Abstract
Puberty is a tightly regulated process that leads to reproductive capacity. Kiss1 neurons are crucial in this process by stimulating GnRH, yet how Kiss1 neurons are regulated remains unknown. Substance P (SP), an important neuropeptide in pain perception, induces gonadotropin release in adult mice in a kisspeptin-dependent manner. Here, we assessed whether SP, through binding to its receptor NK1R (neurokinin 1 receptor), participates in the timing of puberty onset and fertility in the mouse. We observed that 1) selective NK1R agonists induce gonadotropin release in prepubertal females; 2) the expression of Tac1 (encoding SP) and Tacr1 (NK1R) in the arcuate nucleus is maximal before puberty, suggesting increased SP tone; 3) repeated exposure to NK1R agonists prepubertally advances puberty onset; and 4) female Tac1−/− mice display delayed puberty; moreover, 5) SP deficiency leads to subfertility in females, showing fewer corpora lutea and antral follicles and leading to decreased litter size. Thus, our findings support a role for SP in the stimulation of gonadotropins before puberty, acting via Kiss1 neurons to stimulate GnRH release, and its involvement in the attainment of full reproductive capabilities in female mice.
Reproductive function is controlled by the hierarchical interplay of central and peripheral factors that ultimately dictate the timing and pattern of GnRH release from puberty onset onwards. However, the exact mechanisms that communicate this information to GnRH neurons are poorly understood. Hypothalamic Kiss1 neurons (encoding kisspeptins) have been posed as the main conveyors of these regulatory factors by translating their information to effect congruent GnRH pulses (1). Indeed, loss-of-function mutations in the Kiss1 or Kiss1r (kisspeptin receptor) genes lead to hypogonadotropic hypogonadism (HH) in mice and humans (2–5).
Recently, an increasing number of direct hypothalamic modulators of Kiss1 neurons have been identified, uncovering a complex system of neuroendocrine factors that ensure proper gonadotropic function. In this context, Kiss1 neurons in the arcuate nucleus (ARC) coexpress dynorphin (inhibitory) and neurokinin B (NKB) (stimulatory) (6, 7), which have been proposed to act in a coordinated fashion to shape kisspeptin pulses. Interestingly, humans bearing inactivating mutations in TAC3 or TACR3 (encoding NKB and the receptor of NKB, neurokinin 3 receptor (NK3R), respectively) also display HH, characterized by absence of puberty and infertility (8–11). Moreover, mice bearing a null Tac2 or Tacr3 gene display delayed puberty and subfertility (12, 13) and chronic treatment of mice and rats with specific NK3R antagonists delays puberty onset (14, 15). Of note, this action of NKB in the central control of the gonadotropic axis is kisspeptin dependent (16, 17).
Importantly, NKB belongs to a family of closely related peptides termed tachykinins, which comprises also substance P (SP) and neurokinin A (NKA), both encoded by the Tac1 gene (18). A number of studies have associated SP with pain perception and inflammatory processes in the brain (19) as well as with psychiatric disorders (20). Although no human mutations in the genes encoding these ligands (TAC1) or their receptors (TACR1 and TACR2, respectively) have been correlated with reproductive disorders yet, both SP and NKA have been reported to stimulate the gonadotropic axis in several species (18, 21–30) in a kisspeptin-dependent manner (25) and SP is coexpressed in a subset of Kiss1 neurons in the human (31). Indeed, we recently demonstrated that SP, but not NKA, may act directly on Kiss1 neurons to stimulate gonadotropin release, along with NKB, because a subpopulation of these neurons expresses the SP receptor, neurokinin 1 receptor (NK1R) (25), in agreement with recent studies showing activation of Kiss1 neurons by SP (23).
Thus, based on the potent stimulatory action of gonadotropin release that we have previously observed after stimulation of the SP receptor (25); in this work, we focused on deciphering the potential role of SP, as a direct kisspeptin stimulator, in the control of puberty onset and reproductive function through a series of functional tests and genetic studies in the female mouse.
Materials and Methods
Mice
Prepubertal wild-type (WT) female C57Bl/6 mice were purchased from Charles River Laboratories International, Inc. Tac1-deficient (Tac1−/−) mice and WT littermates were generated by crossing Tac1+/− (heterozygous) acquired from The Jackson Laboratory. A detailed characterization of this mouse model can be found elsewhere (32). In addition, we have further confirmed the absence of Tac1 mRNA in mediobasal hypothalamic samples of adult male mice by real-time quantitative PCR (data not shown). All experiments were approved by the Harvard Medical Area Standing Committee on Animals in the Harvard Medical School Center for Animal Resources and Comparative Medicine. Mice were maintained in a 12-hour light, 12-hour dark cycle and were fed a standard rodent diet.
Reagents
The NK1R agonist (GR73632, termed NK1R-A in this study), a highly selective SP receptor agonist (EC50 NK1R = 4nM; NK2R = 960nM; and NK3R > 1000nM) (25), was purchased from Tocris. The doses of GR73632 (600 pmol in 5 μL of physiological saline for intracerebroventricular [icv] and 3 nmol in 50 μL for ip) were selected on the basis of previous references showing effective action of these compounds to induce gonadotropin responses in mice (25).
Experimental design
Experiment 1. Effect of central NK1R-agonist administration on gonadotropin release in prepubertal female mice
Female mice (postnatal d [p]25, n = 10/group) were anesthetized with isoflurane anesthesia and received 5-μL GR73632 (NK1R-A, 600 pmol) or vehicle (0.9% NaCl) through an icv injection, as previously described (33). Briefly, mice were anesthetized with isoflurane delivered by a vaporizer. Upon achieving a surgical plane of anesthesia, a small hole was bored in the skull 1 mm lateral and 0.5 mm posterior to bregma with a Hamilton syringe attached to a 27-gauge needle fitted with polyethylene tubing, leaving 3.5 mm of the needle tip exposed. Once the initial hole was made, all subsequent injections were made at the same site. Mice were allowed to recover for at least 2 days before treatment. For icv injections, mice were anesthetized with isoflurane for a total of 2–3 minutes, during which time 5 μL of solution were slowly and continuously injected into the lateral ventricle. The needle remained inserted for approximately 60 seconds after the injection to minimize backflow up the needle track. Mice typically recovered from the anesthesia within 3 minutes after the injection. Blood samples (200 μL) were collected by retroorbital bleeding (34) 25 minutes after injection (flowchart in Supplemental Figure 1). The dose and time of collection were selected based on our previous studies (25).
