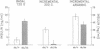Abstract
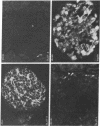
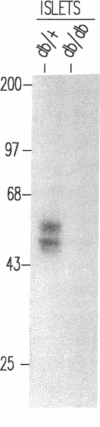
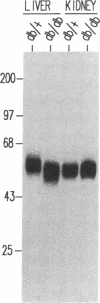
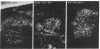
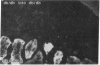
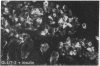
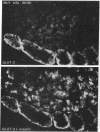
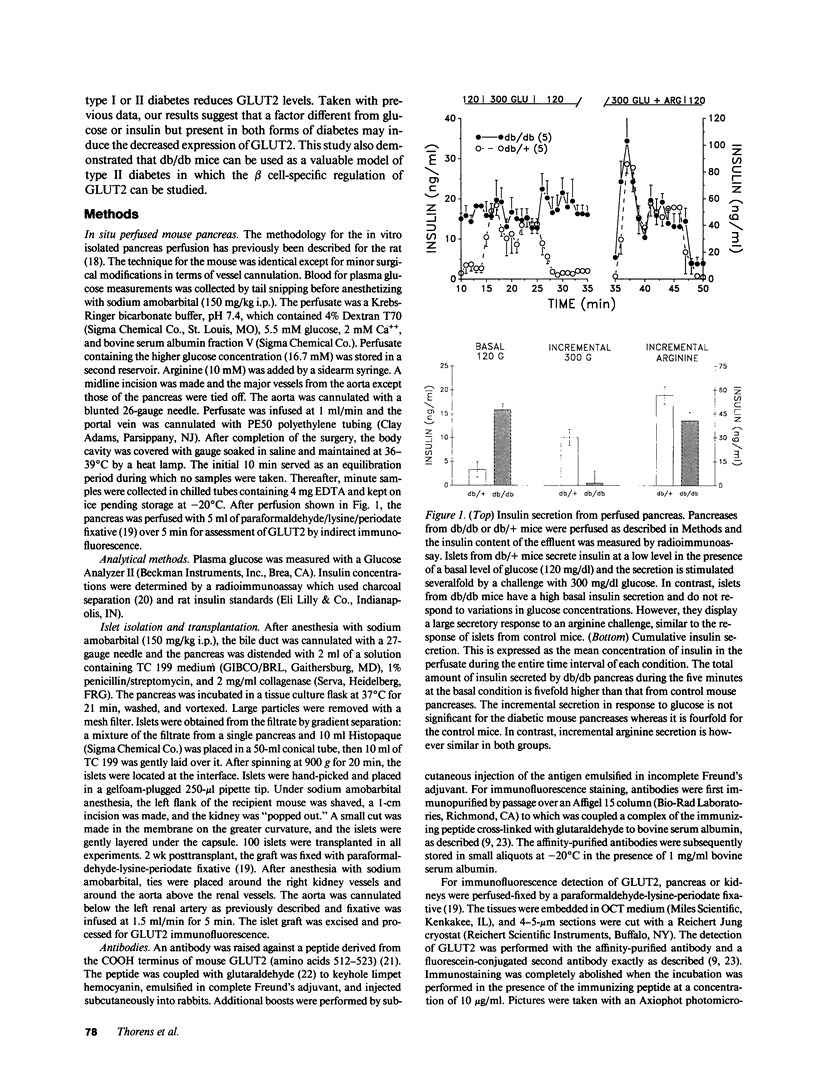
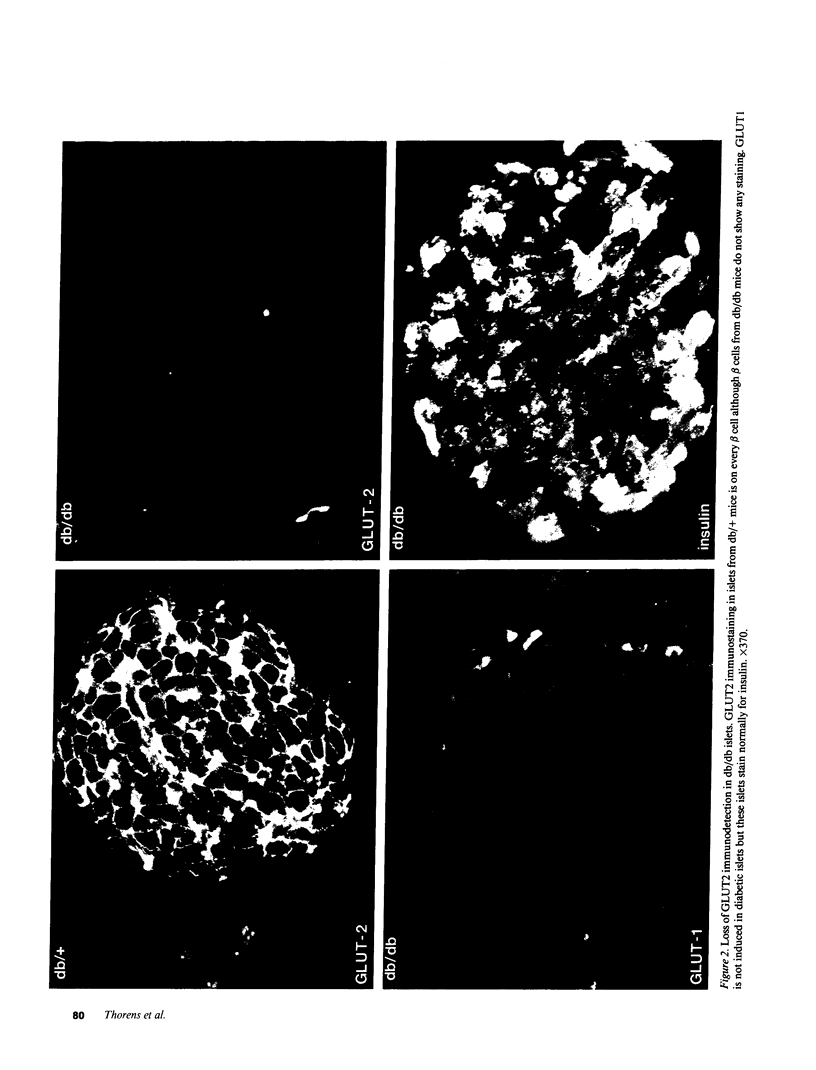
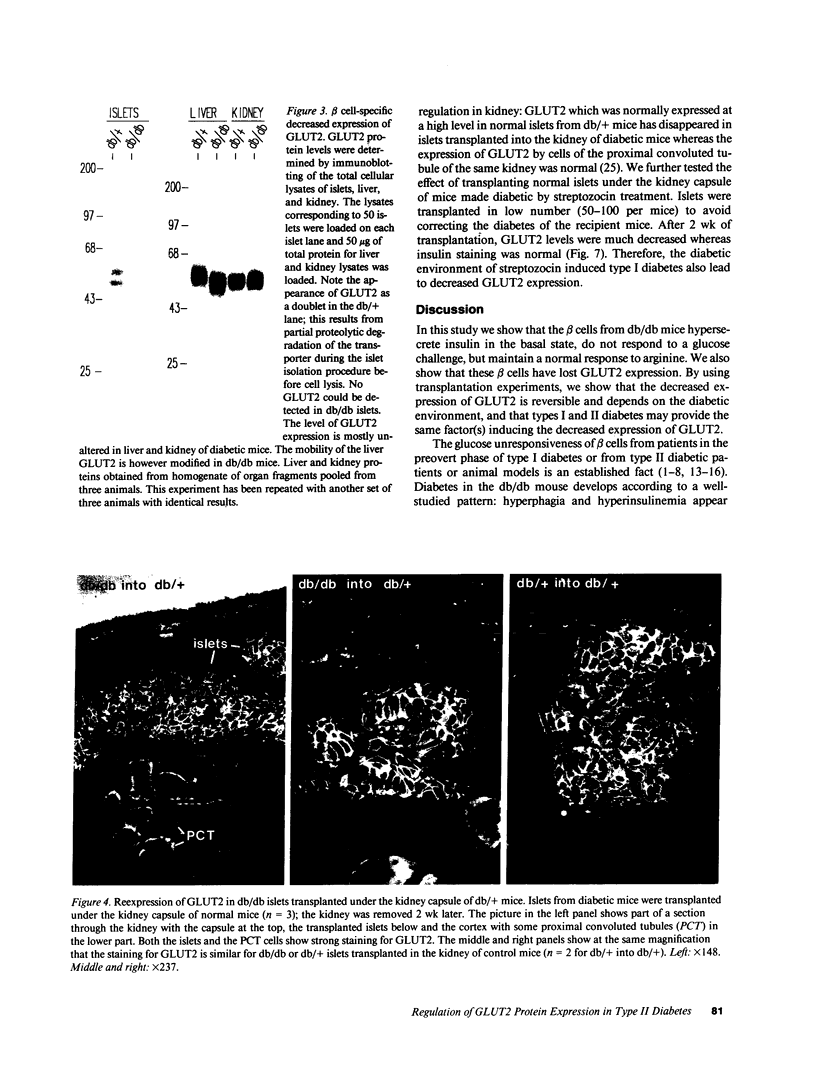
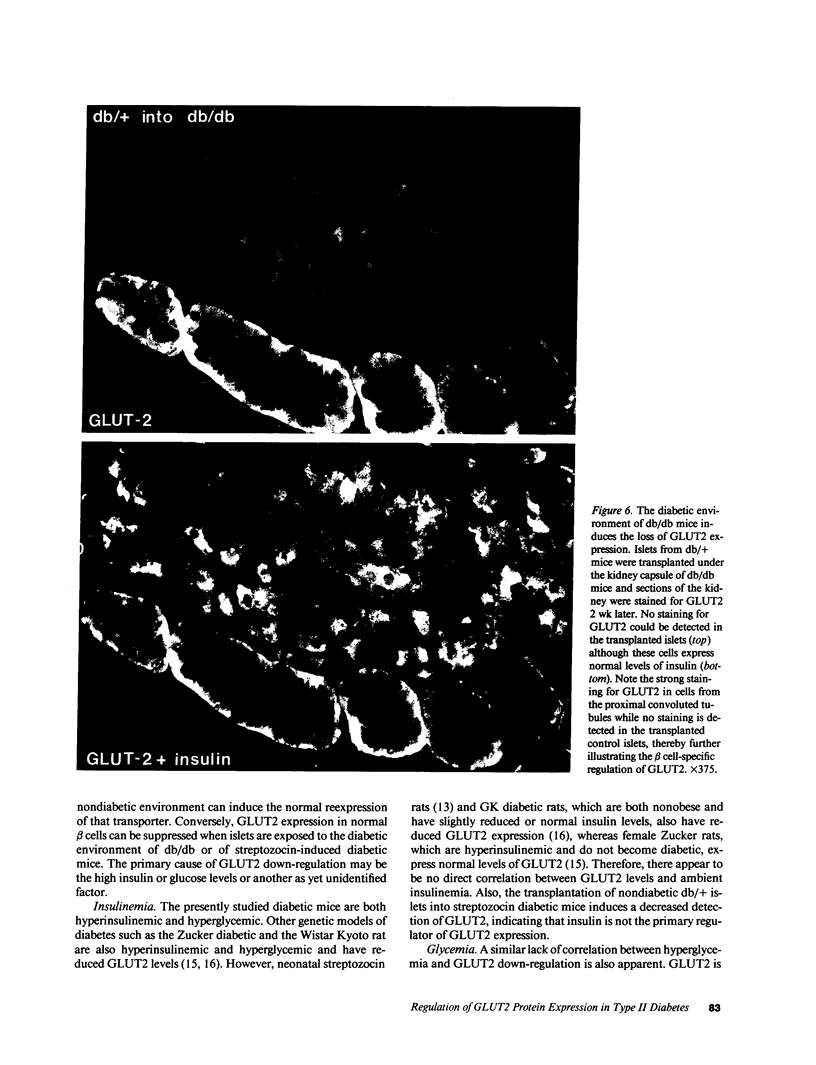
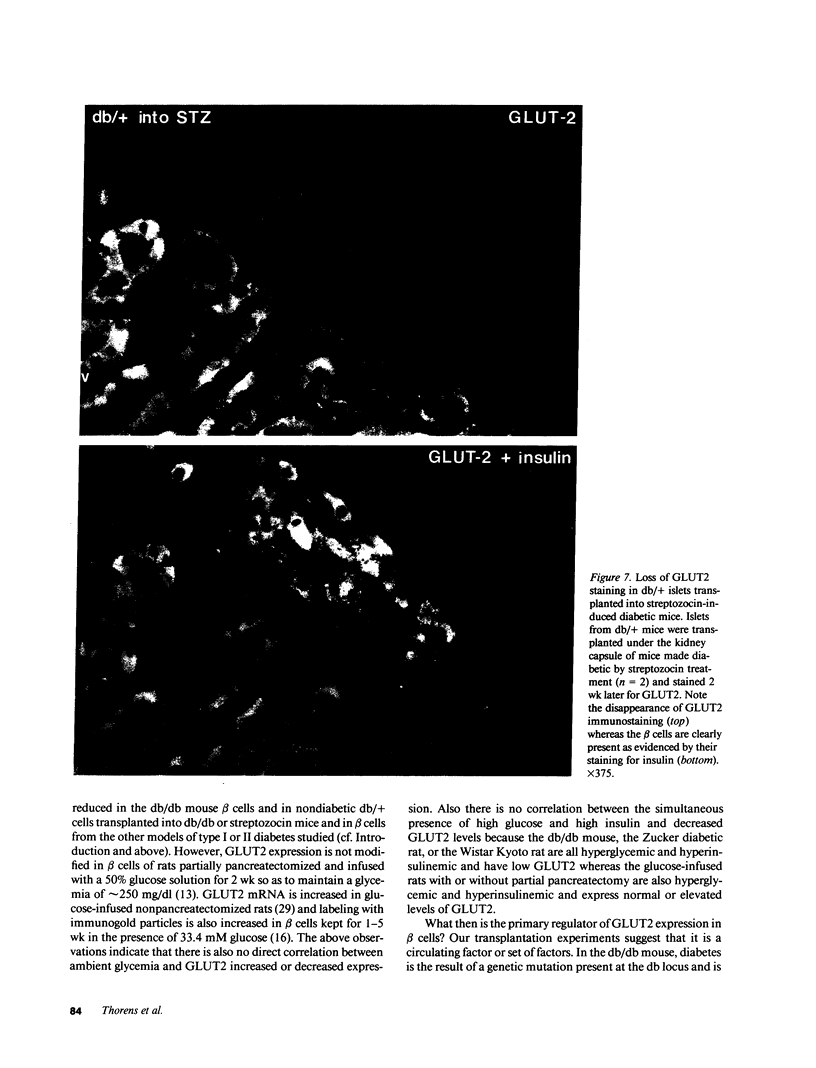
Glucose-induced insulin secretion by beta cells of diabetic db/db mice was studied by a pancreas perfusion technique, and the levels of GLUT2 protein in pancreatic islets were assessed by immunofluorescence microscopy and protein blot analysis. Beta cells from diabetic mice had a high basal rate of insulin secretion; they did not respond to glucose stimulation but displayed a normal secretory response to arginine. At the same time, GLUT2 expression by db/db islets was lost whereas beta cells from nondiabetic db/+ mice expressed high levels of this transporter. GLUT2 levels in liver or kidney of diabetic mice were, however, mostly unaltered. Transplanting islets from db/db mice under the kidney capsule of db/+ mice restored normal GLUT2 levels. Conversely, transplantation of db/+ islets into db/db mice induced the disappearance of GLUT2 expression. When islets from db/+ mice were transplanted under the kidney capsule of streptozocin-diabetic mice, the immunodetection of GLUT2 also disappeared. We conclude