Abstract
Invariant natural killer T (iNKT) cells are a unique population of T lymphocytes, which lie at the interface between the innate and adaptive immune systems, and are important mediators of immune responses and tumor-surveillance. iNKT cells recognize lipid antigens in a CD1d-dependent manner; their subsequent activation results in a rapid and specific downstream response, which enhances both innate and adaptive immunity. The capacity of iNKT cells to modify the immune-microenvironment influences the ability of the host to control tumor growth, making them an important population to be harnessed in the clinic for the development of anti-cancer therapeutics. Indeed, the identification of strong iNKT cell agonists, such as α-galactosylceramide (α-GalCer) and its analogues, has led to the development of synthetic lipids which have shown potential in vaccination and treatment against cancers. In this Masters of Immunology article we discuss these latest findings, and summarise the major discoveries in iNKT cell biology, which have enabled the design of potent strategies for immune-mediated tumor destruction.
Introduction
Invariant Natural Killer T cells (iNKT) cells represent a distinct population of T lymphocytes, which have features of both conventional T cells as well as natural killer (NK) cells [1]. As a result of their unique ability to recognize CD1d-bound endogenous lipid antigens, iNKT cells have a constitutive memory phenotype and are capable of rapidly responding to stimulation, producing a broad range of cytokines. In addition, through direct interactions, in particular via CD1d and CD40L-CD40 signalling, as well as indirect interactions with other immune cells, iNKT cells are capable of maturing dendritic cells (DC) and activating B cells, and thus are crucial in enhancing antigen-specific B- and T-cell responses [2]. The use of iNKT-cell deficient mice and iNKT cell-specific adjuvants has provided compelling evidence demonstrating that iNKT cells play an important role in mounting an antitumor response. Indeed, the importance of iNKT cells in tumor immunosurveillance is further emphasised with the observation that reduced iNKT cell numbers and function have been documented in a large number of cancer patients, including in patients with progressive malignant multiple myeloma [3], prostate cancer [4] and a broad range of other solid malignancies [5]. In this Master of Immunology article, we will discuss the role of iNKT cells in enhancing tumor immunity and introduce clinical strategies that are currently being considered to harness iNKT cells in cancer patients to encourage stronger anti-cancer immune responses.
NKT cells: classification and subsets
In contrast to conventional T cells, which recognize protein-derived antigens presented by major histocompatibility complex (MHC) class I and class II molecules, the T-cell receptors (TCR) on NKT cell recognize both exogenous and endogenous lipids presented in the context of the non-polymorphic, MHC class I-like CD1d molecules [6, 7]. NKT cell development requires thymic selection, similarly to that of conventional T cells, which results in the release and expansion of a population of cells with the ability for specific antigen recognition, but also with a range of innate immune functions [2]. Analysis of the phenotype and cytokine profile of NKT cells has led to the identification of two main NKT-cell subsets: invariant NKT (iNKT) cells, otherwise known as type I NKT cells, and diverse NKT cells, which are more commonly called type II NKT cells [8]. iNKT cells express an antigen-specific TCR composed of a semi-invariant α-chain (Vα14-Jα18 in mice and Vα24-Jα18 in humans) paired with a restricted repertoire of β-chains (Vβ2, Vβ7 and Vβ8.2 in mice, or Vβ11 in humans) [9]. Similarly, type II NKT cells are CD1d-restricted, but in contrast to iNKT cells, they express a polyclonal TCR repertoire, and are more comparable to the highly diverse TCRs of conventional CD4+ and CD8+ T cells [10-12]. The importance of antigen presentation by CD1d molecules in NKT-cell activation and development was highlighted by the observation that Cd1d−/− mice lack both iNKT cells and type II NKT cells [13-15]. Indeed, to distinguish the roles of the two NKT populations, researchers commonly compare the phenotype of Cd1d−/− mice [13-15] with that of Jα18−/− mice [16], which lack only iNKT cells. Notably, recent studies have highlighted that Jα18−/− mice exhibit additional defects in the T-cell repertoire [17], therefore, the iNKT cell relevance of results obtained using Jα18−/− mice should be considered in the context of these findings. The heterogeneity of Vα14+ iNKT cells has been further appreciated with the identification of several subsets of iNKT cells with distinct developmental and functional properties [18-21]. Indeed, a distinct Vα50-Jα10 iNKT-cell subset was identified, which although absent in Cd1d−/− mice, was found to be present in Jα18−/− mice [22]; it is clear that considering these subsets will be critical in order to accurately interpret forthcoming data.
Although a lack of reagents to monitor type II NKT cells has slowed down functional and phenotypic analysis of these cells, access to CD1d tetramers loaded with iNKT-cell agonists has allowed characterisation of the frequency and phenotype of iNKT cells both in mice and humans [23-25]. In mice, iNKT cells comprise approximately 1–3% of the lymphocytes in the circulation and lymphoid organs, and are unusually enriched in the liver where they can comprise up to 30% of resident lymphocytes [26]. Conversely, although found to be enriched in the adipose tissue and omentum [27], the frequency of iNKT cells in the human periphery is lower and more variable than in mice [28].
iNKT cells recognize a diverse range of antigens
Despite their semi-invariant TCRs, iNKT cells are able to recognize a diverse range of antigens [29]. Structural and functional studies have been fundamental in determining which features of lipid recognition modulate the potency and activation of iNKT cells, and importantly, have been crucial in optimising the design of iNKT-cell agonists suitable for use in the clinic [30-36]. α-galactosylceramide (α-GalCer), derived from the glycosphingolipid extract of the marine sponge Agelas mauritianus, was the first lipid identified which potently activates iNKT cells [37]; the α-linked glycan in α-GalCer has since been shown to be a structural motif common to many of the identified α-linked bacterial pathogens, which can directly and potently activate iNKT cells [38-41]. Recently a β-linked lipid, Asperamide B, was identified as the first example of a fungal-derived iNKT-cell agonist [42], although in other models of fungal infection iNKT-cell reactivity was shown to be driven through Dectin-1- and MyD88-mediated upregulation of IL12 by antigen-presenting cells (APC) [43]. In addition to recognising synthetic and microbial-derived antigens, iNKT cells react against CD1d+ APCs in the absence of exogenous antigens, a feature defined as autoreactivity. iNKT-cell autoreactivity underpins the constitutive memory phenotype of iNKT cells and their ability to be activated during a wide variety of immune responses including infections, cancer and autoimmunity [44, 45]. Although complete elucidation of endogenous and exogenous lipids mediating iNKT-cell activation has been challenging due to poor sensitivity of assays, which are often unable to detect low lipid concentrations purified from cellular extracts and pathogens, seminal studies in the last year identified the gut mucosa [46-48] and alternative enzymatic pathways in mammals [49, 50] as potential sources of exogenous and endogenous iNKT-cell lipid agonists. Further investigations are warranted to fully characterise these lipids, which will be highly valuable for understanding the role of iNKT cells in cancers, where endogenous lipids undoubtedly play a key role in triggering the immune response.