Experiment 2. Expression of Tac1 and Tacr1 in the mediobasal hypothalamus (MBH) of female mice across postnatal development
We aimed to determine whether there are changes in the expression of Tac1 and Tacr1 during the postnatal development in the MBH as the site that includes the ARC as the likely hypothalamic area where the mechanisms that initiate puberty take place. Intact infantile (p10), early juvenile (p15), late juvenile (p22), peripubertal (p30), and adult in diestrous (p60) females were killed and brains were collected. The hypothalamic tissue was sectioned using a coronal brain matrix (Braintree Scientific) and immediately frozen in liquid nitrogen and stored at −80°C. Briefly, The ARC fragments were isolated from each section under a dissection microscope with fine instruments by 2 bilateral parasagittal cuts, each 0.5 mm lateral to the midline between the optic chiasm and the posterior commissure, and 1 horizontal cut 1 mm dorsal of the ventral surface, yielding total tissue sizes of approximately 1 mm3 that were immediately frozen in liquid nitrogen and stored at −80°C (35). It is possible that part of the ventromedial hypothalamus (VMH) may have been dissected along with the ARC by this approach; therefore, we have called this area MBH. The expression of the Tac1 and Tacr1 genes in the MBH was assessed by quantitative real-time PCR (n = 3/group).
Experiment 3. Effects of repeated stimulation of prepubertal female mice with NK1R-A
WT females obtained from Charles River Laboratories International at p21 were injected ip with 3 nmol GR73632 (NK1R-A) in 50 μL every 12 hours from p22 to p34 (n = 9/group). Of note, the NK1R-A crosses the blood brain barrier (36); therefore, to facilitate the repeated treatment of the animals, ip injections were performed using the highest dose previously tested in mice (3 nmol). Vaginal opening (VO), as an indirect marker of circulating estradiol (E2) levels and therefore puberty, was monitored daily. The experiment was extended until one of the 2 groups reached 80% (or above) of VO. That day, body weights (BWs) of the animals were measured before the treatment, and they were euthanized 15 minutes after the last NK1R-A injection (Supplemental Figure 1). Gonadal weights were measured and trunk blood collected for hormonal determination.
Experiment 4. Assessment of the pubertal development of Tac1 null female mice
Littermate Tac1+/+ (WT), Tac1+/− (heterozygous, HET), and Tac1−/− (knockout, KO) females (n = 8–12 in each group) were monitored daily from p25 for: 1) BW, 2) progression to VO (as indicated by complete canalization of the vagina), and 3) timing of first estrus (first d with cornified cells determined by daily [in the morning] vaginal cytology after the d of VO) (Supplemental Figure 1).
Experiments 5 and 6. Analysis of the reproductive phenotype of adult Tac1 null female mice
In experiment 5, we characterized the estrous cyclicity of Tac1 null mice and controls by daily vaginal cytology for 4–5 weeks (Supplemental Figure 1). Cytology samples were obtained every morning (10 am) and placed on a glass slide for determination of the estrous cycle under the microscope as previously described (37). In addition, we performed a fertility assessment by breeding adult Tac1−/− or WT females with WT male previously proven to father litters (n = 6/group). The time to the first litter and number of pups per litter was monitored.
Experiment 6
In order to further assess the ovarian physiology of these animals, a histological study was performed on ovaries from adult (diestrus) WT and Tac1−/− mice (n = 5/group). Ovaries were collected, weighed and fixed in Bouin's solution. The tissues were embedded in paraffin and sectioned (10 μm) for hematoxylin and eosin staining (Harvard Medical School Rodent Pathology Core) and images acquired under ×4 magnification. The ovaries were analyzed for presence and type of follicles and for presence of corpora lutea (CLs) per section. Each value represents the number of follicles and CLs of 1 representative section from the middle line of one ovary per animal.
Experiment 7. Characterization of the postgonadectomy response of gonadotropins
LH and FSH levels were measured in intact (diestrus in the morning) WT and Tac1−/− mice compared with 1 week after bilateral ovariectomy (OVX) (n = 5/group) (Supplemental Figure 1). Blood samples were collected by retroorbital bleeding and serum stored at −20°C until hormonal determination.
Hormone measurements
Serum LH and FSH were measured using the Luminex (Luminex Corp) system analysis. Because of the small samples of blood obtained from the mice during the study, only single measurements were performed using 8 μL of serum per individual time point for each animal. The LH was analyzed using xMap technology (Millipore) with the mouse pituitary panel. A standard curve was generated using 5-fold serial dilutions of the LH/FSH standard cocktail provided by the vendor. Standards and samples were incubated with the antibody-coated beads on a microplate shaker overnight at 4°C and washed 3 times using a vacuum manifold apparatus. Detection antibody was then added to the wells and incubated on a microplate shaker at room temperature for 30 minutes. Streptavidin-phycoerythrin solution was then added, and an additional 30-minute incubation at room temperature was performed using the microplate shaker. Plates were then washed 3 times, and sheath fluid was added to each well. Beads were resuspended on the microplate shaker for 5 minutes. Plates were then read on the Luminex 200IS system using xPonent software (Luminex Corp). Data were analyzed using 5-parameter logistic curve fitting. The minimum detectable concentration (pg/mL) for LH was 1.9 and for FSH was 9.5. The intraassay coefficient of variation was less than 15%.
Quantitative real-time RT-PCR
Total RNA from the MBH was isolated using TRIzol reagent (Invitrogen) followed by chloroform/isopropanol extraction. RNA was quantified using NanoDrop 2000 spectrophotometer (Thermo Scientific), and 1 μm of RNA was reverse transcribed using Superscript III cDNA synthesis kit (Invitrogen). Quantitative real-time PCR assays were performed on an ABI Prism 7000 sequence detection system and analyzed using ABI Prism 7000 SDS software (Applied Biosystems). The cycling conditions were as follows: 2 minutes of incubation at 50°C, 10 minutes of incubation at 95°C (hot start), 40 amplification cycles (95°C for 15 s, 60°C for 1 min, and 45 s at 75°C, with fluorescence detection at the end of cycles 3–40), followed by melting curve of the amplified products obtained by ramped increase of the temperature from 55°C to 95°C to confirm the presence of single amplification product per reaction. The Tac1 mRNA NM_009311 (primers, 5′-TTTCTCGTTTCCACTCAACTGTT-3′ and 5′-GTCTTCGGGCGATTCTCTGC-3′) and Tacr1 mRNA NM_009313 (primers, 5′-TTGTGCAACCTACCTGGCAAA-3′ and 5′-CCACTGTATTGAATGCAGCCAT-3′) were detected using SYBR Green Mix (Bio-Rad) according to the manufacturer's instructions. The data were normalized using L19 primers as an internal control (38), and expressed as fold-change relative to p10.
Statistical analysis
All data are expressed as the mean ± SEM for each group. A 2-tailed unpaired Student's t test or a one- or two-way ANOVA test was used followed by Newman-Keuls or Tukey's post hoc tests, respectively, to assess variation among experimental groups. Significance level was set at P < .05. All analyses were performed with GraphPad Prism Software, Inc.