that: (a) GLUT2 expression is decreased in glucose-unresponsive beta cells from db/db mice; (b) the decreased expression of GLUT2 is reversible; (c) the loss of GLUT2 expression is induced by the diabetic environment of db/db and streptozocin-induced diabetic mice. These observations together with previously published data suggest that a factor different from glucose or insulin regulates the beta cell expression of GLUT2.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano J. D., Ekins R. P., Maritz G., Turner R. C. A sensitive, precise radioimmunoassay of serum insulin relying on charcoal separation of bound and free hormone moieties. Acta Endocrinol (Copenh) 1972 Jul;70(3):487–509. doi: 10.1530/acta.0.0700487. [DOI] [PubMed] [Google Scholar]
- Aronoff S. L., Bennett P. H., Rushforth N. B., Miller M., Unger R. H. Normal glucagon response to arginine infusion in "prediabetic" Pima Indians. J Clin Endocrinol Metab. 1976 Aug;43(2):279–286. doi: 10.1210/jcem-43-2-279. [DOI] [PubMed] [Google Scholar]
- Cerasi E., Luft R., Efendic S. Decreased sensitivity of the pancreatic beta cells to glucose in prediabetic and diabetic subjects. A glucose dose-response study. Diabetes. 1972 Apr;21(4):224–234. doi: 10.2337/diab.21.4.224. [DOI] [PubMed] [Google Scholar]
- Chen L., Alam T., Johnson J. H., Hughes S., Newgard C. B., Unger R. H. Regulation of beta-cell glucose transporter gene expression. Proc Natl Acad Sci U S A. 1990 Jun;87(11):4088–4092. doi: 10.1073/pnas.87.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert T., Lauridsen U. B., Madsen S. N., Mogensen P. Insulin response to glucose, tolbutamide, secretin, and isoprenaline in maturity-onset diabetes mellitus. Dan Med Bull. 1972 Oct;19(7):222–226. [PubMed] [Google Scholar]
