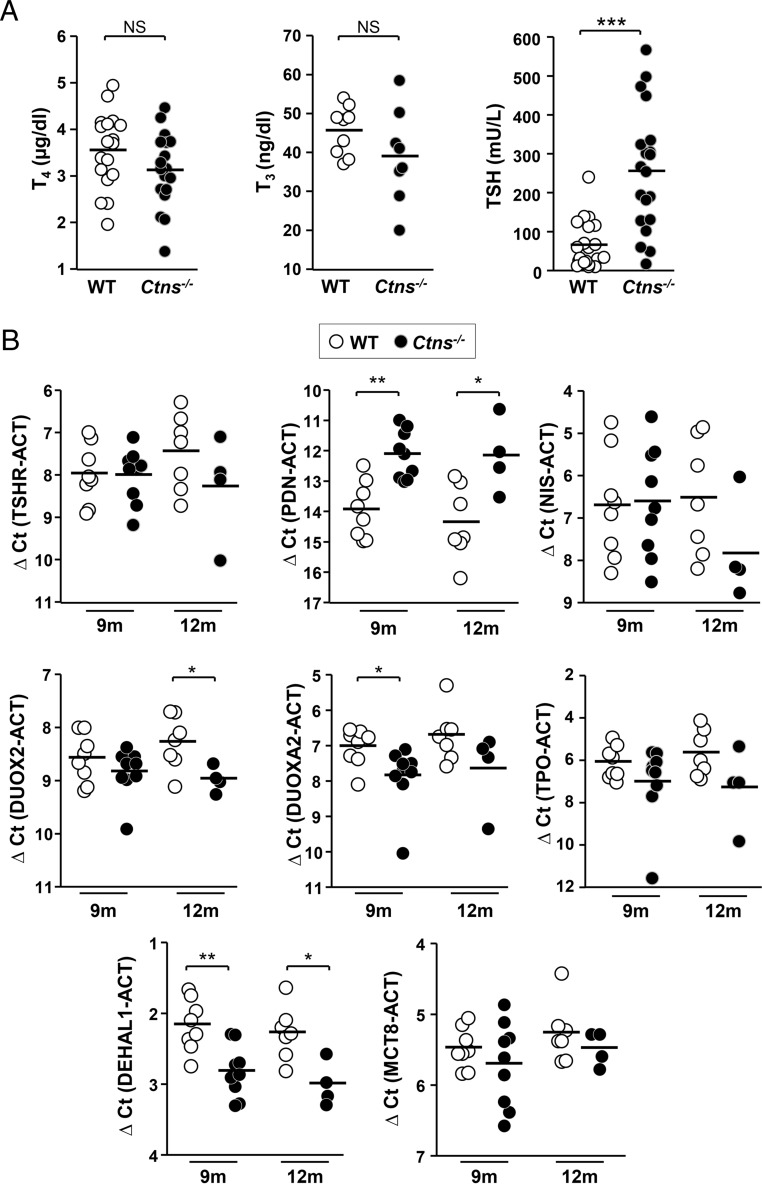
Figure 3.
Compensated primary hypothyroidism and mRNA expression of thyroid hormone synthesis machinery in Ctns−/− mice. A, Ctns−/− mice elicit a compensatory TSH increase. T4, T3, and TSH plasma concentrations were measured in 9- to 10-month WT mice (open symbols) and Ctns−/− littermates (filled symbols) (n = 18 for T4; n = 9 = WT and 8 Ctns−/− for T3; n = 20 for TSH). Ctns−/− mice show an average 4-fold increase in plasma TSH level but still normal thyroid hormone plasma levels, indicating effective thyroid compensation. B, Expression of thyroid-specific genes involved in hormone synthesis. Quantification by RT-qPCR of TSH receptor (TSHR), Na/I symporter (NIS), pendrin (PDN), DUOX2, DUOXA2, thyroperoxidase (TPO), DEHAL1 and MCT8 mRNAs in WT and Ctns−/− thyroids collected at 9 and 12 months, normalized to actin mRNA (ACT) and presented as ΔCt values (difference of cycle threshold).
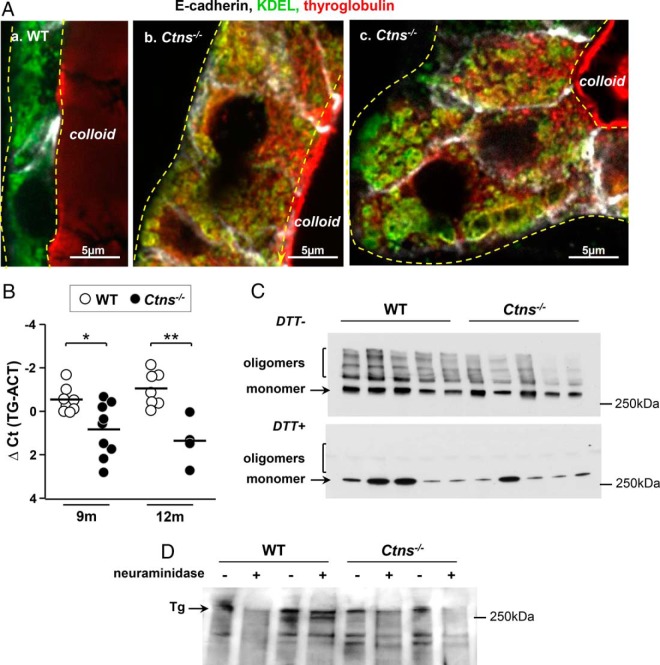
Figure 5.
Tg synthesis is slowed down but maturation is qualitatively preserved in hyperplastic Ctns−/− thyrocytes. A, Tg accumulates in the ER of Ctns−/− thyrocytes. Comparison of WT (a) and Ctns−/− mice (b, c) at 9–10 months for E-cadherin (white), KDEL (green, retention motive used as marker of ER), and Tg (red). Yellow broken lines indicate thyrocyte profiles. Tg labeling of colloid is out of focus because of artifactual colloid stickiness to the coverslip. In WT thyrocytes (a), the ER is usually localized to the basolateral cytoplasm, without resolution of the reticulum at the confocal level and shows barely visible Tg signal in our labeling conditions. In contrast, Ctns−/− hyperplastic thyrocytes (b and c) show ER dilatations, resolved at the confocal level (KDEL, green), containing detected Tg (red), resulting in yellow signal, and expanding up to the apical pole. B, Decreased levels of Tg mRNA in Ctns−/− mice thyroid. Quantification of thyroglobulin (TG) mRNA by RT-qPCR in WT and Ctns−/− thyroids collected at 9 and 12 months, normalized to actin mRNA (ACT), and presented as ΔCt values. *, P < .05; **, P < .01. C and D, Preserved thyroglobulin maturation. C, Disulfide bonding. Thyroid extracts of five WT and five Ctns−/− thyroids were analyzed by Western blotting for Tg, without or after reduction of disulfide bonds by 100 mM DTT. Notice that total Tg is decreased in Ctns−/− thyroid and that Tg oligomers remain detectable in Ctns−/− thyroids, although their proportion is decreased as compared with WT. These effects are attributed to accelerated colloid turnover. Oligomers are fully reduced into 330-kDa monomers upon DTT. Decreasing DTT concentration to 0.3 mM yielded identical results (not shown). D, Terminal N-glycosylation. Thyroid extracts from two WT and two Ctns−/− mice were analyzed by lectin blotting after reduction by DTT, without or with pretreatment with neuraminidase as lectin-specificity control. Notice comparable terminal Tg sialylation. This blot is representative of two experiments.