Results
Experiment 1. Effect of central NK1R-agonist administration on gonadotropin release in prepubertal female mice
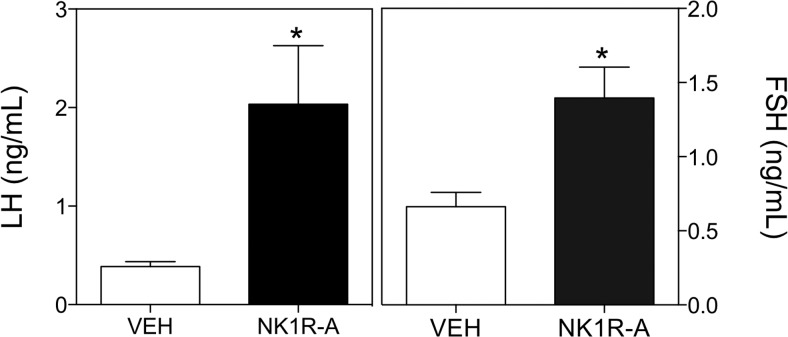
Our previous studies have documented potent LH and FSH release after NK1R-A treatment in adult mice (25). Therefore, the purpose of experiment 1 was to confirm that prepubertal female mice are also able to respond to an SP receptor agonist. In this context, icv administration of NK1R-A (600 pmol) was able to significantly stimulate LH (vehicle, 0.39 ± 0.05 ng/mL vs NK1R-A, 2.03 ± 0.59; t(7) = 2.43; P = .005) and FSH (vehicle, 0.66 ± 0.09 ng/mL vs NK1R-A, 1.40 ± 0.20; t(9) = 2.99; P = .015) release in prepubertal female mice 25 minutes after injection (Figure 1).
Figure 1.
Serum LH (left panel) and FSH (right panel) values of prepubertal female mice 25 minutes after central icv injection of 600 pmol GR73632 (NK1R-A) or vehicle (VEH). Statistical analysis was performed using a 2-tailed t test (*, P < .05 compared with vehicle-treated controls).
Experiment 2. Expression of Tac1 and Tacr1 in the MBH of female mice across postnatal development
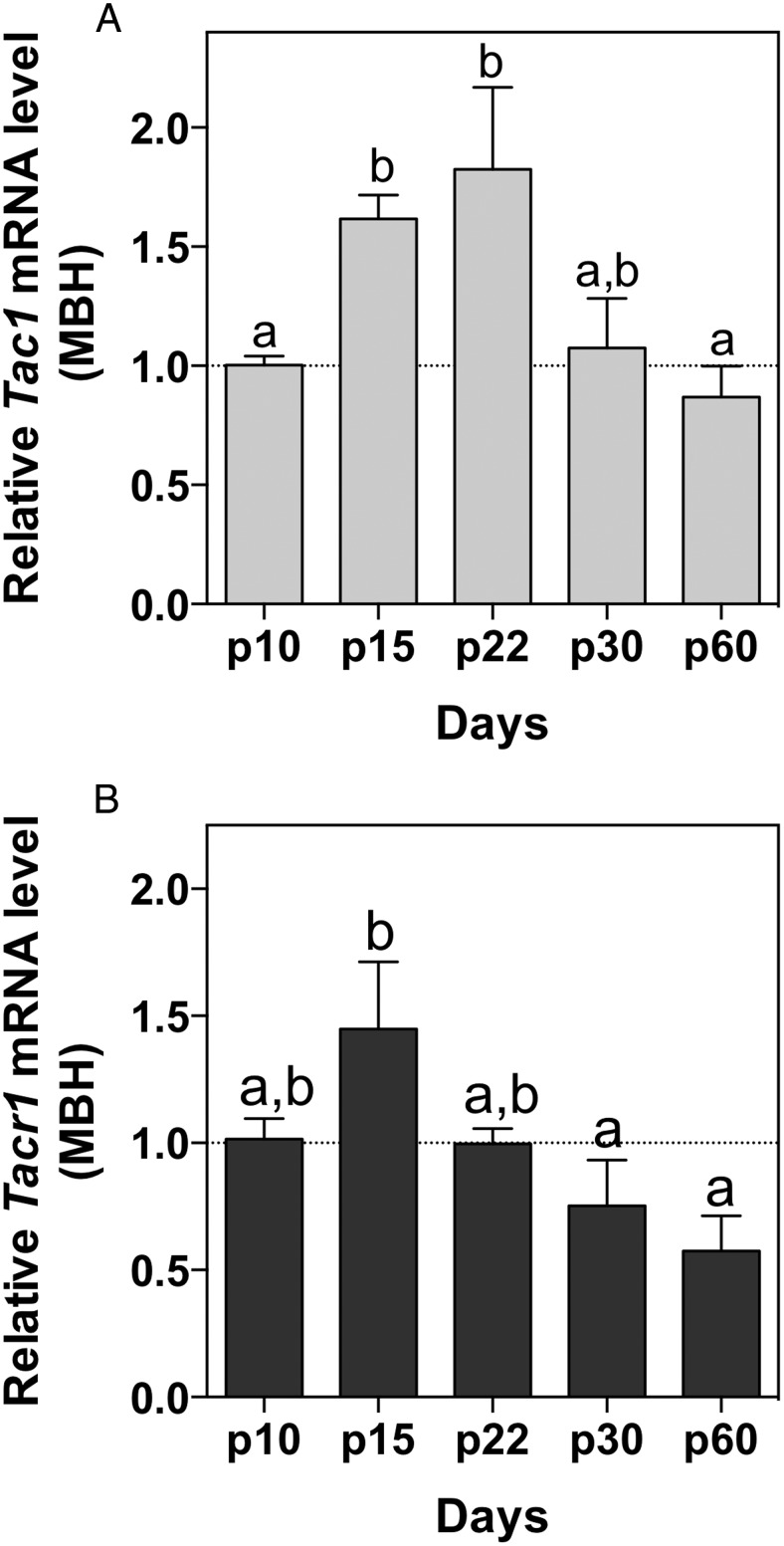
Genes with documented stimulatory action of the gonadotropic axis prepubertally, eg, Kiss1 and Tac2, display significantly higher expression in the hypothalamus before phenotypic manifestations of puberty such as VO in females or preputial separation in males (14, 39). Thus, in experiment 2, we hypothesized that the expression profile of the genes encoding the SP/NK1R system (Tac1 and Tacr1, respectively) would also be augmented in the hypothalamus during this developmental period, if they play roles in the activation of the HPG axis at the onset of puberty. We have previously documented a predominant expression of Tac1 in the ARC and the VMH nuclei within the hypothalamus and the presence of Tacr1 in half of Kiss1 neurons in the ARC (25). Thus, given the predicted role of ARC Kiss1 neurons in triggering puberty onset, we focused on a study of the expression of Tac1 and Tacr1 genes in the MBH (as likely direct mediators of kisspeptin activation). Tac1 expression was significantly higher in the MBH at p15 and p22 (Figure 2A) compared with infantile (p10) and adult (p60) ages (F(4,13) = 6.34; P = .005). Similarly, Tacr1 expression at p15 is significantly higher than at peripubertal or adult ages (F(4,13) = 4.52; P = .02) (Figure 2B).