iNKT-cell activation and down-stream signalling
Activation of iNKT cells can occur directly or indirectly
-
i)
Direct activation of iNKT cells involves the endocytosis of glycolipid antigens by APCs and their presentation to iNKT cells via CD1d-antigen complexes. In addition to direct iNKT-cell activation by exogenous lipid agonists, we and others have shown that signalling events downstream of Toll-like receptors (TLR) [44, 45, 51], inflammasome components NOD1 and NOD2 [52] and the Formyl Peptide Receptor 2 (FPR2), which recognizes Serum Amyloid A-1 [53], results in the loading of CD1d molecules expressed on APCs with endogenous lipid antigens, and subsequent iNKT-cell activation. Additionally, since a number of tumor cells express CD1d [3, 54-57], it is hypothesised that tumor cells may also present endogenous lipids to iNKT cells directly, although to date the identity of such tumor cell-derived endogenous iNKT-cell agonists remains contentious. Importantly, CD1d-dependent activation of iNKT cells triggers release of IFNγ and interleukin (IL)4, as well as of a diverse range of other cytokines including IL2, IL5, IL6, IL10, IL17, IL21, TNFα, TGFβ and granulocyte-macrophage colony stimulating (GM-CSF) [1, 58-60], in addition to chemokines, such as RANTES, Eotaxin, MIP-1α and MIP-1β [61]. IFNγ and IL4 transcription is activated during iNKT-cell thymic development, and preformed IL4 mRNA in the cytoplasm allows for rapid responses upon antigen stimulation [62, 63]. In concert with cytokine release, activation of iNKT cells through TCR stimulation augments the bi-directional cross-talk with DCs in a CD40/CD40L and CD1d-dependent manner; this interaction promotes the maturation, activation and the upregulation of co-stimulatory receptors such as CD80 and CD86 on DCs, as well as the release of IL12. Interestingly, depending on the lipid antigen presented, iNKT cells may also modulate up-regulation of inhibitory molecules (such as PD-L1 and PD-L2) on CD8α+ DCs, which may be the mechanism behind the Th2-polarizing effect of some iNKT-cell agonists [64]. As a result of direct interaction with iNKT cells, DCs can prime antigen-specific CD4+ and CD8+ T cells [65-67]. Licensing by iNKT cells of CD8α+ DCs results in the secretion of the chemokine CCL17, which attracts naive CD8+ T cells expressing the chemokine receptor CCR4 [68]. iNKT cells can also directly provide B-cell help through CD1d expression on B cells [69, 70]. This ability to prime the adaptive immune response indicates that iNKT-cell agonists could be used in the clinic to harness iNKT cells, where they have previously been shown to have adjuvant effects in combination with a number of vaccines [71].
-
ii)
iNKT cells can be activated via soluble factors (indirect NKT-cell activation) released by TLR-activated DCs, such as type I IFN, IL12, and IL18 [44, 45, 51, 72-75], or by co-stimulatory molecules like OX40/OX40L [76].
Structural and functional analyses of the interaction between the iNKT TCRs and CD1d molecules loaded with endogenous and exogenous iNKT-cell agonists are of importance to characterize further how the quality of iNKT-cell activation can be modulated by the binding affinity, concentration, hydrophobicity and stability of glycolipid-CD1d complexes [31, 32, 77, 78]. Indeed, low antigen concentration or weak binding affinity of CD1d/lipid complexes to the iNKT TCRs induce GM-CSF and IL13, whereas a higher antigen concentration or higher binding affinity of CD1d/lipid complexes induce IL4 and IFNγ, along with increased expression of GM-CSF and IL13 [79]. In line with this, the lipid C-glycoside, an analog of α-GalCer, has a weak binding affinity to the iNKT-cell TCR, but as a result of the formation of a stable complex with CD1d, and thus its extended survival in vivo, is still able to induce IFNγ production from iNKT cells [80]. These mechanisms demonstrate how antigenic activation of iNKT cells can enhance both cell-mediated and humoral immunity through direct or indirect interaction with other immune cells.
iNKT cells in tumor immunity
The initial observation that α-GalCer injected into mice could protect against tumor progression [81, 82], led to the subsequent discovery that α-GalCer specifically activated iNKT cells in a CD1d-resticted manner [37]. In addition to exerting a protective role in a range of different tumor models when in vivo activated with α-GalCer [83] or IL12 [16], iNKT cells also play a critical role during tumor immunosurveillance. Indeed, following adoptive transfer of iNKT cells into Jα18−/− mice Crowe and colleagues demonstrated their ability to protect mice from methylcholanthrene (MCA)-induced sarcomas via direct interaction of the iNKT TCR with CD1d molecules [84], confirming and extending previous observations by the same group using MCA tumor models [83]. The role of iNKT cells in tumor immunosurveillance has been confirmed in other murine studies including a p53 deficiency model [85] and a TRAMP model [86], all of which showed enhanced tumor growth in iNKT cell-deficient mice (Jα18−/− mice or Cd1d−/− mice), as compared with wild-type animals. Notably, not all iNKT-cell subsets are equally protective, as rejection of MCA-1 sarcomas and B16F10 melanomas was mediated exclusively by the liver-derived CD4− iNKT-cell subset [87].
Activation of iNKT cells during immunosurveillance can occur either directly, through presentation of self-lipids by CD1d positive tumors, or indirectly, by cross-presentation of tumor lipids by APCs [88]. Evidence for direct presentation stems from the observation that overexpression of CD1d by the B-cell lymphoma NS0 induces cytokine production by iNKT cells and iNKT cell-dependent lysis [89]. Consistent with these findings, in a mouse model of breast cancer metastases, tumor down-regulation of CD1d molecules inhibits iNKT-mediated antitumor immunity and promotes metastatic breast cancer progression [57]. Furthermore, human iNKT cells were found to recognize and kill CD1d+ osteosarcoma cells, but not CD1d− osteoblasts, confirming the CD1d restriction of iNKT cell-dependent cytotoxicity [90]. Notably, these studies and others [91, 92] have confirmed iNKT cell-dependent cytotoxicity against CD1d+ tumor cell lines without pulsing with α-GalCer, underscoring the notion that the iNKT cell TCR can interact with endogenous antigenic lipids expressed by human and mouse tumor cells, which can lead to direct iNKT-cell activation [90].
CD1d is preferentially expressed in hematopoietic cells [93], especially those of myelomonocytic and B-cell lineages, and accordingly, malignancies originating from such tissues have also been found to be CD1d-positive [3, 54, 55, 89, 94, 95]. Interestingly, CD1d expression has also been found on select solid tumors, such as prostate cancer [4, 56], breast cancer [57], renal cell carcinoma [96] and specific nervous system tumors including malignant glioma [97] and paediatric medulloblastoma [98]; however many other human and murine solid tumors are generally thought to be CD1d-negative, or to down-regulate CD1d molecules. Lack of CD1d expression in tumors results in their lack of recognition by iNKT cells, and has, in some models, been correlated with tumor progression. It remains to be determined, however, whether the lack of detection of CD1d molecules on the surface of such tumors could stem from sub-optimal antibody staining or the local down-regulation of CD1d, and thus whether these tumors are able to present endogenous lipid is not yet defined. Given that CD1d molecules are widely expressed by normal cells, it remains unclear as to whether a different set of unidentified self-iNKT-cell agonists can be presented by CD1d molecules expressed by transformed cells, as compared to normal cells. Furthermore, although it is commonly accepted that endogenous lipids are likely to be responsible for activating iNKT cells in the inflammatory tumor microenvironment, the mechanisms by which iNKT cells are activated during tumour growth remain elusive. Further investigations are warranted to elucidate these findings.