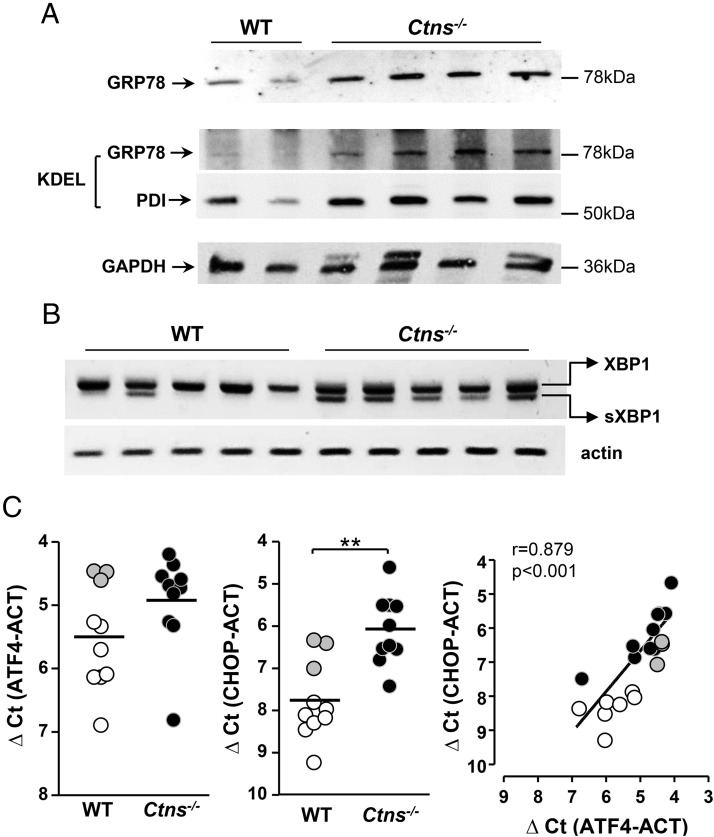
Figure 6.
ER stress is triggered in Ctns−/− thyroid. A, Increased expression of GRP78 and PDI in Ctns−/− mice thyroid. Thyroid lysates from WT and Ctns−/− at 11–12 months were analyzed by Western blotting with antibodies to GRP78 (upper panel) or KDEL (the latter identifies both GRP78 and PDI; medium panel) and to GAPDH (stripped reprobed blot, lower panel). B, Unconventional splicing of XBP-1 mRNA is triggered in Ctns−/− mice thyroid. RT-PCR analysis of XBP-1 mRNA maturation in thyroid of five WT and five Ctns−/− mice at 9 months. Notice the alternative spliced XBP1 lower band in all Ctns−/− samples and only one WT sample. C, Induction of ATF-4 expression triggers CHOP mRNA expression. Quantification of ATF-4 and CHOP mRNAs by RT-qPCR in WT and Ctns−/− thyroids collected at 9 months, normalized to actin mRNA (ACT), and presented as ΔCt values. **, P < .01. Gray dots correspond to outlier WT mice for which both ATF-4 and CHOP mRNAs were increased. At right, increased expression of ATF-4 strongly correlates with increased expression of CHOP.
Figure 7.
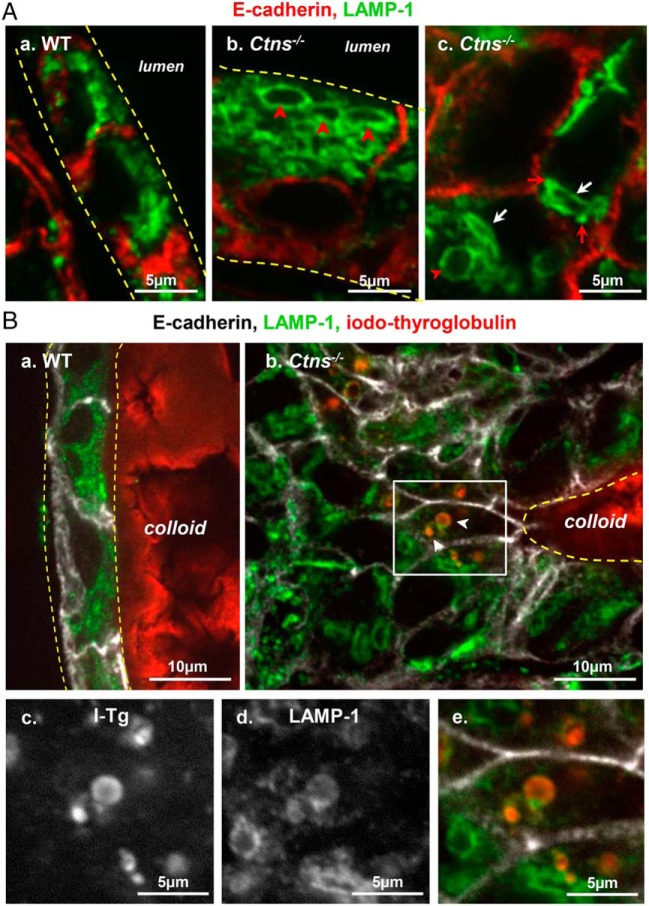
Alterations of the late endocytic apparatus in hyperplastic Ctns−/− thyrocytes. A, Identification of large apical vacuoles and crystal-bearing structures as lysosomes. Comparison of WT (a) and Ctns−/− mice (b and c) at 9 months for immunolabeling of E-cadherin (red) and LAMP-1 (green; late endosome/lysosome membrane). a, In this resting WT follicle, flat thyrocytes show at their apical pole packed lysosomes of uniform small size and round shape. b and c, In activated Ctns−/− thyrocytes, late endosomes-lysosomes are frequently dilated (red arrowheads) and distorted (better seen at panel c, white arrows). Red arrows at panel c suggest docked but not fused late endosomes/lysosomes. For levels of Rab5 and Rab7 mRNAs, see Supplemental Figure 4. B, Iodo-Tg is retained in lysosomes. Comparison of WT (a) and Ctns−/− mice (b–e) at 9–10 months for E-cadherin (white), LAMP-1 (green), and iodo-Tg (red). In resting WT follicles (a), iodo-Tg is stored in the colloid and is never detected within thyrocytes. In activated Ctns−/− thyrocytes (b), vesicles filled with iodo-Tg and labeled by LAMP-1 (thus not primary colloid droplets) are frequently found at the apical pole of hyperplasic thyrocytes. Enlargements of the boxed field (c–e) first show single-channel images of iodo-Tg and LAMP-1 in black and white for optimal resolution and easier pattern comparison and then merged back with E-cadherin in triple colors as above. The apparent defect of (iodo-)Tg labeling of the central lumen is due to artifactual sticking of the colloid to the coverslip, thus out of focus by confocal imaging. For a gallery of representative images of iodo-Tg retained in lysosomes, see Supplemental Figure 3.
Figure 2.
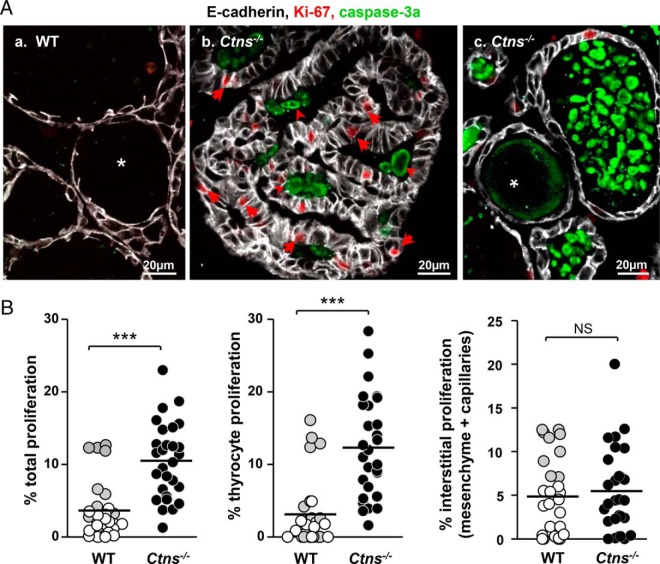
Cystinosis induces follicle-autonomous thyrocyte proliferation and apoptosis. A, Confocal microscopy. Immunofluorescence for E-cadherin (white; thyrocyte basolateral membrane), Ki-67 (red; cell proliferation marker), and activated caspase-3 (green, apoptosis marker) in 9-month WT (a) and Ctns−/− (b, c) thyroids. Notice numerous proliferating cells (arrows) in Ctns−/− hyperplastic follicles, pointing to autonomous follicular response, whereas Ki-67 labeling in WT thyrocytes is very rare. At panel c, large collections of apoptotic bodies in the follicular lumen of Ctns−/− thyroid (arrowheads) indicate accelerated cell turnover. All scale bars, 20 μm. B, Quantification of proliferation. Percentage of total nuclei area stained for Ki-67 in whole tissue (total proliferation) or associated with E-cadherin (thyrocyte proliferation) as estimated by morphometric analysis in 9-month WT (open and gray symbols; gray identifies a special WT individual) and Ctns−/− thyroids (filled symbols) (n = 3). For each mice, 3.4 105 μm2 of area corresponding to nine fields spanning the entire thyroid section were analyzed. Proliferation is specifically increased in Ctns−/− thyrocytes. ***, P < .001.