Figure 2.
Expression profile of Tac1 (A) and Tacr1 (B) in the MBH of female mice across postnatal development. One-way ANOVA + Newman-Keuls post hoc test was performed to compare all groups. Different letters indicate significantly different values (P < .05).
Experiment 3. Effects of repeated stimulation of prepubertal female mice with NK1R-A
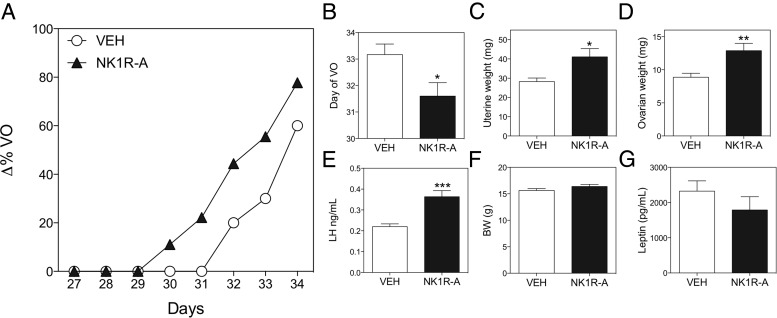
The ability of prepubertal animals to respond to NK1R-A with significantly increased gonadotropin levels (experiment 1) and the apparently higher tone of the SP/NK1R system at the time of puberty initiation (experiment 2) led us to propose that, if SP induces puberty onset, exogenous administration of NK1R-A to mice at an early age (thus increasing SP tone) would lead to advanced puberty. Thus, we observed that chronic NK1R-A administration from p22 significantly advanced puberty onset as indicated by the advanced timing of VO (Figure 3A), also represented in Figure 3B as the day at which 50% of the animals in each group reached VO (vehicle, 33.17 ± 0.4 mg vs NK1R-A, 31.60 ± 0.5 mg; t(9) = 2.45; P = .04); higher uterine (vehicle, 28.22 ± 1.8 vs NK1R-A, 41.11 ± 4.20; t(16) = 2.77; P = .01) and ovarian weights (vehicle, 8.87 ± 0.5 mg vs NK1R-A, 12.89 ± 1.1 mg; t(16) = 3.19; P = .005) (Figure 3, C and D); and higher LH levels (vehicle, 0.22 ± 0.01 ng/mL vs NK1R-A, 0.36 ± 0.02 ng/mL; t(15) = 4.34; P < .001) (Figure 3E). Of note, BW and circulating leptin levels were similar in the control and treated groups (Figure 3, F and G), indicating that there is no metabolic contribution to this phenotype at this age.
Figure 3.
Repeated ip stimulation (every 12 h) of female mice with GR73632 (NK1R-A) from p22 to p34. Progression of VO (A), age (postnatal d) until 50% of the animals in each group displayed VO (B). At p34, uterine weight (C), ovarian weight (D), serum LH levels (E), BW (F), and serum leptin levels (G) were measured. Statistical analysis was performed using a 2-tailed t test (*, P < .05; **, P < .01; ***, P < .001).
Experiment 4. Assessment of the pubertal development of Tac1 null female mice
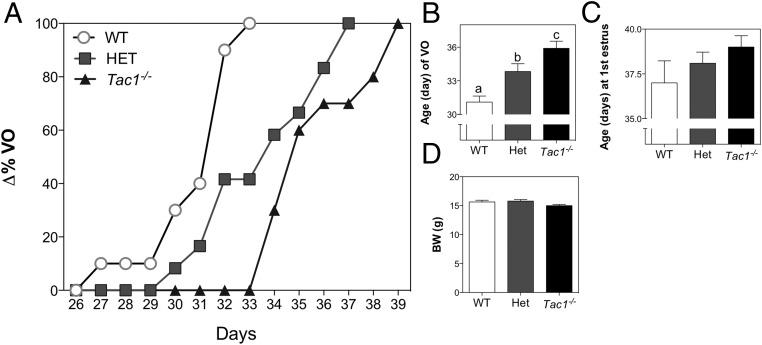
Based on the strong evidence from experiments 1–3, indicating that the SP/NK1R system plays a role in the control of puberty onset in the mouse, we aimed to characterize the reproductive phenotype of mice with congenital absence of SP (Tac1−/−). We observed that both Tac1+/− (HETs) and Tac1−/− (KOs) mice displayed a significant delay in VO compared with Tac1+/+ (WT) (WT, 31.1 ± 0.5 d; HET, 33.83 ± 0.69 d; KO, 35.90 ± 0.64 d; F(2,29) = 1057; P < .0001) (Figure 4, A and B). Once animals reached VO, daily vaginal smears were monitored to determine the age of first estrus, which showed a trend to be delayed in KO mice compared with WT, but did not reach statistical significance (WT, 37.00 ± 1.2 d vs KO, 39.00 ± 0.6 d; t(12) = 1.594; P = .137) (Figure 4C). Of note, the BW of the animals, measured on p32 (when 100% of WT mice had reached VO), was not different between groups, indicating that, at that age, the delay in VO is not attributable to a lower BW (F(2,28) = 0.13; P = .88) (Figure 4D). Of note, WT littermates in this experiment displayed VO at an earlier age than WT mice purchased at p21 from Charles River Laboratories International in experiment 3; nonetheless, the presence of the corresponding controls in each experiment validates the results.
Figure 4.
Pubertal progression of Tac1−/− female mice. A, Percentage of mice showing VO at each age. B, Mean age of VO. C, Age of first estrus. D, BW at p32. One-way ANOVA followed by Newman-Keuls post hoc test was performed to compare all groups. Different letters indicate significantly different values (P < .05).