A hypothesis: The role of the endoplasmic reticulum (ER)-stress response in modulating iNKT-mediated tumor immunity
In non-sterile disease models, pathogen-associated molecular patterns (PAMP) act as TLR agonists, and through the up-regulation of endogenous ligand presentation and the release of soluble factors by APCs, have been shown to enhance the activation of iNKT cells [44, 45, 51]. In light of this, we put forward the hypothesis that a similar mechanism may be involved in iNKT-mediated tumor surveillance. Indeed in recent years a new concept of ‘immunogenic cell death’ [99] has emerged, which links ER stress with the release of damage-associated molecular patterns (DAMP) during anticancer therapy, and through recognition by pattern recognition receptors (PRR), such as TLR4, the release of DAMPs by dying cancer cells results in the activation of a cancer-specific immune response [100]. Although it remains unclear whether these DAMPs can influence iNKT cell antitumor responses, in support of this idea, we and others have shown that stimulation of TLR4 on APCs can enhance presentation of iNKT-cell agonists and stimulate iNKT-cell activation [44, 45, 101]. In line with this, the Unfolded Protein Response (UPR), which is also triggered by ER stress, increases the activity of the ER lipid transfer protein microsomal triglyceride transfer protein (MTP) [102], which is involved in CD1d loading [103, 104]. Lastly, an additional UPR component, XBP-1, which modulates phospholipid synthesis and is required for ER membrane expansion under ER stress [105], has been shown to positively control hepatic lipogenesis at basal levels [106]. Disruption of XBP-1 led to decreased fatty acids and sterols in primary hepatocytes, possibly by directly trans-activating key genes in this metabolic pathway [106].
As well as tumor-intrinsic ER-stress signalling, which promotes tumor survival and proliferation, the tumor-cell UPR can function in a cell-extrinsic manner, transmitting ER stress to tumor-infiltrating myeloid cells, in a mechanism termed transmissible ER stress (TERS) [107]. Although not yet assessed in the context of cancer, ER stress was correlated with abnormalities in the function and frequency of NKT cells in hepatic steatosis, where it was suggested that ER disruption might lead to dysregulation of iNKT-mediated innate immunity through decreased expression of membrane CD1d resulting in reduced iNKT-cell activation [108]. While in this model ER stress had a negative effect on iNKT-cell activation, in light of the reported effects of ER stress on lipid metabolism and CD1d loading discussed above, further experimentation needs to be performed to dissect whether changes in lipid metabolism due to ER stress in cancer cells may modulate iNKT-cell activity.
NKT cell-mediated adjuvant effects on innate and adaptive immunity against cancer in mice
The ability of iNKT cells to activate antitumor immune responses can be jump started by using exogenous iNKT-cells agonists, such as the prototypic ligand α-GalCer [109-112]. Injection of α-GalCer was found to inhibit tumor metastases and increase survival in a range of murine cancer models, including models of B16 tumor challenge [109], spontaneous sarcomas in p53−/− mice [113] and the colon carcinoma model C26GM [114]. In line with this, injection of α-GalCer-pulsed DCs [115], or intravenous administration of either live or irradiated B16 tumor cells loaded with α-GalCer [116] was shown to elicit an innate iNKT and NK cell response that rejects the tumor. The α-GalCer-mediated antitumor activity of iNKT cells has since been shown to be dependent on IFNγ production and NK cells [110, 117, 118], dendritic cell maturation, activation and IL12 release, and ultimately the activation of CD8+ cytotoxic T cells, CD4+ Th1 cells, and gamma-delta (γδ) T cells that further target and kill tumor cells [65, 116, 119]. Indeed, administration of α-GalCer into mice injected with a T-cell lymphoma enhanced the generation of tumor-specific cytotoxic T cells in an IFNγ- and NK-cell-dependent manner [120]. This pathway was further emphasised in murine models of lung and liver metastasis, where the anti-metastatic activity of α-GalCer was dependent on IL12- and IL18-mediated enhancement of IFNγ production by iNKT and NK cells [118].
Upon activation, both murine and human iNKT cells can exhibit potent cytotoxic functions to promote the killing of tumor cells, such as acute myeloid leukaemia, through the expression of tumor necrosis factor–related apoptosis-inducing ligand (TRAIL) [121]. This observation was also confirmed with iNKT cells from patients with malignant melanoma, whereby upon α-GalCer/DC activation, the patient-derived iNKT cells displayed potent perforin-dependent cytotoxic activity against a range of tumor cell lines [122]. Interestingly, the transfer of perforin-deficient iNKT cells into Jα18−/− mice with MCA-induced tumors restored tumor resistance, suggesting that in this model direct perforin-dependent tumor lysis by iNKT cells is not critical [84]. Taken together, these observations imply that both direct and indirect mechanisms of iNKT-cells activation play a key critical role in iNKT cell-mediated tumor immunosurveillance [88, 116].
Studies aimed at enhancing iNKT cell-mediated antitumor immunity have shown that the use of soluble α-GalCer leads to potent stimulation of iNKT-cell subsets and may result in iNKT-cell over-activation and anergy [123, 124]. Given these considerations, the search for efficient iNKT agonists with functional differences compared with α-GalCer is an ongoing goal in the field, which attracts the work of many laboratories. Indeed, in recent years, many α-GalCer analogs have been formulated that exhibit different properties, including optimised cytokine induction profiles, which are aimed at targeting specific subsets of iNKT cells in a number of different clinical settings [125-133].
Harnessing iNKT cells to optimize vaccination strategies in cancer patients
Activity of iNKT cells in cancer patients
A large number of pre-clinical and clinical trials have been performed to investigate whether activation of iNKT cells could be a therapeutically beneficial approach in human patients suffering from cancer and other infectious diseases. Reduced iNKT-cell frequency and function has been observed in patients with haematologic cancers [3, 134] and a range of solid tumors [4, 135], as compared with that of healthy volunteers, independent of tumor type and tumor load. In line with these observations, reduced iNKT-cell frequency was shown to correlate with poor overall survival in acute myeloid leukaemia [136], and head and neck squamous cell carcinoma [137], while increased numbers of intra-tumor or circulating iNKT cells have been associated with improved prognosis in colon cancer, prostate cancer, haematologic malignancies, and neuroblastoma [138-140]. Whether immune-cell subsets found in peripheral blood are accurate representative of systemic cancer immunity in humans remains to be established in all cancer models [141]; relative NKT-cell deficiencies have, however, also been observed locally in solid tumors and the surrounding tissues, such as in neuroblastoma [142] and colorectal cancer [27]. Interestingly, other investigators have reported elevated iNKT-cell frequency in some tumors [143, 144]; and increased iNKT-cell frequency in the microenvironment of colorectal cancers is thought to be a positive prognostic indicator [145, 146]. The high variability in iNKT-cell frequencies in humans, in addition to the defective numbers shown in cancer and other diseases, reduces the effectiveness of targeting iNKT cells in these individuals. Indeed, studies have reported that NKT cell-based treatments may only be beneficial for patients with high iNKT-cell frequency [147]. To overcome these limitations, universal efforts have been directed at optimising the development of synthetic iNKT-cell agonists to enhance iNKT-cell activation and antitumor function.
iNKT cell-based cancer immunotherapy
Three main iNKT cells-directed therapeutics have been exploited thus far; these include, but are not limited to: administration of iNKT cell-activating ligands (all human studies described to date have used α-GalCer), administration of APCs pulsed with α-GalCer, transfer of ex vivo-expanded and/or activated iNKT cells, and finally a combination of these methods.
1. Intravenous injection of α-GalCer
α-GalCer remains the best-characterised iNKT agonist in tumor immunity to date. Although promising data utilising this agonist have been generated in murine models and in vitro, the fundamental question remains whether iNKT-cell activation by select agonists is relevant in the clinic. The first clinical study of α-GalCer used repeated intravenous (IV) injection of α-GalCer at varying doses in patients with solid tumors [148]. No dose-limiting toxicity was observed, suggesting that activation of iNKT cells through IV injection of α-GalCer is a safe, well-tolerated treatment in humans. Although iNKT cell numbers appeared to decrease in the periphery, likely resulting from down-regulation of the TCR following iNKT-cell activation [149], Giaccone and colleagues observed elevated serum levels of iNKT cell-associated cytokines, including TNFα and GM-CSF [148], and disease stabilisation for a median of 123 days in 7 of 24 patients. Similar to murine studies in which injection of soluble, but not cell-associated α-GalCer leads to iNKT-cell anergy [123] in a PD-1/PDL-1-dependent manner [150], follow up studies in humans identified α-GalCer-induced iNKT-cell anergy using this administration method [151].