Results
Ctns−/− thyroids develop multifocal hyperplasia/hypertrophy with colloid exhaustion and proportional vascular expansion
There was neither macroscopic change nor goiter at 9 and 12 months (Figure 1A). Thyroid glands were analyzed by conventional histopathology at 3, 6, 9, and 15 months (Figure 1B and Supplemental Figure 1). There were no detectable lesions at 3 months. Between 6 and 9 months, Ctns−/− mice consistently developed multifocal thyrocyte hypertrophy and hyperplasia with pseudostratification up to papillary lesions, luminal cell remnants (Figure 1B, b and c), and colloid exhaustion (Figure 1Bb). As better seen with 1-μm plastic sections, hypertrophic thyrocytes exhibited apical vacuolation, suggesting (TSH)-induced macropinocytosis/phagocytosis, and irregular basal cytoplasm clarification, suggesting ER dilatation (Figure 1Bc). As disease progressed, luminal cell remnants accumulated (Figure 1Bc) and colloid vanished (Supplemental Figure 1). At 15 months, most Ctns−/− follicles were hyperplastic or dedifferentiated, with few resting follicles remaining visible. In 15-month WT thyroids, most follicles remained quiescent and few peripheral follicles were activated. To focus on consistent early physiopathological mechanisms, mice were further analyzed at approximately 9 months.
The importance of thyroid capillaries as integrated part of autonomous angiofollicular units has emerged (46). Because blood capillaries are barely visible by conventional histology, we looked for vascular changes by triple immunofluorescence confocal imaging, using E-cadherin and ezrin as markers of thyrocyte membrane domains, together with PECAM for blood capillaries. Ctns−/− hyperplastic follicles were consistently associated with prominent dilated capillaries as compared with resting follicles, indicating synchronous activation of the angiofollicular system (Figure 1C). Proangiogenic Vegf-a was up-regulated in hypertrophic thyrocytes, mostly in papillary projections, in full agreement with recruitment/expansion of blood capillaries (Supplemental Figure 2). Thus, Ctns−/− mice exhibited integrated angiofollicular activation.
To quantitate tissue changes by taking into account disease-induced heterogeneity between mice and between follicles, we exploited E-cadherin immunofluorescence. As shown by Figure 1D, thyrocyte fractional volume was increased by 2.2-fold in Ctns−/− mice (19.9% in WT vs 42.9% in Ctns−/− mice) with a concomitant decrease of fractional luminal volume (64.5% in WT vs 39.9% in Ctns−/− mice). Both parameters showed strong negative correlation (r = 0.93; P < .0001; data not illustrated). No significant difference was observed between WT and Ctns−/− mice for interstitial area (combined mesenchyme and blood capillaries). We thus focused on thyrocytes for further structural studies.
Increased proliferation and apoptosis in Ctns−/− thyrocytes reveals accelerated cell turnover
Cell division is rare in normal adult thyrocytes (47) but was expected to increase to support hyperplasia in Ctns−/− thyroids. Conversely, the striking abundance of luminal remnants was reminiscent of in vitro and in vivo evidence that cystinosis triggers apoptosis in other cells/tissues (33, 48, 49). To define the impact of cystinosis on thyrocyte turnover, we analyzed the proliferation and apoptosis (Figure 2, A and B). Thyrocyte proliferation, monitored by Ki-67 immunolabeling, was barely detected in WT (<3%) but significantly increased in Ctns−/− mice (by 4.5-fold). Interstitial cell proliferation did not reach a significant difference between WT and Ctns−/− mice (Figure 3B, except if values of one outlier WT mouse were excluded; P < .01). Likewise, in WT thyroids, apoptotic events (monitored by active caspase-3 immunolabeling) were very rare (Figure 2Aa), consistent with a resting cell population. In contrast, caspase-3a-labeled apoptotic bodies accumulated in Ctns−/− follicular lumen, confirming accelerated cell turnover (Figure 2A, b and c). The distribution of apoptotic cells among follicles was much more heterogeneous than proliferative events, probably due to follicle heterogeneity in disease progression and unequal long-term retention of apoptotic bodies in follicular lumina [in contrast to continuous shedding in kidney proximal tubules; (33)].
Ctns−/− mice develop subclinical hypothyroidism
Because histological alterations of Ctns−/− thyroid suggested TSH activation and because cystinotic children develop subclinical hypothyroidism, we next evaluated the thyroid hormonal status in Ctns−/− mice and analyzed the expression of critical genes involved in thyroid hormone synthesis. As shown in Figure 3A, plasma TSH concentrations of Ctns−/− mice at 9–10 months were significantly increased (by 4.4-fold) as compared with WT mice, a feedback response sufficient to maintain normal plasma T4 and T3 concentrations. Although mRNA of some components of thyroid hormone biosynthetic machinery were moderately altered [pendrin, dual oxidase (DUOX)-2, DUOXA2, dehalogenase 1 (DEHAL1)], none (including the basolateral transporter of TH, Mct-8) was really defective in Ctns−/− mice (Figure 3B). We concluded that Ctns−/− mice mimic subclinical hypothyroidism of cystinotic children.
Tg synthesis is quantitatively but not qualitatively altered in Ctns−/− mice
As an explanation for colloid exhaustion observed in Ctns−/− thyroids, we first looked at Tg biosynthetic machinery, including ER structure, disulfide-bonding, and N-glycosylation. Electron microscopy revealed prominent ER dilatation in the hypertrophic Ctns−/− thyrocytes, contrasting with normal Golgi complex (Figure 4, A vs B). Also by confocal microscopy, immunolabeling for the C-terminal ER-retention motive, KDEL, confirmed that basolateral dilations seen in semithin plastic sections of hypertrophic thyrocytes (Figure 1C), reflected a major enlargement of this compartment (Figure 5A, b and c). Simultaneous Tg immunolabeling disclosed its accumulation in the dilated ER, compatible with either increased synthesis upon TSH stimulation or defective export, eg, upon ER stress (50) (Figure 5A, b and c). To discriminate between these two hypotheses, we quantified Tg mRNA expression in thyroids at 9 and 12 months and found a significant decrease in Ctns−/− as compared with WT mice (Figure 5B). Western blotting on thyroid lysates confirmed a decreased total Tg content and revealed a decreased proportion of high-molecular-weight Tg (ie, cross-linked) in Ctns−/− thyroids (Figure 5C). Irrespectively of the cystinosin status, Tg could be completely reduced by DTT into 330-kDa monomers (Figure 5C) by as low as 0.3 mM DTT (not shown). These data indicated that Tg could still oligomerize in Ctns−/− follicle lumina and suggested accelerated colloid turnover. Of note, the extent of cross-linking differed between mice studied here (low) and young human adults (higher) (21): species differences should be kept in mind when extrapolating conclusions from cystinotic mice to patients. Analysis of Tg terminal N-glycosylation by sialic acid lectin blotting of thyroid lysates revealed no major difference between WT and Ctns−/− mice (Figure 5D). We thus concluded that Tg processing was qualitatively preserved in Ctns−/− mice and that ER enlargement was not due to increased Tg synthesis, pointing instead to quantitative defect in export, possibly upon ER stress.
Figure 4.