Experiments 5 and 6. Analysis of the reproductive phenotype of adult Tac1 null female mice
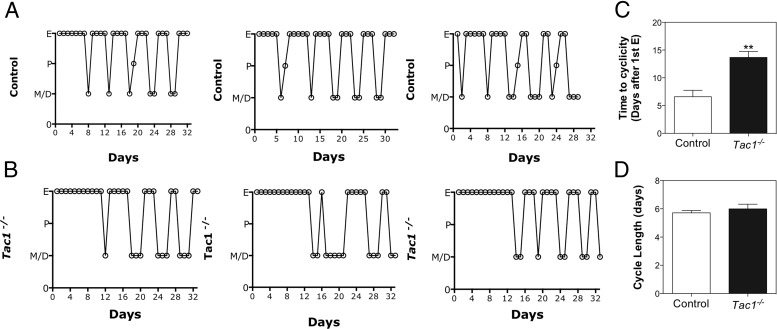
Experiments 3 and 4 indicated a likely role of SP in the timing of puberty onset; therefore, in order to determine whether SP also plays a role in the attainment of fertility, the reproductive phenotype of Tac1 null female mice was assessed in experiments 6 and 7. WT and HET mice displayed regular cycles (pooled and referred to as controls) (Figure 5A); interestingly, Tac1−/− mice exhibited a sustained phase of estrus after the first estrus (Figure 5, B and C) that was significantly longer than that observed in control animals (n = 5–8/group) (control, 6.57 ± 1.17 d vs KO, 13.67 ± 1.08 d; t(11) = 4.38; P = .001). However, after this period, Tac1 null mice initiated cyclicity in most cases with similar cycle lengths (from estrus to estrus of the next cycle) to control animals (control, 5.71 ± 0.16 d vs KO, 6.00 ± 0.33 d; t(26) = 0.77; P = nonsignificant) (Figure 5D).
Figure 5.
Estrous cyclicity of control (A) and Tac1−/− (B) female mice. C, Time from first estrus to initiate regular cyclicity. D, Cycle length (from estrus to the next estrus). Statistical analysis was performed using a 2-tailed t test (**, P < .01 compared with controls).
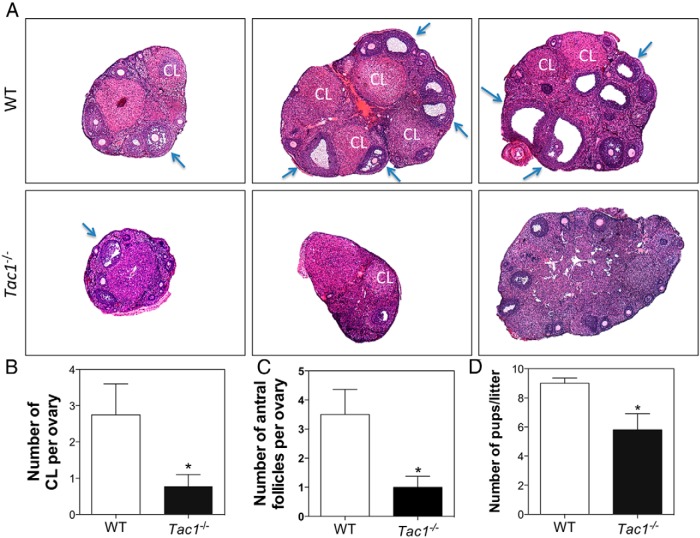
In order to further assess the ovarian physiology of these animals, a histological study was performed on ovaries from adult (diestrus) WT and Tac1−/− mice. Tac1−/− mice had smaller ovaries with fewer CLs (control, 2.75 ± 0.85 CL vs KO, 0.78 ± 0.32 CL; t(11) = 2.70; P = .02) (Figure 6, A and B) and fewer antral follicles (AFs) (control, 3.50 ± 0.86 AF vs KO, 1.00 ± 0.37; t(9) = 3.09; P = .01) (Figure 6, A and C).
Figure 6.
A, Ovarian histology of WT and Tac1−/− adult mice. B, Number of corporal lutea per ovary. C, Number of AFs per ovary. D, Litter sizes of WT and Tac1−/− female mice mated with adult experienced WT males. Blue arrows are examples of AFs. Statistical analysis was performed using a 2-tailed t test (*, P < .05 compared with controls).
These data suggested possible subfertility in the Tac1 knockout animals. Hence, we conducted a further fertility assessment by breeding WT and Tac1−/− female mice (n = 6) with experienced adult WT males. Tac1−/− mice delivered a significantly lower number of pups per litter (WT, 9.00 ± 0.36 pups/litter vs KO, 5.80 ± 1.11 pups/litter; t(9) = 2.95; P = .02) (Figure 6D). Of note, all females in both groups achieved pregnancy with no significant differences in the time to deliver the pups (data not shown).
Experiment 7. Characterization of the postgonadectomy response of gonadotropins
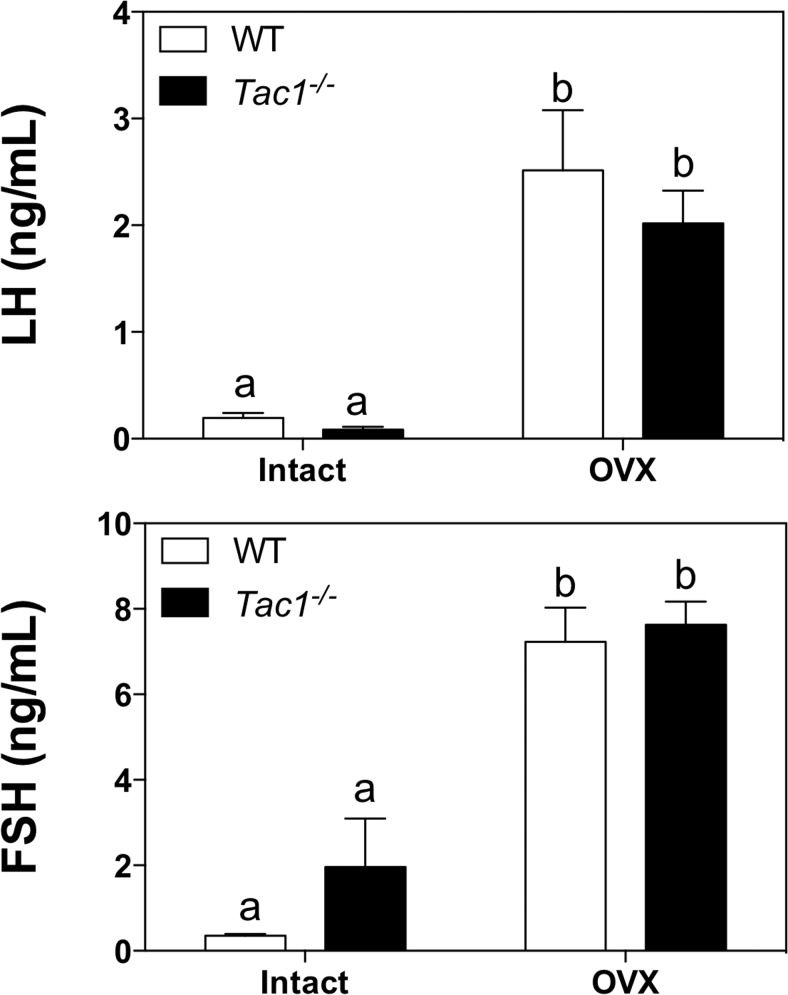
Experiments 4–6 indicate a likely reproductive impairment in Tac1−/− mice that leads to a subfertile phenotype. In order to address whether the absence of SP leads to a diminished ability of these animals to release kisspeptin (and/or GnRH), we measured LH and FSH levels in intact (diestrus) WT and Tac1−/− mice compared with 1 week after OVX, as a model of elevated gonadotropin release. Control (WT) and Tac1 null mice were ovariectomized for 1 week, and serum LH and FSH levels were compared between groups. Interestingly, both groups of animals displayed a similar compensatory rise of gonadotropin levels with no significant difference between the 2 genotypes (F(1,18) = 0.73; P = .40) (Figure 7), suggesting normal increases in kisspeptin and GnRH release after estrogen withdrawal.