2. Adoptive transfer of α-GalCer-pulsed APCs
Studies with murine tumor models demonstrated that co-injection of α-GalCer and tumor antigens [65], or alternatively administration of α-GalCer-pulsed DCs [152], induced prolonged cytokine responses as compared with injection of soluble α-GalCer. Although the reasoning behind the differing immune responses is unclear, it has been hypothesised that the type of APC and method of administration could play an important role. Indeed, whereas IV injection of pulsed DCs induced a strong cytokine response, α-GalCer-pulsed DCs injected subcutaneously in mice did not stimulate a particularly effective iNKT-cell response [152]. In addition, DCs were found to stimulate a stronger iNKT-cell response in comparison to B cells [153]. A large number of clinical trials have since utilised ex-vivo-generated, or isolated APCs pulsed with α-GalCer, which has thus far been shown to be safe and well tolerated.
The first phase-I trial reported utilised IV administration of α-GalCer-pulsed monocyte-derived DCs, which were given at two weekly intervals to patients with metastatic tumors [154]. Although activation of iNKT cells increased serum levels of cytokines including IFNγ and IL12 and the trans-activation of both T and NK cells, only 2 of the 12 patients enrolled exhibited a decrease in serum tumor markers, indicating minimal efficacy of this treatment [154]. Two later studies using α-GalCer-pulsed, monocyte-derived DCs were published; the first, using weekly IV injections of IL2-cultured DCs in patients with advanced or recurrent non-small-cell lung cancer, demonstrated an expansion of iNKT-cell frequency and elevated IFNγ levels by PCR analysis [151]. IFNγ ceased to be detected onwards of the second injection, possibly consistent with the onset of iNKT-cell anergy [151]. Comparably, Chang and colleagues reported that the injection of α-GalCer-pulsed monocyte-derived DCs also induced elevation of iNKT-cell frequency to greater than 100-fold, as well as higher serum concentrations of IFNγ and IL12 [155]. iNKT-cell activation could be seen for up to 6 months in some patients and was consistent with an increase in the levels of IL12p40, IP-10, and MIP-1β, and an increase in cytomegalovirus-specific CD8+ memory T cells [155]. Uchida and colleagues modified the administration approach by utilising injection of α-GalCer-pulsed peripheral blood APCs directly into the nasal sub-mucosa of patients with head and neck cancer [156]. Elevation in iNKT cell numbers and NK activation was observed in approximately half of the patients, and a reduction or stabilisation of tumor growth was seen in 6 of 9 patients [156]. A follow up study demonstrated that administration via the nasal sub-mucosa was optimal over administration via the oral sub-mucosa [157]; notably, authors also reported that oral-administration was linked to the expansion of CD4+ CD25+ FoxP3+ regulatory T cells [157].
More recently, four additional studies were published in which cancer patients were injected with APCs pulsed with α-GalCer either IV or intradermally (ID) [158-161]. Injection of APCs generated in the presence of GM-CSF and IL2 into patients with non-small-cell lung cancer demonstrated expansion of iNKT cells, and in patients with elevated level of IFNγ, a possible prolongation in survival was observed, although no partial or complete clinical responses were detected [161]. Elevated IFNγ production, as well as expansion and infiltration of iNKT cells were also observed following injection of GM-CSF/IL2-generated α-GalCer-pulsed APCs prior to surgery [159]. For patients with cancers of differing origin and metastatic potential, Nicol and colleagues reported that IV injection of pulsed APCs stimulated antitumor activity both at the main tumor site, and in sites of metastasis [158]; more than half of the patients showed disease stabilisation or a reduction in tumor mass [158]. Finally treatment of patients with multiple myeloma using the combined regimen of α-GalCer-pulsed APCs and the immune-modulatory drug lenalidomide elicited elevated IL2 in the serum, as well as a decrease in tumor-associated monoclonal immunoglobin levels (M spike) [160, 162]. Taken together, these findings demonstrate that α-GalCer-pulsed APCs represent a possible therapeutic strategy to enhance antitumor immunity. While further optimisation of loading and delivery and a more detailed understanding of the mechanisms of action are required, α-GalCer-pulsed APCs show promise for reducing tumor growth and metastasis.
3. Adoptive transfer of ex-vivo-activated iNKT cells
An alternative strategy to compensate for the decreased iNKT-cell frequency observed in patients with cancer involves expanding autologous iNKT-cell populations in vitro. Firstly, adoptive transfer of in vitro-activated iNKT cells into patients with non-small-cell lung cancer resulted in in vivo iNKT-cell expansion, downstream activation of NK cells and IFNγ release [163]. Interestingly, the combined transfer of iNKT cells and α-GalCer-pulsed DCs has been reported to induce substantial antitumor immunity in patients with head and neck squamous cell carcinomas [164, 165]. In these studies, patients demonstrated a partial response or stabilisation of the disease, and in some cases, tumor regression [164, 165]. Optimisation of the current protocols holds high potential in tumor immunotherapy. Indeed, functionally competent iNKT cells have recently been differentiated from induced pluripotent stem cells (iPSC) in mice, which may represent a novel approach to expand iNKT cells for cancer therapy in humans [166].
Conclusion & future perspectives
Murine studies and clinical trials performed to date have demonstrated that therapies involving the manipulation of iNKT cells are not only feasible but also appear to be generally well tolerated by mice and human patients alike, and in some cases induce significant tumor regression, disease stabilization, or possible prolongation of survival. Many of the approaches used thus far induce iNKT-cell activation; however it remains to be determined which route of administration, APC type, and dosing interval are the most efficacious. Although pre-clinical studies in animal models may help answer these questions, ultimately, appropriately designed clinical trials in humans will guide protocol optimization. Our ability to manipulate these cells in antitumor therapeutics is critically dependent on our understanding of iNKT cell biology and of the factors which activate and regulate these cells; the identification and optimisation of iNKT-cell agonists which can promote Th1 immune responses without inducing iNKT-cell anergy is of high priority. Notably, despite the clear ability of exogenously-activated iNKT cells to initiate potent antitumor activity in response to immunotherapeutic stimuli, whether this represents a physiologic role for NKT cells in tumor rejection, and if so, which signalling cascades are required, remains unclear. Additionally, in light of the identification of developmentally and functionally distinct subsets of iNKT cells and type II NKT cells, emphasis should be put on characterising the roles and interactions of these cells during immunosurveillance therefore improving the specificity of NKT-targeted agonists.