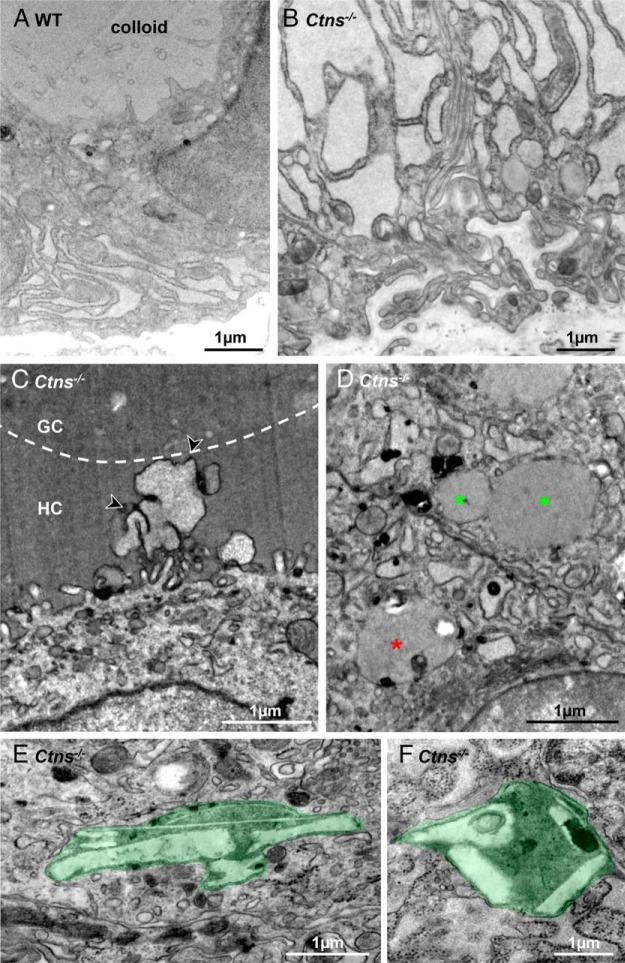
Ultrastructural alterations in Ctns−/− thyrocytes. Representative EM views of resting WT (A) and activated Ctns−/− thyrocytes (B–F). A, In this resting WT thyrocyte at 12 months, notice limited ER expansion and thin apical projections. B, Hyperplastic Ctns−/− thyrocyte at 12 months showing strong dilation of endoplasmic reticulum lumen. Notice characteristic tortuous basal plasma membrane and basal lamina. C, This activated Ctns−/− thyrocyte at 8 months projects a lamellipodium, characteristic of macropinocytosis (arrowheads) across a homogenous peripheral colloid ring (HC) up to central granular colloid (GC), indicated by the broken line. D, Asterisks indicate colloid droplets in two adjacent activated thyrocytes, two with homogenous colloid content and undergoing homotypic fusion (green asterisks), one bearing additional luminal structures indicating fusion with lysosomes (red asterisk). E and F, Cystine crystals-bearing lysosomes in Ctns−/− thyrocytes at 12 months.
Ctns−/− thyrocytes develop the unfolded protein response to ER stress
Secretory cells are particularly prone to ER stress, previously documented in activated thyrocytes (38, 50). Moreover, cystinosis has been associated with ER stress (51). We thus evaluated whether the UPR was activated in response to ER stress in Ctns−/− thyroid by looking at UPR-target genes and products (52, 53). The ER-resident chaperone GRP78 and PDI, both bearing the KDEL ER-retention motive, were increased at the protein level in Ctns−/− thyroid homogenates (Figure 6A). Downstream in the UPR pathway, unconventional splicing of transcription factor XBP-1 mRNA, yielding the spliced XBP-1 form, was detected in almost all Ctns−/− thyroids but only in a minority of WT thyroids. As illustrated by Figure 6B, at 9 months, 10 of 13 Ctns−/− vs 3 of 9 WT mice thyroids exhibited nonconventional XBP-1 mRNA splicing (P < .001); at 12 months, the proportion were 8 of 9 Ctns−/− vs 2 of 8 WT (P < .01; not shown). Further in the UPR pathway, induction of ATF-4 expression strongly correlated with increased expression of its downstream effector, the transcription factor CHOP (Figure 6C). These data together supported the hypothesis that activation of ER stress/UPR pathway in Ctns−/− thyrocytes not only leads to defective Tg synthesis and secretion, thus colloid exhaustion, but also contributes to apoptosis triggering.
Lysosomal Tg processing is altered in Ctns−/− mice
In addition to UPR effects, our alternative working hypothesis for primary hypothyroidism was the defective release of thyroid hormones from iodo-Tg. To this aim, we looked for ultrastructural alterations of the endocytic apparatus (Figure 4) and at LAMP-1 immunofluorescence for late endosomes/lysosomes (Figure 7). By EM, although the apical surface of resting WT thyrocytes showed only sparse thin microvilli (Figure 4A), Ctns−/− thyrocytes in activated follicles frequently showed apical lamellipodia (Figure 4C), sequestration of primary colloid droplets, and their fusion into phagolysosomes (Figure 4D). These are characteristic of TSH-induced macropinocytosis, the structural equivalent of accelerated endocytosis. In contrast, we detected no change in the abundance of Rab5 and Rab7 mRNAs, which finely tune micropinocytosis (Supplemental Figure 4). By immunofluorescence, induced macropinocytosis correlated with enlarged LAMP-1-labeled apical structures (Figure 7Ab), absent in resting WT thyrocytes (Figure 7Aa). In addition, LAMP-1 labeled some distorted structures (Figure 7Ac), either strongly elongated or showing angular membranes, reminiscent of the abundant lysosome-bearing crystals in Ctns−/− kidney proximal tubular cells (33). Electron microscopy of Ctns−/− thyrocytes yielded several examples of severely distorted secondary lysosomes, identified by a limiting membrane and heterogeneous content and containing characteristic electron-lucent needles or long polyhedric objects, ie, bona fide cystine crystals (Figure 4, E and F). Remarkably, small LAMP-1-labeled vesicles appeared closely apposed to distorted LAMP-1-labeled structures, suggesting lysosomal docking but impaired fusion (Figure 7Ac) as seen in Ctns−/− PTCs in which a late endocytic trafficking defect has been evidenced.
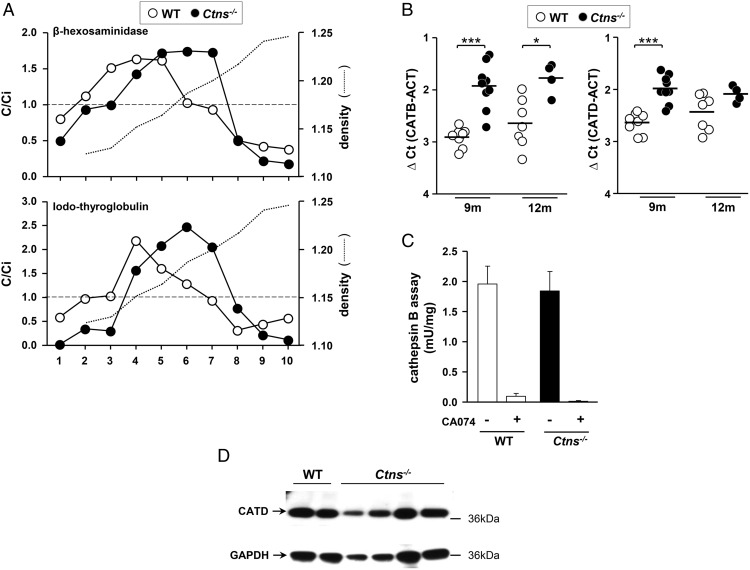
Because late endocytic trafficking seemed affected in Ctns−/− thyrocytes, we looked for a functional impact on iodo-Tg processing to explain TSH feedback and partial TSH refractoriness. By immunofluorescence, we frequently found iodo-Tg retained in LAMP-1-labeled lysosomes selectively in Ctns−/− hypertrophic thyrocytes, suggesting defective prohormone processing (Figure 7B, b–e, and Supplemental Figure 3). This was never observed in WT thyrocytes. Furthermore, by subcellular fractionation using isopycnic centrifugation combined with Western blotting, we demonstrated that Ctns−/− thyroid lysosomes were denser and contained more iodo-Tg as compared with WT (Figure 8A and Supplemental Figure 5). To examine whether accumulation of iodo-Tg in lysosomes could be due to impaired lysosomal enzymatic machinery, we also looked at the expression and activity of cathepsin B and D. Both cathepsins were actually increased at the mRNA level, but cathepsin B activity and cathepsin D protein level were not appreciably affected in Ctns−/− thyrocytes. We concluded that TH release from Tg in lysosomes is further impaired in Ctns−/− thyrocytes, whereas major cathepsins are preserved, pointing instead to a defect in the lysosomal milieu, likely its redox status.
Figure 8.