Figure 7.
Serum LH (upper panel) and FSH (lower panel) values of adult intact and OVX (1 wk) WT and Tac1−/− female mice. Statistical analysis was performed using two-way ANOVA followed by Tukey's post hoc test. Different letters indicate significantly different values (P < .05).
Discussion
Understanding the neuroendocrine events that determine the timing of puberty onset, and the subsequent achievement of reproductive capacity, has been a matter of intense research in recent decades; however, although a number of stimulatory and inhibitory factors have been reported to interplay during this developmental process (40), the exact mechanism/s that trigger sexual maturation remain incompletely understood. Here, we provide evidence that SP also participates in the pubertal activation of the gonadotropic axis in the mouse. We have previously documented that: 1) agonists of the SP receptor stimulate gonadotropin release in adult mice and in prepubertal rats (25, 27); 2) this action is kisspeptin dependent (25); and 3) the gene encoding the SP receptor (Tacr1) is expressed in approximately 50% of Kiss1 neurons in the ARC and 33% of Kiss1 neurons in the anteroventral periventricular nucleus (25). These data are consistent with a direct effect of SP on Kiss1 neurons, as recently demonstrated through electrophysiological studies (23).
Kiss1 neurons are the main gatekeepers of reproduction and loss-of-function mutations in the Kiss1 or Kiss1r genes lead to the absence of reproductive maturation (1). Considerable interest has emerged to identify the factors involved in the control of kisspeptin release as the putative central regulator of the gonadotropic axis. In this context, the tachykinin NKB has been reported to stimulate kisspeptin prepubertally (15) and the expression of Tac2 (encoding NKB) increases before Kiss1 (14), suggesting a likely role of this tachykinin in the pubertal activation of kisspeptin-GnRH secretion, as also supported by human studies describing HH in patients with impaired function of the NKB/NK3R system (8, 9).
In this study, we demonstrate that the activation of the receptor of SP, a counterpart of NKB in the tachykinin family, is able to accelerate the onset of puberty in mice, suggesting that the NK1R is present, and functional, during this developmental period; moreover, the expression of the genes encoding SP and the SP receptor are significantly higher at the time of the initiation of puberty onset than they are at earlier or later stages of postnatal development. This suggests the existence of a higher SP tone, and greater sensitivity to hypothalamic SP, at the time of puberty initiation, putatively leading to an increase in GnRH pulses and activation of the gonadotropic axis. Interestingly, unlike Kiss1 and Tac2, the genes encoding the SP/NK1R system decrease in the ARC of the mouse after the initiation of puberty, perhaps indicating a predominant role of this molecule early in puberty onset. Indeed, supporting this contention, we have demonstrated in the present study that 1) stimulation of the SP receptor (NK1R) during this prepubertal period advances puberty onset, possibly through the early activation of Kiss1 neurons, resembling previous kisspeptin treatments in prepubertal rodents (41); and 2) congenital SP-deficiency (Tac1−/− mice) leads to delayed puberty. Of note, due to the limitation of chronic animal manipulations before weaning, the repeated injections of NK1R-A were performed from p22 onwards, which is beyond the reported peak of Tac1 and Tacr1 expression. Still, this treatment successfully advanced puberty onset, probably due to the sustained higher activation of NK1R compared with controls. Admittedly, although p30 has been widely considered as a peripubertal age based on external signs of puberty onset in rodents, ie, VO, the initiation of puberty onset centrally must occur at an earlier age, thereby leading to the increase of GnRH release that will evoke the appearance of these secondary sexual signs. However, the timing of the appearance of these central mechanisms remains largely unknown, and it is very likely that it occurs during what is typically termed the juvenile state, around the time that Tac1 and Tacr1 show maximal expression. Interestingly, NK1R has been described in both Kiss1 neuronal populations (ARC and anteroventral periventricular nucleus) as well as in a subset of GnRH neurons (25). Therefore, whether the action of SP in the regulation of puberty onset occurs on one, or all, of these neuronal groups remains to be deciphered.
In addition, SP is critical for the maintenance of proper reproductive function. SP null female mice required more time to initiate estrous cyclicity, during which they remained in constant estrus. This suggests that although E2 is produced by the ovary, this alone may not be sufficient to trigger an LH surge and, therefore, ovulation. Indeed, histological examination of the ovaries revealed fewer numbers of CLs and AFs in Tac1 knockout mice. Of note, these data in Tac1 null mice suggest that compensation by other tachykinins, eg, NKB, does not occur in this model. In fact, we have observed similar Tac2 mRNA levels (encoding NKB in rodents) in MBH samples of adult WT and Tac1 null male mice (data not shown) that support this contention, although further characterization of all tachykinin ligand-receptor systems needs to be performed in the female. Nonetheless, the data mentioned above, together with the significantly reduced litter size in SP-deficient mice, indicate a level of subfertility in the absence of SP, which may be, at least in part, due to an impairment in the positive feedback action of sex steroids. Along these lines, SP levels have been described to fluctuate in the hypothalamus of rats during the estrous cycle (42), and we have documented that Tac1 expression in the ARC and ventromedial nucleus is inhibited by E2 (25), in agreement with previous studies showing increased SP levels in hypothalami of postmenopausal women (43). These data are also in agreement with human and monkey studies demonstrating that plasma SP levels vary across the menstrual cycle, with higher levels of SP during the follicular phase that correlated negatively with the E2 levels (44, 45). However, the site of origin of the SP measured in the serum in these studies is unknown. Altogether, these studies support a role for this tachykinin in the control of the gonadotropic axis, at least in part by regulating kisspeptin release centrally.
The identification of all the potential site/s of action of SP for its reproductive role remains to be fully deciphered. Strong evidence indicates that SP can activate the gonadotropic axis centrally because, first, central activation of the SP receptor (NK1R) causes a robust gonadotropin induction (that is prevented in the absence of kisspeptin) (25), and second, SP activates Kiss1 neurons (23). Admittedly, given the expression of Tac1 in other areas of the brain, eg, VMH and lateral hypothalamus (25), the possibility that these populations of Tac1 neurons outside the ARC also play a role in the control of kisspeptin/GnRH release cannot be excluded.