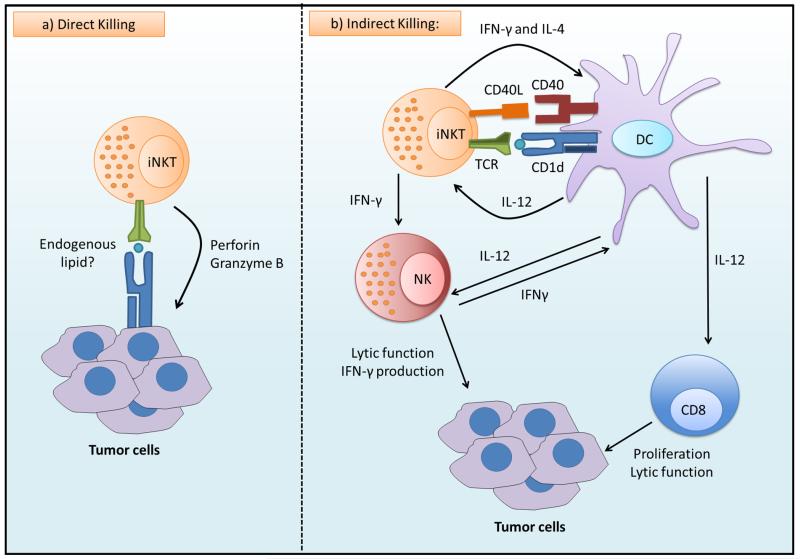
Figure 1. Antitumor activities of iNKT cells.
a) Invariant natural killer T (iNKT) cells can recognize endogenous lipids presented by CD1d molecules on tumor cells and subsequently eliminate tumor cells directly through iNKT cell-mediated lysis. b) In the absence of CD1d expression on tumor cells, iNKT cells may become activated in response to CD1d-expressing or Toll-like receptor (TLR)-activated antigen-presenting cells (APC). Bi-directional activation of iNKT cells and APCs promotes NK-cell activation and the activation of the tumor-specific T-cell response, thereby indirectly mediating tumor-cell killing. (This figure is created by Hemza Ghadbane of the Weatherall Institute of Molecular Medicine and the University of Oxford.)
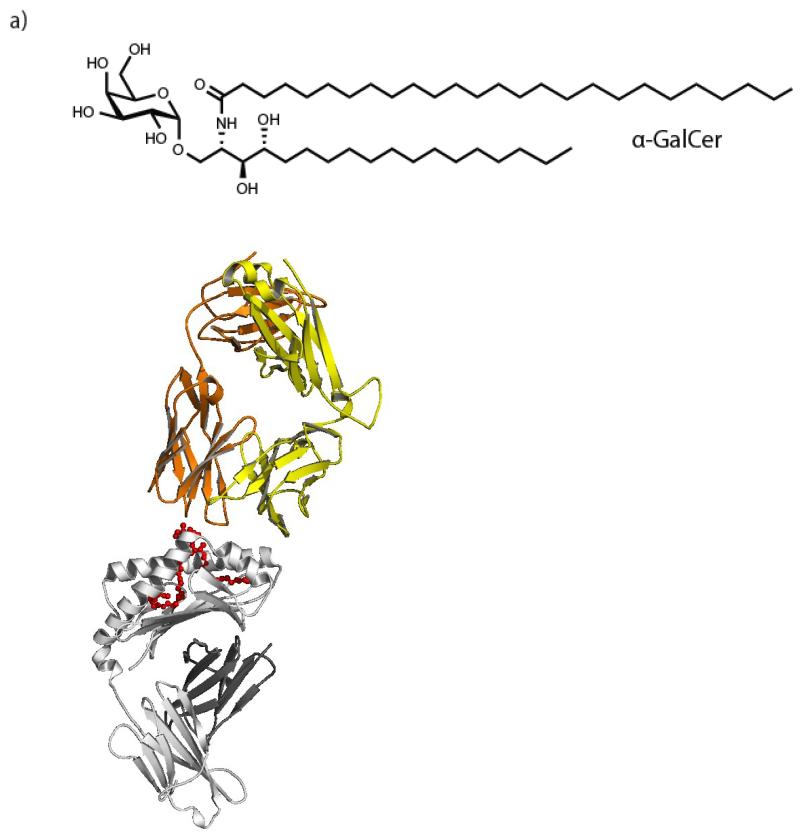
Figure 2. Structure and interactions of the prototypic iNKT-cell agonist α-GalCer with CD1d molecule and TCR.
a) The biochemical structure of the prototypic iNKT-cell agonist, α-GalCer. b) The crystal structure of α-GalCer (red) loaded onto human CD1d molecules (grey) and binding to the iNKT-cell TCR (yellow/orange). Figure was generated using PyMOL and the Protein Data Bank using accession number 2PO6 from [78], and adapted by permission from Macmillan Publishers Ltd: Nature [78], copyright 2007. The head group of the lipid is exposed and allows for interaction with the iNKT-cell TCR. Modifications to the head-group, tail length or saturation affect the ability of iNKT-cell agonists to activate iNKT cells [31], a property which has been utilised to optimise anti-cancer therapeutics. (Panel a of this figure is generated by Hemza Ghadbane of the Weatherall Institute of Molecular Medicine and the University of Oxford.)
Acknowledgements
We apologize to colleagues whose works were not cited due to space constraints or omission. We thank Dr Hemza Ghadbane for assistance with generating figures. This work was supported by Cancer Research UK [Programme Grant C399/A2291 (to V.C.)], the Medical Research Council, The Harry Mahon Cancer Research Trust UK, and the Wellcome Trust [84923 (to V.C.)].
References
- 1.Bendelac A, Savage PB, Teyton L. The biology of NKT cells. Annu Rev Immunol. 2007;25:297–336. doi: 10.1146/annurev.immunol.25.022106.141711. [DOI] [PubMed] [Google Scholar]
- 2.Salio M, Silk JD, Jones EY, Cerundolo V. Biology of CD1- and MR1-restricted T cells. Annu Rev Immunol. 2014;32:323–66. doi: 10.1146/annurev-immunol-032713-120243. [DOI] [PubMed] [Google Scholar]
- 3.Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–76. doi: 10.1084/jem.20021650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tahir SM, Cheng O, Shaulov A, Koezuka Y, Bubley GJ. Loss of IFN-γ production by invariant NK T cells in advanced cancer. J Immunol. 2001;167:4046–50. doi: 10.4049/jimmunol.167.7.4046. [DOI] [PubMed] [Google Scholar]
- 5.Crough T, Purdie DM, Okai M, Maksoud A, Nieda M, Nicol AJ. Modulation of human Vα24+Vβ11+ NKT cells by age, malignancy and conventional anticancer therapies. Br J Cancer. 2004;91:1880–6. doi: 10.1038/sj.bjc.6602218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bendelac A, Lantz O, Quimby ME, Yewdell JW, Bennink JR, Brutkiewicz RR. CD1 recognition by mouse NK1+ T lymphocytes. Science. 1995;268:863–5. doi: 10.1126/science.7538697. [DOI] [PubMed] [Google Scholar]
- 7.Exley M, Garcia J, Balk SP, Porcelli S. Requirements for CD1d recognition by human invariant Vα24+ CD4−CD8− T cells. J Exp Med. 1997;186:109–20. doi: 10.1084/jem.186.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Godfrey DI, MacDonald HR, Kronenberg M, Smyth MJ, Van Kaer L. NKT cells: What’s in a name. Nat Rev Immunol. 2004;4:231–7. doi: 10.1038/nri1309. [DOI] [PubMed] [Google Scholar]
- 9.Lantz O, Bendelac A. An invariant T cell receptor alpha chain is used by a unique subset of major histocompatibility complex class I-specific CD4+ and CD4-8- T cells in mice and humans. J Exp Med. 1994;180:1097–106. doi: 10.1084/jem.180.3.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cardell S, Tangri S, Chan S, Kronenberg M, Benoist C, Mathis D. CD1-restricted CD4+ T cells in major histocompatibility complex class II-deficient mice. J Exp Med. 1995;182:993–1004. doi: 10.1084/jem.182.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu YH, Jayawardena J, Weiss A, Lee D, Park SH. Distinct subsets of CD1d-restricted T cells recognize self-antigens loaded in different cellular compartments. J Exp Med. 1999;189:103–10. doi: 10.1084/jem.189.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Behar SM, Podrebarac RA, Roy CJ, Wang CR, Brenner MB. Diverse TCRs recognize murine CD1. J Immunol. 1999;162:161–7. [PubMed] [Google Scholar]
- 13.Chen YH, Chiu NM, Mandal M, Wang N, Wang CR. Impaired NK1+ T cell development and early IL-4 production in CD1-deficient mice. Immunity. 1997;6:459–67. doi: 10.1016/s1074-7613(00)80289-7. [DOI] [PubMed] [Google Scholar]
- 14.Mendiratta SK, Martin WD, Hong S, Boesteanu A, Joyce S, Van Kaer L. CD1d1 mutant mice are deficient in natural T cells that promptly produce IL-4. Immunity. 1997;6:469–77. doi: 10.1016/s1074-7613(00)80290-3. [DOI] [PubMed] [Google Scholar]
- 15.Smiley ST, Kaplan MH, Grusby MJ. Immunoglobulin E production in the absence of interleukin-4-secreting CD1-dependent cells. Science. 1997;275:977–9. doi: 10.1126/science.275.5302.977. [DOI] [PubMed] [Google Scholar]
- 16.Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Vα14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6. doi: 10.1126/science.278.5343.1623. [DOI] [PubMed] [Google Scholar]