- Gapp D. A., Leiter E. H., Coleman D. L., Schwizer R. W. Temporal changes in pancreatic islet composition in C57BL/6J-db/db (diabetes) mice. Diabetologia. 1983 Nov;25(5):439–443. doi: 10.1007/BF00282525. [DOI] [PubMed] [Google Scholar]
- Hogan A., Heyner S., Charron M. J., Copeland N. G., Gilbert D. J., Jenkins N. A., Thorens B., Schultz G. A. Glucose transporter gene expression in early mouse embryos. Development. 1991 Sep;113(1):363–372. doi: 10.1242/dev.113.1.363. [DOI] [PubMed] [Google Scholar]
- Johnson J. H., Newgard C. B., Milburn J. L., Lodish H. F., Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J Biol Chem. 1990 Apr 25;265(12):6548–6551. [PubMed] [Google Scholar]
- Johnson J. H., Ogawa A., Chen L., Orci L., Newgard C. B., Alam T., Unger R. H. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990 Oct 26;250(4980):546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- Koranyi L., James D., Mueckler M., Permutt M. A. Glucose transporter levels in spontaneously obese (db/db) insulin-resistant mice. J Clin Invest. 1990 Mar;85(3):962–967. doi: 10.1172/JCI114526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy J. L. Natural history of beta-cell dysfunction in NIDDM. Diabetes Care. 1990 Sep;13(9):992–1010. doi: 10.2337/diacare.13.9.992. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Baetens D., Inman L., Amherdt M., Peterson R. G., Newgard C. B., Johnson J. H., Unger R. H. Evidence that down-regulation of beta-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L., Thorens B., Ravazzola M., Lodish H. F. Localization of the pancreatic beta cell glucose transporter to specific plasma membrane domains. Science. 1989 Jul 21;245(4915):295–297. doi: 10.1126/science.2665080. [DOI] [PubMed] [Google Scholar]
- Orci L., Unger R. H., Ravazzola M., Ogawa A., Komiya I., Baetens D., Lodish H. F., Thorens B. Reduced beta-cell glucose transporter in new onset diabetic BB rats. J Clin Invest. 1990 Nov;86(5):1615–1622. doi: 10.1172/JCI114883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973 Apr;52(4):870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikanta S., Ganda O. P., Eisenbarth G. S., Soeldner J. S. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for Type I diabetes mellitus. N Engl J Med. 1983 Feb 10;308(6):322–325. doi: 10.1056/NEJM198302103080607. [DOI] [PubMed] [Google Scholar]
- Suzue K., Lodish H. F., Thorens B. Sequence of the mouse liver glucose transporter. Nucleic Acids Res. 1989 Dec 11;17(23):10099–10099. doi: 10.1093/nar/17.23.10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B., Charron M. J., Lodish H. F. Molecular physiology of glucose transporters. Diabetes Care. 1990 Mar;13(3):209–218. doi: 10.2337/diacare.13.3.209. [DOI] [PubMed] [Google Scholar]
- Thorens B., Cheng Z. Q., Brown D., Lodish H. F. Liver glucose transporter: a basolateral protein in hepatocytes and intestine and kidney cells. Am J Physiol. 1990 Dec;259(6 Pt 1):C279–C285. doi: 10.1152/ajpcell.1990.259.2.C279. [DOI] [PubMed] [Google Scholar]