Subcellular fractionation of iodo-thyroglobulin distribution and assessment of hormonogenic cathepsins. A, Sucrose density gradients. Thyroids glands pooled from four WT (open symbols) or four Ctns−/− mice (filled symbols) were homogenized and postnuclear particles were resolved by isopycnic centrifugation into linear sucrose gradients. Ten fractions were collected from the bottom and analyzed for density (dotted lines), β-Hexosaminidase activity as lysosomal marker (upper panel), and iodo-Tg (lower panel; densitometry of Western blot bands with molecular weight >250 kDa, ie, the sum of monomer and oligomers). Results are expressed by reference to the sum of all fractions so that C/Ci indices greater than 1 indicate enrichment level over initial concentration (broken lines). Corresponding Western blots are shown in Supplemental Figure 5. In Ctns−/− mice, iodo-Tg accumulates in fractions corresponding to lysosomes, equilibrating at higher density as compared with WT mice. Representative experiment of two is shown. B–D, Cathepsins B/D expression and activity are not defective in Ctns−/− thyroid glands. B, Expression of cathepsin B and D mRNAs. Quantification by RT-qPCR of cathepsin B (CATB) and cathepsin D (CATD) mRNAs in WT and Ctns−/− thyroids collected at 9 and 12 months, normalized to actin mRNA (ACT) and presented as ΔCt values. C, Cathepsin B activity. Cathepsin B activity, assayed using benzyloxycarbonyl-L-phenylalanyl-L-arginine 4-methylcoumaryl-7-amide, is given by the difference in absence or presence of the specific inhibitor, CA-074; data normalized to protein concentration. D, Western blot for cathepsin D. Thyroid extracts from two WT and four Ctns−/− individual mice at 11–12 months were analyzed by Western blotting with antibodies to cathepsin D (CATD), using GAPDH for normalization in parallel blots.
Discussion
In this study, we report for the first time that C57BL/6 Ctns−/− mice recapitulate the earliest and almost obligatory endocrine complication of cystinotic children, namely primary hypothyroidism. The longitudinal study of knockout mice thus allowed to delineate the early events of thyroid changes, presumably also occurring in affected children before the end-stage atrophy mostly documented in pathological samples. Two complementary pathogenic mechanisms were found to operate in Ctns−/− mice: 1) impaired Tg biosynthesis involving the unfolded protein response to ER stress and contributing to progressive colloid exhaustion; and 2) impaired lysosomal iodo-Tg proteolytic processing, thus defective TH release. Adaptation mechanisms include TSH increase, accelerated colloid uptake by macropinocytosis, thyrocyte hyperplasia/hyperthrophy combined with microvascular basket expansion, and accelerated cell turnover/apoptosis.
In 9-month Ctns−/− mice, TSH was moderately increased with T4 and T3 values remaining normal. Increased TSH induced follicle-autonomous hyperplasia/hypertrophy and microvascular basket expansion but was not associated with significant decrease in global expression of iodo-Tg synthesis-related genes except Tg itself, nor cathepsins B and D or of the thyroid hormone transporter, Mct-8. Thus, primary hypothyroidism was adequately compensated at this age (subclinical) and originated from a more subtle mechanism. Immunofluorescence proved particularly useful to demonstrate proliferation, apoptosis, and microvasculature changes. In the integrated angiofollicular units, capillaries not only serve to passively feed thyrocytes and to collect TH but also take part in an active, bidirectional paracrine cross-talk that instructs follicular embryological differentiation (54). Furthermore, follicular changes upon iodine deficiency/goitrogenesis are closely associated with increased thyroid blood flow and vascular expansion (55, 56). Thus, expansion of follicular capillaries not only reflects increased tissue demands but also can be a useful independent functional marker of follicle activation.
The first key pathogenic finding of this study was activation of the UPR. Newly synthesized Tg accounts for greater than 50% of normal thyrocyte protein content (57) and can be further increased by TSH (58, 59). Professional secretory cells such as thyrocytes have a well-developed pathway for protein export and rely on a sophisticated quality control machinery to escape ER stress when overstimulated. However, when ER folding capacity is exceeded or fully abrogated by Tg point mutations (38, 60), UPR is triggered to attenuate protein synthesis, up-regulate folding capacity, and increase protein degradation by proteasomes. We here demonstrate that Tg accumulates in the dilated ER of Ctns−/− thyrocytes yet with decreased mRNA level despite higher TSH stimulation. Folding of core-glycosylated Tg necessitates the simultaneous assistance of a variety of ER-resident chaperones (eg, GRP78) and foldase (PDI), both of which were strongly increased in Ctns−/− thyroids. GRP78 is a major quality-control monitor of Tg folding status (61, 62). Formation of mixed-disulfide folding intermediates between Tg and the ER oxidoreductase, PDI, is crucial for Tg maturation and export (63). These chaperones functionally depend on ER redox homeostasis and high ATP levels, both of which are impaired in cystinosis (51, 64, 65). Combined with general concepts from literature, our data on Ctns−/− thyrocytes are thus consistent with the following hypotheses: 1) correct Tg disulfide bonding is slower due to impaired luminal redox, which results in misfolded/unfolded Tg accumulation in ER, triggering the UPR response; 2) consequently, slower ER exit/impaired secretion leads to ER dilatation and contributes to colloid exhaustion.
Activation of ER-resident chaperones associated with UPR has been analyzed in detail in a congenital hypothyroidism goiter due to a mutated Tg trafficking defect (38, 66, 67). In contrast, immortalized FRTL5 cells showed increased expression of ER chaperones upon activation of Tg synthesis by TSH independently of UPR (no alternative XBP-1 splicing or CHOP expression) (68). In most 9- and 12-month-old Ctns−/− thyroids, we here report the alternative splicing of XBP-1 as well as coordinated increased expression of ATF-4 and CHOP, strongly supporting activation of UPR in response to ER stress. This conclusion has several implications. XBP-1 transcriptional activation triggers ER expansion (69, 70), as we observed in Ctns−/− thyrocytes, but also promotes gene transcription of ER resident chaperones (71) and of proteins involved in ER-associated degradation of misfolded/unfolded protein by proteasome. Moreover, induced CHOP expression triggers apoptosis, in particular via the down-regulation of the major antiapoptotic regulator, Bcl-2 (for a review, see reference 40). This mechanism likely contributes to apoptotic thyrocyte shedding, as evidenced by the accumulation of cell remnants immunolabeled for cleaved caspase-3 in the Ctns−/− colloid.
Also consistent with a role for UPR in cystinotic thyroid physiopathology, activation of UPR has been demonstrated in cystinotic proximal tubular cells (37, 51, 72) and in several noncystinotic lysosomal storage diseases (72). Interestingly, rescue of Rab27a-dependent vesicular trafficking alleviated defective lysosomal transport and reduced ER stress in cystinotic proximal tubular cells (37). Rab27a is a Ras-related small GTPase that regulates vesicular transport and exocytosis in a variety of secretory cells, including thyrocytes. We therefore raise the possibility that, in the thyroid gland, lysosomal vesicular transport defect due to cystine overload may be linked to ER stress and UPR activation.
Our second key pathogenic observations in Ctns−/− thyrocytes relate to structural and functional endocytic alterations. These include the following: 1) induced macropinocytosis as expected for TSH stimulation; 2) retention of undigested iodo-Tg in enlarged (LAMP-1 immunolabeling) and denser endosomes/lysosomes (fractionation data), indicating defective Tg lysosomal degradation without alteration of lysosomal cathepsin expression and activity; and 3) progressive build-up of lysosomal cystine needles and exclusion of cystine-crystal bearing lysosomes from endocytic trafficking. Increased lysosomal density is better accounted for by protein (density 1.33 g/mL) than cystine crystal accumulation [1.73 g/mL (73)]. Similar alterations in the apical endocytic pathway have recently been demonstrated in Ctns−/− kidney PTCs, but lesions appeared earlier on and crystals were more prominent than in thyrocytes (33, 34). Although kidney PTCs and thyrocytes are both specialized for apical endocytosis, the constitutive endocytic rate is faster in PTCs, and the sequence of kidney then thyroid lesions in Ctns−/− mice also mimics the order of appearance of clinical signs in cystinotic children (4).