- 17.Bedel R, Matsuda J, Brigl M, White J, Kappler J, Marrack P, et al. Lower TCR repertoire diversity in TRAJ18-deficient mice. Nat Immunol. 2012;13:705–6. doi: 10.1038/ni.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watarai H, Sekine-Kondo E, Shigeura T, Motomura Y, Yasuda T, Satoh R, et al. Development and function of invariant natural killer T cells producing T(h)2- and T(h)17-cytokines. PLoS Biol. 2012;10:e1001255. doi: 10.1371/journal.pbio.1001255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee YJ, Holzapfel KL, Zhu J, Jameson SC, Hogquist HA. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat Immunol. 2013;14:1146–54. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lynch L, Michelet X, Zhang S, Brennan PJ, Moseman A, Lester C, et al. Regulatory iNKT cells lack expression of the transcription factor PLZF and control the homeostasis of Treg cells and macrophages in adipose tissue. Nat Immunol. 2015;16:85–95. doi: 10.1038/ni.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sag D, Krause P, Hedrick CC, Kronenberg M, Wingender G. IL-10-producing NKT10 cells are a distinct regulatory invariant NKT cell subset. J Clin Invest. 2014;124:3725–40. doi: 10.1172/JCI72308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uldrich AP, Patel O, Cameron G, Pellicci DG, Day EB, Sullivan LC, et al. A semi-invariant Vα10+ T cell antigen receptor defines a population of natural killer T cells with distinct glycolipid antigen-recognition properties. Nat Immunol. 2011;12:616–23. doi: 10.1038/ni.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benlagha K, Weiss A, Beavis A, Teyton L, Bendelac A. In vivo identification of glycolipid antigen specific T cells using fluorescent CD1d tetramers. J Exp Med. 2000;191:1895–903. doi: 10.1084/jem.191.11.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Matsuda JL, Naidenko OV, Gapin L, Nakayama T, Taniguchi M, Wang CR, et al. Tracking the response of natural killer T cells to a glycolipid antigen using CD1d tetramers. J Exp Med. 2000;192:741–53. doi: 10.1084/jem.192.5.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karadimitris A, Gadola S, Altamirano M, Brown D, Woolfson A, Klenerman P, et al. Human CD1d-glycolipid tetramers generated by in vitro oxidative refolding chromatography. Proc Natl Acad Sci U S A. 2001;98:3294–8. doi: 10.1073/pnas.051604498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bendelac A, Rivera MN, Park SH, Roark JH. Mouse CD1-specific NK1 T cells: development, specificity, and function. Annu Rev Immunol. 1997;15:535–62. doi: 10.1146/annurev.immunol.15.1.535. [DOI] [PubMed] [Google Scholar]
- 27.Lynch L, O’Shea D, Winter DC, Geoghegan J, Doherty DG, O’Farrelly C. Invariant NKT cells and CD1d+ cells amass in human omentum and are depleted in patients with cancer and obesity. Eur J Immunol. 2009;39:1893–901. doi: 10.1002/eji.200939349. [DOI] [PubMed] [Google Scholar]
- 28.Sandberg JK, Bhardwaj N, Nixon DF. Dominant effector memory characteristics, capacity for dynamic adaptive expansion, and sex bias in the innate Vα24 NKT cell compartment. Eur J Immunol. 2003;33:588–96. doi: 10.1002/eji.200323707. [DOI] [PubMed] [Google Scholar]
- 29.Rossjohn J, Pellicci DG, Patel O, Gapin L, Godfrey DI. Recognition of CD1d-restricted antigens by natural killer T cells. Nat Rev Immunol. 2012;12:845–57. doi: 10.1038/nri3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Koch M, Stronge VS, Shepherd D, Gadola SD, Mathew B, Ritter G, et al. The crystal structure of human CD1d with and without alpha-galactosylceramide. Nat Immunol. 2005;6:819–26. doi: 10.1038/ni1225. [DOI] [PubMed] [Google Scholar]
- 31.McCarthy C, Shepherd D, Fleire S, Stronge VS, Koch M, Illarionov PA, et al. The length of lipids bound to human CD1d molecules modulates the affinity of NKT cell TCR and the threshold of NKT cell activation. J Exp Med. 2007;204:1131–44. doi: 10.1084/jem.20062342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zajonc DM, Cantu C, 3rd, Mattner J, Zhou D, Savage PB, Bendelac A, et al. Structure and function of a potent agonist for the semi-invariant natural killer T cell receptor. Nat Immunol. 2005;6:810–8. doi: 10.1038/ni1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kjer-Nielsen L, Borg NA, Pellicci DG, Beddoe T, Kostenko L. A structural basis for selection and cross-species reactivity of the semi-invariant NKT cell receptor in CD1d/glycolipid recognition. J Exp Med. 2006;203:661–73. doi: 10.1084/jem.20051777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wun KS, Borg NA, Kjer-Nielsen L, Beddoe T, Koh R, Richardson SK, et al. A minimal binding footprint on CD1d-glycolipid is a basis for selection of the unique human NKT TCR. J Exp Med. 2008;205:939–49. doi: 10.1084/jem.20072141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mallevaey T, Clarke AJ, Scott-Browne JP, Young MH, Roisman LC, Pellicci DG, et al. A molecular basis for NKT cell recognition of CD1d-self-antigen. Immunity. 2011;34:315–26. doi: 10.1016/j.immuni.2011.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scott-Browne JP, Matsuda JL, Mallevaey T, White J, Borg NA, McCluskey J, et al. Germline-encoded recognition of diverse glycolipids by natural killer T cells. Nat Immunol. 2007;8:1105–13. doi: 10.1038/ni1510. [DOI] [PubMed] [Google Scholar]
- 37.Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y. CD1d-restricted and TCR-mediated activation of Vα14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- 38.Kinjo Y, Wu D, Kim G, Xing GW, Poles MA, Ho DD, et al. Recognition of bacterial glycosphingolipids by natural killer T cells. Nature. 2005;434:520–5. doi: 10.1038/nature03407. [DOI] [PubMed] [Google Scholar]
- 39.Mattner J, DeBord KL, Ismail N, Goff RD, Cantu C, Zhou D, et al. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature. 2005;434:525–9. doi: 10.1038/nature03408. [DOI] [PubMed] [Google Scholar]
- 40.Chang YJ, Kim HY, Albacker LA, Lee HH, Baumgarth N, Akira S, et al. Influenza infection in suckling mice expands an NKT cell subset that protects against airway hyperreactivity. J Clin Invest. 2011;121:57–69. doi: 10.1172/JCI44845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinjo Y, Illarionov P, Vela JL, Pei B, Girardi E, Li X, et al. Invariant natural killer T cells recognize glycolipids from pathogenic Gram-positive bacteria. Nat Immunol. 2011;12:966–74. doi: 10.1038/ni.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Albacker LA, Chaudhary V, Chang YJ, Kim HY, Chuang YT, Pichavant M, et al. Invariant natural killer T cells recognize a fungal glycosphingolipid that can induce airway hyperreactivity. Nat Med. 2013;19:1297–304. doi: 10.1038/nm.3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cohen NR, Tatituri RV, Rivera A, Watts GF, Kim EY, Chiba A, et al. Innate recognition of cell wall beta-glucans drives invariant natural killer T cell responses against fungi. Cell Host Microbe. 2011;10:437–50. doi: 10.1016/j.chom.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Paget C, Mallevaey T, Speak AO, Torres D, Fontaine J, Sheehan KC, et al. Activation of invariant NKT cells by toll-like receptor 9-stimulated dendritic cells requires type I interferon and charged glycosphingolipids. Immunity. 2007;27:597–609. doi: 10.1016/j.immuni.