- Thorens B., Lodish H. F., Brown D. Differential localization of two glucose transporter isoforms in rat kidney. Am J Physiol. 1990 Dec;259(6 Pt 1):C286–C294. doi: 10.1152/ajpcell.1990.259.2.C286. [DOI] [PubMed] [Google Scholar]
- Thorens B., Sarkar H. K., Kaback H. R., Lodish H. F. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988 Oct 21;55(2):281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Thorens B., Weir G. C., Leahy J. L., Lodish H. F., Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga M., Komiya I., Johnson J. H., Inman L., Alam T., Moltz J., Crider B., Stefan Y., Baetens D., McCorkle K. Loss of insulin response to glucose but not arginine during the development of autoimmune diabetes in BB/W rats: relationships to islet volume and glucose transport rate. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9749–9753. doi: 10.1073/pnas.83.24.9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991 Mar 8;251(4998):1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- Weir G. C., Knowlton S. D., Martin D. B. Glucagon secretion from the perfused rat pancreas. Studies with glucose and catecholamines. J Clin Invest. 1974 Dec;54(6):1403–1412. doi: 10.1172/JCI107887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir G. C., Leahy J. L., Bonner-Weir S. Experimental reduction of B-cell mass: implications for the pathogenesis of diabetes. Diabetes Metab Rev. 1986;2(1-2):125–161. doi: 10.1002/dmr.5610020108. [DOI] [PubMed] [Google Scholar]