Lysosomal cargo retention despite conservation of hydrolytic equipment implies an altered lysosomal milieu, probably an impaired redox environment as immediate consequence of cystine sequestration. Alteration of intracellular redox potential due to defective lysosomal cystine export has been broadly associated to cystinosis physiopathology (64). Thyroid hormone release from Tg requires stepwise proteolytic processing by synergistic endopeptidases acting at specific sites around conserved N-terminal and C-terminal hormonogenic residues, allowing for final pruning by exopeptidases (23–27). However, the crucial endopeptidases cathepsin B and D were not appreciably affected in Ctns−/− thyrocytes. The need for a lysosomal supply of reducing equivalents to expose buried cathepsin-sensitive peptide is supported by the acceleration of Tg degradation by lysosomal proteinases in a reducing environment (22, 74). In vitro, the addition of reduced glutathione boosted degradation of Tg by a thyroid phagolysosome-enriched fraction (22). This effect was originally attributed to substrate unfolding, a conclusion confirmed with highly purified cathepsin D and 125I-thyroglobulin, supporting the concept that Tg unfolding by disulfide bond reduction renders it more susceptible to proteolysis (75). However, a combined effect on activation of cysteine proteinases is now well accepted (15, 23). A lysosomal cysteine import system has been demonstrated (14), but associated gene(s) remain to be identified. We thus propose that, in Ctns−/− thyrocytes, alteration of lysosomal redox status upon cystine accumulation impairs cathepsin action. In turn, defective lysosomal processing of iodo-Tg leads to decreased TH production, thus primary hypothyroidism, eliciting a compensatory TSH response, thyrocyte hypertrophy/hyperplasia, and integrated vascular expansion as well as accelerated endocytosis by macropinocytosis.
Endocrine dysfunction related to a lysosomal storage disorder is not unique to cystinosis. Subclinical hypothyroidism with elevated TSH has been reported in patients affected by Fabry disease [lysosomal galactosidase-A deficiency (76, 77) and Hurler syndrome/mucopolysaccharidosis type IH (α-L-iduronidase deficiency (78)], which are more frequent than cystinosis. However, to the best of our knowledge, their underlying thyroid physiopathology has not been explored. Comparison of Tg synthesis and processing into TH in corresponding knockout mouse models would be interesting.
In conclusion, 9-month-old C57BL/6 Ctns−/− mice recapitulate several key features of infantile cystinosis underlying compensated/subclinical hypothyroidism, namely chronically increased TSH, follicular activation and proliferation, and eventual thyrocyte lysosomal crystals. They also disclose early pathogenic, so far unreported mechanisms, such as ER stress triggering UPR, itself contributing to apoptosis; and impaired endolysosomal trafficking associated with defective lysosomal Tg processing. Combination of impaired Tg secretion and accelerated endocytosis provide a satisfactory explanation for colloid exhaustion. We suggest that defective Tg processing following silent accumulation of Tg-derived cystine (single substrate) is the primum movens event of the other functional and structural changes. Thus, C57BL/6 Ctns−/− mice are a useful model to better understand early pathogenic vs adaptative cascades leading to eventual cystinotic thyroid atrophy and to evaluate the stage-specific benefit (and limitations) of conventional (cysteamine) or new drugs to be developed as well as novel therapies such as gene and stem cell therapy (79–81). A particularly promising generic approach for gene therapy, validated in mice, is based on hematopoietic stem cell correction, by which immunocompatible grafted cells bearing normal cystinosin (or other) genes are selectively attracted to diseased tissue areas. We recently reported that hematopoietic stem cells project expansions, known as tunneling nanotubes, whereby they physically interconnect with diseased epithelial cells across basement laminae and can bidirectionally exchange lysosomes by tubulin-based motion (82).
Acknowledgments
We thank Dr E. Marbaix for helpful advice in thyroid pathology, Dr T. Arnould for valuable suggestions on endoplasmic reticulum stress, Dr C. Ris-Stalpers for providing anti-iodo-Tg antibodies, and Mrs L. Thanh for assistance with the electron microscopy.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
H.P.G.C. is Postdoctoral Researcher and C.E.P. is Senior Research Associate at Belgian Fonds de la Recherche Scientifique (Belgium).
This work was mainly supported by the Cystinosis Research Foundation, Belgian Science Policy Office-Interuniversity Attraction Poles Program Grant IAP P7/43-BeMGI, Belgian Fonds de la Recherche Scientifique and Actions de Recherche Concertées (to C.E.P. and P.J.C.), National Institutes of Health Grants RO1-DK090058, R21-DK090548, and RO1-DK099338 (to S.C.). This work was also supported in part by Grants R37-DK15070 from the National Institutes of Health (to S.R.). The Platform for Imaging Cells and Tissues was financed by National Lottery, Région Bruxelloise, Région Wallonne, Université Catholique de Louvain, and de Duve Institute (to P.J.C.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATF
- activating transcription factor
- CHOP
- C/EBP homologous protein
- ΔCt
- difference of cycle threshold
- DEHAL1
- dehalogenase 1
- DTT
- dithiothreitol
- DUOX
- dual oxidase
- EM
- electron microscopy
- ER
- endoplasmic reticulum
- GAPDH
- glyceraldehyde-3 phosphate deshydrogenase
- GRP78
- glucose-regulated protein of 78 kDa
- LAMP-1
- lysosome-associated membrane protein-1
- Mct-8
- monocarboxylate transporter-8
- PDI
- protein disulfide isomerase
- PECAM-1
- platelet endothelial cell adhesion molecule-1
- PTC
- proximal tubular cell
- RT-qPCR
- real-time quantitative PCR
- Tg
- thyroglobulin
- TH
- thyroid hormone
- UPR
- unfolded protein response
- XBP-1
- X-box binding protein-1
- WT
- wild type.
References
- 1. Van Vliet G. Development of the thyroid gland: lessons from congenitally hypothyroid mice and men. Clin Genet. 2003;63(6):445–455. [DOI] [PubMed] [Google Scholar]
- 2. Grasberger H, Refetoff S. Congenital defects of thyroid hormone synthesis. In: Weiss RE, Refetoff S. eds. Genetics Diagnosis of Endocrine Disorders. Amsterdam, The Netherlands: Academic Press, Elsevier, Inc; 2010:87–95. [Google Scholar]
- 3. Gahl WA, Thoene JG, Schneider JA. Cystinosis. N Engl J Med. 2002;347(2):111–121. [DOI] [PubMed] [Google Scholar]
- 4. Gahl WA, Thoene J. Cystinosis: a disorder of lysosomal membrane transport. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Childs B, Kinzler KW, Vogelstein B, eds. The Metabolic and Molecular Basis of Inherited Disease. Vol 3, 8th ed New York: McGraw-Hill Companies, Inc; 2001:5085–5108 Online update 2013. [Google Scholar]
- 5. Chan AM, Lynch MJ, Bailey JD, Ezrin C, Fraser D. Hypothyroidism in cystinosis. A clinical, endocrinologic and histologic study involving sixteen patients with cystinosis. Am J Med. 1970;48(6):678–692. [DOI] [PubMed] [Google Scholar]
- 6. Broyer M, Niaudet P. Cystinosis. In: Saudubray JM, van den Berghe G, Walter JH, eds. Inborn Metabolic Diseases: Berlin Heidelberg: Springer; 2012:617–624. [Google Scholar]
- 7. Cherqui S, Kalatzis V, Trugnan G, Antignac C. The targeting of cystinosin to the lysosomal membrane requires a tyrosine-based signal and a novel sorting motif. J Biol Chem. 2001;276(16):13314–13321. [DOI] [PubMed] [Google Scholar]
- 8. Kalatzis V, Cherqui S, Antignac C, Gasnier B. Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter. EMBO J. 2001;20(21):5940–5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jezegou A, Llinares E, Anne C, et al. Heptahelical protein PQLC2 is a lysosomal cationic amino acid exporter underlying the action of cysteamine in cystinosis therapy. Proc Natl Acad Sci USA. 2012;109(50):E3434–E3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lucky AW, Howley PM, Megyesi K, Spielberg SP, Schulman JD. Endocrine studies in cystinosis: compensated primary hypothyroidism. J Pediatr. 1977;91(2):204–210. [DOI] [PubMed] [Google Scholar]