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 45.Salio M, Speak AO, Shepherd D, Polzella P, Illarionov PA, Veerapen N, et al. Modulation of human natural killer T cell ligands on TLR-mediated antigen-presenting cell activation. Proc Natl Acad Sci U S A. 2007;104:20490–5. doi: 10.1073/pnas.0710145104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Olszak T, An D, Zeissig S, Vera MP, Richter J, Franke A, et al. Microbial exposure during early life has persistent effects on natural killer T cell function. Science. 2012;336:489–93. doi: 10.1126/science.1219328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wieland Brown LC, Penaranda C, Kashyap PC, Williams BB, Clardy J, Kronenberg M, et al. Production of α-galactosylceramide by a prominent member of the human gut microbiota. PLoS Biol. 2013;11:e1001610. doi: 10.1371/journal.pbio.1001610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.An D, Oh SF, Olszak T, Neves JF, Avci FY, Erturk-Hasdemir D, et al. Sphingolipids from a symbiotic microbe regulate homeostasis of host intestinal natural killer T cells. Cell. 2014;156:123–33. doi: 10.1016/j.cell.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brennan PJ, Tatituri RVV, Heiss C, Watts GFM, Hsu F-F, Veerapen N, et al. Activation of iNKT cells by a distinct constituent of the endogenous glucosylceramide fraction. Proc Natl Acad Sci U S A. 2014;111:13433–8. doi: 10.1073/pnas.1415357111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kain L, Webb B, Anderson BL, Deng S, Holt M, Costanzo A, et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian α-linked glycosylceramides. Immunity. 2014;41:543–54. doi: 10.1016/j.immuni.2014.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brigl M, Bry L, Kent SC, Gumperz JE, Brenner MB. Mechanism of CD1d-restricted natural killer T cell activation during microbial infection. Nat. Immunol. 2003;4:1230–7. doi: 10.1038/ni1002. [DOI] [PubMed] [Google Scholar]
- 52.Selvanantham T, Escalante NK, Cruz Tleugabulova M, Fieve S, Girardin SE, Philpott DJ, et al. Nod1 and Nod2 enhance TLR-mediated invariant NKT cell activation during bacterial infection. J Immunol. 2013;191:5646–54. doi: 10.4049/jimmunol.1301412. [DOI] [PubMed] [Google Scholar]
- 53.De Santo C, Arscott R, Booth S, Karydis I, Jones M, Asher R, et al. Invariant NKT cells modulate the suppressive activity of IL-10-secreting neutrophils differentiated with serum amyloid A. Nat Immunol. 2010;11:1039–46. doi: 10.1038/ni.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fais F, Tenca C, Cimino G, Coletti V, Zanardi S, Bagnara D, et al. CD1d expression on B-precursor acute lymphoblastic leukemia subsets with poor prognosis. Leukemia. 2005;19:551–6. doi: 10.1038/sj.leu.2403671. [DOI] [PubMed] [Google Scholar]
- 55.Xu C, de Vries R, Visser L, Diepstra A, Gadola SD, Poppema S, et al. Expression of CD1d and presence of invariant NKT cells in classical Hodgkin lymphoma. Am J Hematol. 2010;85:539–41. doi: 10.1002/ajh.21743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nowak M, Arredouani MS, Tun-Kyi A, Schmidt-Wolf I, Sanda MG, Balk SP, et al. Defective NKT cell activation by CD1d+TRAMP prostate tumor cells is corrected by interleukin-12 with α-galactosylceramide. Plos One. 2010;5:e11311. doi: 10.1371/journal.pone.0011311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hix LM, Shi YH, Brutkiewicz RR, Stein PL, Wang C-R, Zhang M. CD1d-expressing breast cancer cells modulate NKT cell-mediated antitumor immunity in a murine model of breast cancer metastasis. PLoS ONE. 2011;6:e20702. doi: 10.1371/journal.pone.0020702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Coquet JM, Kyparissoudis K, Pellicci DG, Besra G, Berzins SP, Smyth MJ, et al. IL-21 is produced by NKT cells and modulates NKT cell activation and cytokine production. J Immunol. 2007;178:2827–34. doi: 10.4049/jimmunol.178.5.2827. [DOI] [PubMed] [Google Scholar]
- 59.Michel ML, Keller AC, Paget C, Fujio M, Trottein F, Savage PB, et al. Identification of an IL-17-producing NK1.1− iNKT cell population involved in airway neutrophilia. J Exp Med. 2007;204:995–1001. doi: 10.1084/jem.20061551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sakuishi K, Oki S, Araki M, Porcelli SA, Miyake S, Yamamura T. Invariant NKT cells biased for IL-5 production act as crucial regulators of inflammation. J Immunol. 2007;179:3452–62. doi: 10.4049/jimmunol.179.6.3452. [DOI] [PubMed] [Google Scholar]
- 61.Chang YJ, Huang JR, Tsai YC, Hung JT, Wu D, Fujio M, et al. Potent immune-modulating and anticancer effects of NKT cell stimulatory glycolipids. Proc Natl Acad Sci U S A. 2007;104:10299–304. doi: 10.1073/pnas.0703824104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matsuda JL, Gapin L, Baron JL, Sidobre S, Stetson DB, Mohrs M, et al. Mouse Vα14i natural killer T cells are resistant to cytokine polarization in vivo. Proc Natl Acad Sci U S A. 2003;100:8395–400. doi: 10.1073/pnas.1332805100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stetson DB, Mohrs M, Reinhardt RL, Baron JL, Wang ZE. Constitutive cytokine mRNAs mark natural killer (NK) and NK T cells poised for rapid effector function. J Exp Med. 2003;198:1069–76. doi: 10.1084/jem.20030630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arora P, Baena A, Yu KO, Saini NK, Kharkwal SS, Goldberg MF, et al. A single subset of dendritic cells controls the cytokine bias of natural killer T cell responses to diverse glycolipid antigens. Immunity. 2014;40:105–16. doi: 10.1016/j.immuni.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujii S, Shimizu K, Smith C, Bonifaz L, Steinman RM. Activation of natural killer T cells by α-galactosylceramide rapidly induces the full maturation of dendritic cells in vivo and thereby acts as an adjuvant for combined CD4 and CD8 T cell immunity to a co-administered protein. J Exp Med. 2003;198:267–79. doi: 10.1084/jem.20030324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermans IF, Silk JD, Gileadi U, Salio M, Mathew B, Ritter G, et al. NKT cells enhance CD4+ and CD8+ T cell responses to soluble antigen in vivo through direct interaction with dendritic cells. J Immunol. 2003;171:5140–7. doi: 10.4049/jimmunol.171.10.5140. [DOI] [PubMed] [Google Scholar]
- 67.Silk JD, Hermans IF, Gileadi U, Chong RW, Shepherd D, Salio M, et al. Utilizing the adjuvant properties of CD1d-dependent NK T cells in T cell-mediated immunotherapy. Journal of Clinical Investigation. 2004;114:1800–11. doi: 10.1172/JCI22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, et al. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol. 2010;11:313–20. doi: 10.1038/ni.1848. [DOI] [PubMed] [Google Scholar]