Supporting the role of SP in the central control of puberty onset is the fact that higher SP levels detected in the brain of patients after traumatic brain injury (46–48) correlate with the significantly higher ratio of children displaying precocious puberty after traumatic brain injury (49, 50). This correlation may suggest a causative effect of higher central SP levels on earlier pubertal onset (replicated by our present data in prepubertal female mice) and support a role for SP in the control of the gonadotropic axis across species.
Nonetheless, despite the compelling evidence for a central role of SP, we cannot rule out the possibility of actions of SP in other organs of the gonadotropic axis, such as the ovary. Indeed, SP and NK1R have been identified in the mouse ovary (51, 52) and could, potentially, contribute to the subfertility phenotype observed in SP deficient female mice.
In summary, we offer evidence that supports a role for the SP/NK1R system in the activation of the gonadotropic axis to trigger puberty onset and the attainment of full reproductive capabilities, at least in the female mouse.
Acknowledgments
Present address for S.S.: Department of Obstetrics and Gynecology, Pamukkale University School of Medicine, Denizli, Turkey.
This work was supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through Cooperative Agreement U54 HD028138 as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research grants from the National Institute of Health (NIH) Grant R01 HD019938 (to U.B.K.). V.M.N. was supported by NIH Grant K99 HD071970, Charles H. Hood Foundation for Child Health Research Program and the Microgrant Program from The Biomedical Research Institute and the Center for Faculty Development and Diversity's Office for Research Careers at the Brigham and Women's Hospital. S.S. was supported by TUBITAK (The Scientific and Technological Research Council of Turkey) Grant 2219.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AF
- antral follicle
- ARC
- arcuate nucleus
- BW
- body weight
- CL
- corpora luteum
- E2
- estradiol
- HET
- heterozygous
- HH
- hypogonadotropic hypogonadism
- icv
- intracerebroventricular
- KO
- knockout
- MBH
- mediobasal hypothalamus
- NKA
- neurokinin A
- NKB
- neurokinin B
- NK1R
- neurokinin 1 receptor
- NK3R
- neurokinin 3 receptor
- OVX
- ovariectomy
- p
- postnatal day
- SP
- substance P
- VMH
- ventromedial hypothalamus
- VO
- vaginal opening
- WT
- wild type.
References
- 1. Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiol Rev. 2012;92(3):1235–1316. [DOI] [PubMed] [Google Scholar]
- 2. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci USA. 2003;100(19):10972–10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614–1627. [DOI] [PubMed] [Google Scholar]
- 4. Funes S, Hedrick JA, Vassileva G, et al. The KiSS-1 receptor GPR54 is essential for the development of the murine reproductive system. Biochem Biophys Res Commun. 2003;312(4):1357–1363. [DOI] [PubMed] [Google Scholar]
- 5. Lapatto R, Pallais JC, Zhang D, et al. Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology. 2007;148(10):4927–4936. [DOI] [PubMed] [Google Scholar]
- 6. Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Navarro VM. New insights into the control of pulsatile GnRH release: the role of Kiss1/neurokinin B neurons. Front Endocrinol (Lausanne). 2012;3:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Topaloglu AK, Reimann F, Guclu M, et al. TAC3 and TACR3 mutations in familial hypogonadotropic hypogonadism reveal a key role for neurokinin B in the central control of reproduction. Nat Genet. 2009;41(3):354–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Young J, Bouligand J, Francou B, et al. TAC3 and TACR3 defects cause hypothalamic congenital hypogonadotropic hypogonadism in humans. J Clin Endocrinol Metab. 2010;95(5):2287–2295. [DOI] [PubMed] [Google Scholar]
- 10. Gianetti E, Tusset C, Noel SD, et al. TAC3/TACR3 mutations reveal preferential activation of gonadotropin-releasing hormone release by neurokinin B in neonatal life followed by reversal in adulthood. J Clin Endocrinol Metab. 2010;95(6):2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Noel SD, Abreu AP, Xu S, et al. TACR3 mutations disrupt NK3R function through distinct mechanisms in GnRH-deficient patients. FASEB J. 2014;28(4):1924–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang JJ, Caligioni CS, Chan YM, Seminara SB. Uncovering novel reproductive defects in neurokinin B receptor null mice: closing the gap between mice and men. Endocrinology. 2012;153(3):1498–1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. True C, Nasrin Alam S, Cox K, Chan YM, Seminara S. Neurokinin B is critical for normal timing of sexual maturation but dispensable for adult reproductive function in female mice. Endocrinology. 2015:156(4):1386–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gill JC, Navarro VM, Kwong C, et al. Increased neurokinin B (Tac2) expression in the mouse arcuate nucleus is an early marker of pubertal onset with differential sensitivity to sex steroid-negative feedback than Kiss1. Endocrinology. 2012;153(10):4883–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Navarro VM, Ruiz-Pino F, Sánchez-Garrido MA, et al. Role of neurokinin B in the control of female puberty and its modulation by metabolic status. J Neurosci. 2012;32(7):2388–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. García-Galiano D, van Ingen Schenau D, Leon S, et al. Kisspeptin signaling is indispensable for neurokinin B, but not glutamate, stimulation of gonadotropin secretion in mice. Endocrinology. 2012;153(1):316–328. [DOI] [PubMed] [Google Scholar]
- 17. Grachev P, Li XF, Lin YS, et al. GPR54-dependent stimulation of luteinizing hormone secretion by neurokinin B in prepubertal rats. PLoS One. 2012;7(9):e44344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lasaga M, Debeljuk L. Tachykinins and the hypothalamo-pituitary-gonadal axis: an update. Peptides. 2011;32(9):1972–1978. [DOI] [PubMed] [Google Scholar]
- 19. De Felipe C, Herrero JF, O'Brien JA, et al. Altered nociception, analgesia and aggression in mice lacking the receptor for substance P. Nature. 1998;392(6674):394–397. [DOI] [PubMed] [Google Scholar]
- 20. Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–272. [DOI] [PubMed] [Google Scholar]
- 21. Arisawa M, De Palatis L, Ho R, et al. Stimulatory role of substance P on gonadotropin release in ovariectomized rats. Neuroendocrinology. 1990;51(5):523–529. [DOI] [PubMed] [Google Scholar]