- 11. Kimonis VE, Troendle J, Rose SR, Yang ML, Markello TC, Gahl WA. Effects of early cysteamine therapy on thyroid function and growth in nephropathic cystinosis. J Clin Endocrinol Metab. 1995;80(11):3257–3261. [DOI] [PubMed] [Google Scholar]
- 12. Brodin-Sartorius A, Tete MJ, Niaudet P, et al. Cysteamine therapy delays the progression of nephropathic cystinosis in late adolescents and adults. Kidney Int. 2012;81(2):179–189. [DOI] [PubMed] [Google Scholar]
- 13. Emma F, Nesterova G, Langman C, et al. Nephropathic cystinosis: an international consensus document. Nephrol Dialysis Transplant. 2014;29(suppl 4):iv87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pisoni RL, Acker TL, Lisowski KM, Lemons RM, Thoene JG. A cysteine-specific lysosomal transport system provides a major route for the delivery of thiol to human fibroblast lysosomes: possible role in supporting lysosomal proteolysis. J Cell Biol. 1990;110(2):327–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Brix K, Lemansky P, Herzog V. Evidence for extracellularly acting cathepsins mediating thyroid hormone liberation in thyroid epithelial cells. Endocrinology. 1996;137(5):1963–1974. [DOI] [PubMed] [Google Scholar]
- 16. Friedrichs B, Tepel C, Reinheckel T, et al. Thyroid functions of mouse cathepsins B, K, and L. J Clin Invest. 2003;111(11):1733–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Edelhoch H, Rall J. The proteins and enzymes of the thyroid In: Pitt-Rivers R, Trotter W, eds. The Thyroid Gland. London: Butterworths; 1964:113–130. [Google Scholar]
- 18. Herzog V, Berndorfer U, Saber Y. Isolation of insoluble secretory product from bovine thyroid: extracellular storage of thyroglobulin in covalently cross-linked form. J Cell Biol. 1992;118(5):1071–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gerard AC, Denef JF, Colin IM, van den Hove MF. Evidence for processing of compact insoluble thyroglobulin globules in relation with follicular cell functional activity in the human and the mouse thyroid. Eur J Endocrinol. 2004;150(1):73–80. [DOI] [PubMed] [Google Scholar]
- 20. Klein M, Gestmann I, Berndorfer U, Schmitz A, Herzog V. The thioredoxin boxes of thyroglobulin: possible implications for intermolecular disulfide bond formation in the follicle lumen. Biol Chem. 2000;381(7):593–601. [DOI] [PubMed] [Google Scholar]
- 21. Berndorfer U, Wilms H, Herzog V. Multimerization of thyroglobulin (TG) during extracellular storage: isolation of highly cross-linked TG from human thyroids. J Clin Endocrinol Metab. 1996;81(5):1918–1926. [DOI] [PubMed] [Google Scholar]
- 22. Peake BL, Balasubramaniam K, Deiss WP. Effect of reduced glutathione on the proteolysis of intraparticulate and native thyroglobulin. Biochim Biophys Acta. 1967;148:689–702. [Google Scholar]
- 23. Yoshinari M, Taurog A. Lysosomal digestion of thyroglobulin: role of cathepsin D and thiol proteases. Endocrinology. 1985;117(4):1621–1631. [DOI] [PubMed] [Google Scholar]
- 24. Dunn AD, Dunn JT. Cysteine proteinases from human thyroids and their actions on thyroglobulin. Endocrinology. 1988;123(2):1089–1097. [DOI] [PubMed] [Google Scholar]
- 25. Dunn AD, Crutchfield HE, Dunn JT. Proteolytic processing of thyroglobulin by extracts of thyroid lysosomes. Endocrinology. 1991;128(6):3073–3080. [DOI] [PubMed] [Google Scholar]
- 26. Dunn AD, Myers HE, Dunn JT. The combined action of two thyroidal proteases releases T4 from the dominant hormone-forming site of thyroglobulin. Endocrinology. 1996;137(8):3279–3285. [DOI] [PubMed] [Google Scholar]
- 27. Nakagawa H, Ohtaki S. Thyroxine (T4) release from thyroglobulin and its T4-containing peptide by thyroid thiol proteases. Endocrinology. 1985;116(4):1433–1439. [DOI] [PubMed] [Google Scholar]
- 28. Marino M, McCluskey RT. Role of thyroglobulin endocytic pathways in the control of thyroid hormone release. Am J Physiol Cell Physiol. 2000;279(5):C1295–C1306. [DOI] [PubMed] [Google Scholar]
- 29. Croizet-Berger K, Daumerie C, Couvreur M, Courtoy PJ, van den Hove MF. The endocytic catalysts, Rab5a and Rab7, are tandem regulators of thyroid hormone production. Proc Natl Acad Sci USA. 2002;99(12):8277–8282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. van den Hove MF, Croizet-Berger K, Tyteca D, Selvais C, de Diesbach P, Courtoy PJ. Thyrotropin activates guanosine 5′-diphosphate/guanosine 5′-triphosphate exchange on the rate-limiting endocytic catalyst, Rab5a, in human thyrocytes in vivo and in vitro. J Clin Endocrinol Metab. 2007;92(7):2803–2810. [DOI] [PubMed] [Google Scholar]
- 31. Fujita H. Functional morphology of the thyroid. Int Rev Cytol. 1988;113:145–185. [DOI] [PubMed] [Google Scholar]
- 32. Di Cosmo C, Liao XH, Dumitrescu AM, Philp NJ, Weiss RE, Refetoff S. Mice deficient in MCT8 reveal a mechanism regulating thyroid hormone secretion. J Clin Invest. 2010;120(9):3377–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gaide Chevronnay HP, Janssens V, Van Der Smissen P, et al. Time course of pathogenic and adaptation mechanisms in cystinotic mouse kidneys. J Am Soc Nephrol. 2014;25(6):1256–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Raggi C, Luciani A, Nevo N, Antignac C, Terryn S, Devuyst O. Dedifferentiation and aberrations of the endolysosomal compartment characterize the early stage of nephropathic cystinosis. Hum Mol Genet. 2014;23(9):2266–2278. [DOI] [PubMed] [Google Scholar]
- 35. Cherqui S, Sevin C, Hamard G, et al. Intralysosomal cystine accumulation in mice lacking cystinosin, the protein defective in cystinosis. Mol Cell Biol. 2002;22(21):7622–7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nevo N, Chol M, Bailleux A, et al. Renal phenotype of the cystinosis mouse model is dependent upon genetic background. Nephrol Dialysis Transpl. 2010;25(4):1059–1066. [DOI] [PubMed] [Google Scholar]
- 37. Johnson JL, Napolitano G, Monfregola J, Rocca CJ, Cherqui S, Catz SD. Upregulation of the Rab27a-dependent trafficking and secretory mechanisms improves lysosomal transport, alleviates endoplasmic reticulum stress, and reduces lysosome overload in cystinosis. Mol Cell Biol. 2013;33(15):2950–2962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baryshev M, Sargsyan E, Wallin G, et al. Unfolded protein response is involved in the pathology of human congenital hypothyroid goiter and rat non-goitrous congenital hypothyroidism. J Mol Endocrinol. 2004;32(3):903–920. [DOI] [PubMed] [Google Scholar]
- 39. Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Bio. 2012;13(2):89–102. [DOI] [PubMed] [Google Scholar]
- 40. Szegezdi E, Logue SE, Gorman AM, Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7(9):880–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pohlenz J, Maqueem A, Cua K, Weiss RE, Van Sande J, Refetoff S. Improved radioimmunoassay for measurement of mouse thyrotropin in serum: strain differences in thyrotropin concentration and thyrotroph sensitivity to thyroid hormone. Thyroid. 1999;9(12):1265–1271. [DOI] [PubMed] [Google Scholar]
- 42. Pierreux CE, Cordi S, Hick AC, et al. Epithelial: endothelial cross-talk regulates exocrine differentiation in developing pancreas. Dev Biol. 2010;347(1):216–227. [DOI] [PubMed] [Google Scholar]
- 43. Gaide Chevronnay HP, Cornet PB, Delvaux D, et al. Opposite regulation of transforming growth factors-β2 and -β3 expression in the human endometrium. Endocrinology. 2008;149(3):1015–1025. [DOI] [PubMed] [Google Scholar]