- 22. Coiro V, Volpi R, Capretti L, et al. Luteinizing hormone response to an intravenous infusion of substance P in normal men. Metabolism. 1992;41(7):689–691. [DOI] [PubMed] [Google Scholar]
- 23. de Croft S, Boehm U, Herbison AE. Neurokinin B activates arcuate kisspeptin neurons through multiple tachykinin receptors in the male mouse. Endocrinology. 2013;154(8):2750–2760. [DOI] [PubMed] [Google Scholar]
- 24. Kalra PS, Sahu A, Bonavera JJ, Kalra SP. Diverse effects of tachykinins on luteinizing hormone release in male rats: mechanism of action. Endocrinology. 1992;131(3):1195–1201. [DOI] [PubMed] [Google Scholar]
- 25. Navarro VM, Bosch MA, León S, et al. The integrated hypothalamic tachykinin-kisspeptin system as a central coordinator for reproduction. Endocrinology. 2015;156(2):627–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ohtsuka S, Miyake A, Nishizaki T, Tasaka K, Aono T, Tanizawa O. Substance P stimulates gonadotropin-releasing hormone release from rat hypothalamus in vitro with involvement of oestrogen. Acta Endocrinol (Copenh). 1987;115(2):247–252. [DOI] [PubMed] [Google Scholar]
- 27. Ruiz-Pino F, Garcia-Galiano D, Manfredi-Lozano M, et al. Effects and interactions of tachykinins and dynorphin on FSH and LH secretion in developing and adult rats. Endocrinology. 2015;156(2):576–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Sahu A, Kalra SP. Effects of tachykinins on luteinizing hormone release in female rats: potent inhibitory action of neuropeptide K. Endocrinology. 1992;130(3):1571–1577. [DOI] [PubMed] [Google Scholar]
- 29. Traczyk WZ, Pau KY, Kaynard AH, Spies HG. Modulatory role of substance P on gonadotropin and prolactin secretion in the rabbit. J Physiol Pharmacol. 1992;43(3):279–297. [PubMed] [Google Scholar]
- 30. Tsuruo Y, Kawano H, Hisano S, et al. Substance P-containing neurons innervating LHRH-containing neurons in the septo-preoptic area of rats. Neuroendocrinology. 1991;53(3):236–245. [DOI] [PubMed] [Google Scholar]
- 31. Hrabovszky E, Borsay BA, Racz K, et al. Substance p immunoreactivity exhibits frequent colocalization with kisspeptin and neurokinin B in the human infundibular region. PLoS One. 2013;8(8):e72369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–394. [DOI] [PubMed] [Google Scholar]
- 33. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073–4077. [DOI] [PubMed] [Google Scholar]
- 34. van Herck H, Baumans V, Brandt CJ, et al. Orbital sinus blood sampling in rats as performed by different animal technicians: the influence of technique and expertise. Lab Anim. 1998;32(4):377–386. [DOI] [PubMed] [Google Scholar]
- 35. Gill JC, Wang O, Kakar S, Martinelli E, Carroll RS, Kaiser UB. Reproductive hormone-dependent and -independent contributions to developmental changes in kisspeptin in GnRH-deficient hypogonadal mice. PLoS One. 2010;5(7):e11911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Darmani NA, Wang Y, Abad J, Ray AP, Thrush GR, Ramirez J. Utilization of the least shrew as a rapid and selective screening model for the antiemetic potential and brain penetration of substance P and NK1 receptor antagonists. Brain Res. 2008;1214:58–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Martin C, Navarro VM, Simavli S, et al. Leptin-responsive GABAergic neurons regulate fertility through pathways that result in reduced kisspeptinergic tone. J Neurosci. 2014;34(17):6047–6056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368(26):2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Navarro VM, Castellano JM, Fernández-Fernández R, et al. Developmental and hormonally regulated messenger ribonucleic acid expression of KiSS-1 and its putative receptor, GPR54, in rat hypothalamus and potent luteinizing hormone-releasing activity of KiSS-1 peptide. Endocrinology. 2004;145(10):4565–4574. [DOI] [PubMed] [Google Scholar]
- 40. Ojeda SR, Lomniczi A. Puberty in 2013: unravelling the mystery of puberty. Nat Rev Endocrinol. 2014;10(2):67–69. [DOI] [PubMed] [Google Scholar]
- 41. Navarro VM, Fernandez-Fernandez R, Castellano JM, et al. Advanced vaginal opening and precocious activation of the reproductive axis by KiSS-1 peptide, the endogenous ligand of GPR54. J Physiol. 2004;561(pt 2):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tsuruo Y, Hisano S, Okamura Y, Tsukamoto N, Daikoku S. Hypothalamic substance P-containing neurons. Sex-dependent topographical differences and ultrastructural transformations associated with stages of the estrous cycle. Brain Res. 1984;305(2):331–341. [DOI] [PubMed] [Google Scholar]
- 43. Rance NE, Young WS., 3rd Hypertrophy and increased gene expression of neurons containing neurokinin-B and substance-P messenger ribonucleic acids in the hypothalami of postmenopausal women. Endocrinology. 1991;128(5):2239–2247. [DOI] [PubMed] [Google Scholar]
- 44. Kerdelhué B, Williams RF, Lenoir V, et al. Variations in plasma levels of substance P and effects of a specific substance P antagonist of the NK(1) receptor on preovulatory LH and FSH surges and progesterone secretion in the cycling cynomolgus monkey. Neuroendocrinology. 2000;71(4):228–236. [DOI] [PubMed] [Google Scholar]
- 45. Kerdelhué B, Lenoir V, Scholler R, Jones HW., Jr Substance P plasma concentration during the LH preovulatory surge of the menstrual cycle in the human. Neuro Endocrinol Lett. 2006;27(3):359–364. [PubMed] [Google Scholar]
- 46. Gabrielian L, Helps SC, Thornton E, Turner RJ, Leonard AV, Vink R. Substance P antagonists as a novel intervention for brain edema and raised intracranial pressure. Acta Neurochir Suppl. 2013;118:201–204. [DOI] [PubMed] [Google Scholar]
- 47. Vink R, van den Heuvel C. Substance P antagonists as a therapeutic approach to improving outcome following traumatic brain injury. Neurotherapeutics. 2010;7(1):74–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zacest AC, Vink R, Manavis J, Sarvestani GT, Blumbergs PC. Substance P immunoreactivity increases following human traumatic brain injury. Acta Neurochir Suppl. 2010;106:211–216. [DOI] [PubMed] [Google Scholar]
- 49. Blendonohy PM, Philip PA. Precocious puberty in children after traumatic brain injury. Brain Inj. 1991;5(1):63–68. [DOI] [PubMed] [Google Scholar]
- 50. Kaulfers AM, Backeljauw PF, Reifschneider K, et al. Endocrine dysfunction following traumatic brain injury in children. J Pediatr. 2010;157(6):894–899. [DOI] [PubMed] [Google Scholar]
- 51. Debeljuk L. Tachykinins in the normal and gonadotropin-stimulated ovary of the mouse. Peptides. 2003;24(9):1445–1448. [DOI] [PubMed] [Google Scholar]
- 52. Debeljuk L. Tachykinins and ovarian function in mammals. Peptides. 2006;27(4):736–742. [DOI] [PubMed] [Google Scholar]