- 44. Zachara NE, Vosseller K, Hart GW. Detection and analysis of proteins modified by O-linked N-acetylglucosamine. Curr Protoc Protein Sci. 2011;Chapter 12:Unit12.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Barrett AJ. Fluorimetric assays for cathepsin B and cathepsin H with methylcoumarylamide substrates. Biochem J. 1980;187(3):909–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Colin IM, Denef JF, Lengele B, Many MC, Gerard AC. Recent insights into the cell biology of thyroid angiofollicular units. Endocr Rev. 2013;34(2):209–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Coclet J, Foureau F, Ketelbant P, Galand P, Dumont JE. Cell population kinetics in dog and human adult thyroid. Clin Endocrinol (Oxf). 1989;31(6):655–665. [DOI] [PubMed] [Google Scholar]
- 48. Park MA, Thoene JG. Potential role of apoptosis in development of the cystinotic phenotype. Pediatr Nephrol. 2005;20(4):441–446. [DOI] [PubMed] [Google Scholar]
- 49. Sansanwal P, Kambham N, Sarwal MM. Caspase-4 may play a role in loss of proximal tubules and renal injury in nephropathic cystinosis. Pediatr Nephrol. 2010;25(1):105–109. [DOI] [PubMed] [Google Scholar]
- 50. Leonardi A, Vito P, Mauro C, et al. Endoplasmic reticulum stress causes thyroglobulin retention in this organelle and triggers activation of nuclear factor-κB via tumor necrosis factor receptor-associated factor 2. Endocrinology. 2002;143(6):2169–2177. [DOI] [PubMed] [Google Scholar]
- 51. Sansanwal P, Li L, Hsieh SC, Sarwal MM. Insights into novel cellular injury mechanisms by gene expression profiling in nephropathic cystinosis. J Inherit Metab Dis. 2010;33(6):775–786. [DOI] [PubMed] [Google Scholar]
- 52. Oslowski CM, Urano F. Measuring ER stress and the unfolded protein response using mammalian tissue culture system. Methods Enzymol. 2011;490:71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Samali A, Fitzgerald U, Deegan S, Gupta S. Methods for monitoring endoplasmic reticulum stress and the unfolded protein response. Int J Cell Biol. 2010;2010:830307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hick AC, Delmarcelle AS, Bouquet M, et al. Reciprocal epithelial:endothelial paracrine interactions during thyroid development govern follicular organization and C-cells differentiation. Dev Biol. 2013;381(1):227–240. [DOI] [PubMed] [Google Scholar]
- 55. Ramsden JD, Buchanan MA, Egginton S, Watkinson JC, Mautner V, Eggo MC. Complete inhibition of goiter in mice requires combined gene therapy modification of angiopoietin, vascular endothelial growth factor, and fibroblast growth factor signaling. Endocrinology. 2005;146(7):2895–2902. [DOI] [PubMed] [Google Scholar]
- 56. Imada M, Kurosumi M, Fujita H. Three-dimensional aspects of blood vessels in thyroids from normal, low iodine diet-treated, TSH-treated, and PTU-treated rats. Cell Tissue Res. 1986;245(2):291–296. [DOI] [PubMed] [Google Scholar]
- 57. Van Herle AJ, Vassart G, Dumont JE. Control of thyroglobulin synthesis and secretion (first of two parts). N Engl J Med. 1979;301(5):239–249. [DOI] [PubMed] [Google Scholar]
- 58. Van Heuverswyn B, Streydio C, Brocas H, Refetoff S, Dumont J, Vassart G. Thyrotropin controls transcription of the thyroglobulin gene. Proc Natl Acad Sci USA. 1984;81(19):5941–5945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Van Heuverswyn B, Leriche A, Van Sande J, Dumont JE, Vassart G. Transcriptional control of thyroglobulin gene expression by cyclic AMP. FEBS Lett. 1985;188(2):192–196. [DOI] [PubMed] [Google Scholar]
- 60. Kim PS, Lee J, Jongsamak P, et al. Defective protein folding and intracellular retention of thyroglobulin-R19K mutant as a cause of human congenital goiter. Mol Endocrinol. 2008;22(2):477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kim PS, Bole D, Arvan P. Transient aggregation of nascent thyroglobulin in the endoplasmic reticulum: relationship to the molecular chaperone, BiP. J Cell Biol. 1992;118(3):541–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kim PS, Arvan P. Calnexin and BiP act as sequential molecular chaperones during thyroglobulin folding in the endoplasmic reticulum. J Cell Biol. 1995;128(1–2):29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Di Jeso B, Park YN, Ulianich L, et al. Mixed-disulfide folding intermediates between thyroglobulin and endoplasmic reticulum resident oxidoreductases ERp57 and protein disulfide isomerase. Mol Cell Biol. 2005;25(22):9793–9805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levtchenko E, de Graaf-Hess A, Wilmer M, van den Heuvel L, Monnens L, Blom H. Altered status of glutathione and its metabolites in cystinotic cells. Nephrol Dialysis Transpl. 2005;20(9):1828–1832. [DOI] [PubMed] [Google Scholar]
- 65. Levtchenko EN, Wilmer MJ, Janssen AJ, et al. Decreased intracellular ATP content and intact mitochondrial energy generating capacity in human cystinotic fibroblasts. Pediatr Res. 2006;59(2):287–292. [DOI] [PubMed] [Google Scholar]
- 66. Medeiros-Neto G, Kim PS, Yoo SE, et al. Congenital hypothyroid goiter with deficient thyroglobulin. Identification of an endoplasmic reticulum storage disease with induction of molecular chaperones. J Clin Invest. 1996;98(12):2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim PS, Kwon OY, Arvan P. An endoplasmic reticulum storage disease causing congenital goiter with hypothyroidism. J Cell Biol. 1996;133(3):517–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Christis C, Fullaondo A, Schildknegt D, Mkrtchian S, Heck AJ, Braakman I. Regulated increase in folding capacity prevents unfolded protein stress in the ER. J Cell Sci. 2010;123(Pt 5):787–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167(1):35–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Shaffer AL, Shapiro-Shelef M, Iwakoshi NN, et al. XBP1, downstream of Blimp-1, expands the secretory apparatus and other organelles, and increases protein synthesis in plasma cell differentiation. Immunity. 2004;21(1):81–93. [DOI] [PubMed] [Google Scholar]
- 71. Lee AH, Iwakoshi NN, Glimcher LH. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol. 2003;23(21):7448–7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17(4):469–477. [DOI] [PubMed] [Google Scholar]
- 73. Schulman JD, Bradley KH, Seegmiller JE. Cystine: compartmentalization within lysosomes in cystinotic leukocytes. Science. 1969;166(3909):1152–1154. [DOI] [PubMed] [Google Scholar]
- 74. Pisarev MA, Dumont JE. The role of reduced glutathione in thyroglobulin proteolysis iv vitro. Acta Endocrinol. 1975;79(1):76–85. [DOI] [PubMed] [Google Scholar]
- 75. Dunn AD, Dunn JT. Thyroglobulin degradation by thyroidal proteases: action of purified cathepsin D. Endocrinology. 1982;111(1):280–289. [DOI] [PubMed] [Google Scholar]
- 76. Faggiano A, Pisani A, Milone F, et al. Endocrine dysfunction in patients with Fabry disease. J Clin Endocrinol Metab. 2006;91(11):4319–4325. [DOI] [PubMed] [Google Scholar]
- 77. Hauser AC, Gessl A, Lorenz M, Voigtlander T, Fodinger M, Sunder-Plassmann G. High prevalence of subclinical hypothyroidism in patients with Anderson-Fabry disease. J Inherit Metab Dis. 2005;28(5):715–722. [DOI] [PubMed] [Google Scholar]
- 78. Polgreen LE, Tolar J, Plog M, et al. Growth and endocrine function in patients with Hurler syndrome after hematopoietic stem cell transplantation. Bone Marrow Transpl. 2008;41(12):1005–1011. [DOI] [PubMed] [Google Scholar]
- 79. Syres K, Harrison F, Tadlock M, et al. Successful treatment of the murine model of cystinosis using bone marrow cell transplantation. Blood. 2009;114(12):2542–2552. [DOI] [PubMed] [Google Scholar]
- 80. Yeagy BA, Harrison F, Gubler MC, Koziol JA, Salomon DR, Cherqui S. Kidney preservation by bone marrow cell transplantation in hereditary nephropathy. Kidney Int. 2011;79(11):1198–1206. [DOI] [PubMed] [Google Scholar]
- 81. Harrison F, Yeagy BA, Rocca CJ, Kohn DB, Salomon DR, Cherqui S. Hematopoietic stem cell gene therapy for the multisystemic lysosomal storage disorder cystinosis. Mol Ther. 2013;21(2):433–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Naphade S, Sharma J, Gaide Chevronnay HP, et al. Brief reports: lysosomal cross-correction by hematopoietic stem cell-derived macrophages via tunneling nanotubes. Stem Cells. 2015;33(1):301–309. [DOI] [PMC free article] [PubMed] [Google Scholar]