- 69.Barral P, Eckl-Dorna J, Harwood NE, De Santo C, Salio M, Illarionov P, et al. B cell receptor-mediated uptake of CD1d-restricted antigen augments antibody responses by recruiting invariant NKT cell help in vivo. Proc Natl Acad Sci U S A. 2008;105:8345–50. doi: 10.1073/pnas.0802968105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leadbetter EA, Brigl M, Illarionov P, Cohen N, Luteran MC, Pillai S, et al. NK T cells provide lipid antigen-specific cognate help for B cells. Proc Natl Acad Sci U S A. 2008;105:8339–44. doi: 10.1073/pnas.0801375105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jukes JP, Silk JD, Salio M, Cerundolo V. In: Invariant NKT Cell-Based Vaccine Strategies. Natural Killer T Cells: Balancing the regulation of tumor immunity. Terabe M, Berzofsky JA, editors. 2012. pp. 39–53. [Google Scholar]
- 72.Nagarajan NA, Kronenberg M. Invariant NKT cells amplify the innate immune response to lipopolysalecharide. Journal of Immunology. 2007;178:2706–13. doi: 10.4049/jimmunol.178.5.2706. [DOI] [PubMed] [Google Scholar]
- 73.Tyznik AJ, Tupin E, Nagarajan NA, Her MJ, Benedict CA, Kronenberg M. Cutting edge: the mechanism of invariant NKT cell responses to viral danger signals. J Immunol. 2008;181:4452–6. doi: 10.4049/jimmunol.181.7.4452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Holzapfel KL, Tyznik AJ, Kronenberg M, Hogquist KA. Antigen-dependent versus - independent activation of invariant NKT cells during infection. J Immunol. 2014;192:5490–8. doi: 10.4049/jimmunol.1400722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tyznik AJ, Verma S, Wang Q, Kronenberg M, Benedict CA. Distinct requirements for activation of NKT and NK cells during viral infection. J Immunol. 2014;192:3676–85. doi: 10.4049/jimmunol.1300837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Diana J, Griseri T, Lagaye S, Beaudoin L, Autrusseau E, Gautron A-S, et al. NKT cell-plasmacytoid dendritic cell cooperation via OX40 controls viral infection in a tissue-specific manner. Immunity. 2009;30:289–99. doi: 10.1016/j.immuni.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 77.Im JS, Arora P, Bricard G, Molano A, Venkataswamy MM, Baine I, et al. Kinetics and cellular site of glycolipid loading control the outcome of natural killer T cell activation. Immunity. 2009;30:888–98. doi: 10.1016/j.immuni.2009.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Borg NA, Wun KS, Kjer-Nielsen L, Wilce MCJ, Pellicci DG, Koh R, et al. CD1d-lipid-antigen recognition by the semi-invariant NKT T-cell receptor. Nature. 2007;448:44–9. doi: 10.1038/nature05907. [DOI] [PubMed] [Google Scholar]
- 79.Wang X, Chen X, Rodenkirch L, Simonson W, Wernimont S, Ndonye RM, et al. Natural killer T-cell autoreactivity leads to a specialized activation state. Blood. 2008;112:4128–38. doi: 10.1182/blood-2008-05-157529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sullivan BA, Nagarajan NA, Wingender G, Wang J, Scott I, Tsuji M, et al. Mechanisms for glycolipid antigen-driven cytokine polarization by Vα14i NKT cells. J Immunol. 2010;184:141–53. doi: 10.4049/jimmunol.0902880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi E, Motoki K, Uchida T, Fukushima H, Koezuka Y. KRN7000, a novel immunomodulator, and its antitumor activities. Oncol. Res. 1995;7:529–34. [PubMed] [Google Scholar]
- 82.Morita M, Motoki K, Akimoto K, Natori T, Sakai T. Structure-activity relationship of α-galactosylceramides against B16-bearing mice. J. Med. Chem. 1995;38:2176–87. doi: 10.1021/jm00012a018. [DOI] [PubMed] [Google Scholar]
- 83.Smyth MJ, Thia KYT, Street SEA, Cretney E, Trapani JA, Taniguchi M, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8. doi: 10.1084/jem.191.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Crowe NY, Smyth MJ, Godfrey DI. A critical role for natural killer T cells in immunosurveillance of methylcholanthrene-induced sarcomas. J Exp Med. 2002;196:119–27. doi: 10.1084/jem.20020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Swann JB, Uldrich AP, van Dommelen S, Sharkey J, Murray WK, Godfrey DI, et al. Type I natural killer T cells suppress tumors caused by p53 loss in mice. Blood. 2009;113:6382–5. doi: 10.1182/blood-2009-01-198564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bellone M, Ceccon M, Grioni M, Jachetti E, Calcinotto A, Napolitano A, et al. iNKT Cells Control Mouse Spontaneous Carcinoma Independently of Tumor-Specific Cytotoxic T Cells. Plos One. 2010;5:e8646. doi: 10.1371/journal.pone.0008646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crowe NY, Coquet JM, Berzins SP, Kyparissoudis K, Keating R. Differential antitumor immunity mediated by NKT cell subsets in vivo. J. Exp. Med. 2005;202:1279–88. doi: 10.1084/jem.20050953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wu DY, Segal NH, Sidobre S, Kronenberg M, Chapman PB. Cross-presentation of disialoganglioside GD3 to natural killer T cells. J Ex. Med. 2003;198:173–81. doi: 10.1084/jem.20030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Renukaradhya GJ, Khan MA, Vieira M, Du W, Gervay-Hague J, Brutkiewicz RR. Type I NKT cells protect (and type II NKT cells suppress) the host’s innate antitumor immune response to a B-cell lymphoma. Blood. 2008;111:5637–45. doi: 10.1182/blood-2007-05-092866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fallarini S, Paoletti T, Orsi Battaglini N, Lombardi G. Invariant NKT cells increase drug-induced osteosarcoma cell death. Br J Pharmacol. 2012;167:1533–49. doi: 10.1111/j.1476-5381.2012.02108.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nicol A, Nieda M, Koezuka Y, Porcelli SA, Suzuki K, Tadokoro K, et al. Human invariant Vα24+ natural killer T cells activated by alpha-galactosylceramide (KRN7000) have cytotoxic anti-tumour activity through mechanisms distinct from T cells and natural killer cells. Immunology. 2000;99:229–34. doi: 10.1046/j.1365-2567.2000.00952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Metelitsa LS, Naidenko OV, Kant A, Wu HW, Loza MJ, Perussia B, et al. Human NKT cells mediate antitumor cytotoxicity directly by recognizing target cell CD1d with bound ligand or indirectly by producing IL-2 to activate NK cells. J Immunol. 2001;167:3114–22. doi: 10.4049/jimmunol.167.6.3114. [DOI] [PubMed] [Google Scholar]
- 93.Canchis PW, Bhan AK, Landau SB, Yang L, Balk SP, Blumberg RS. Tissue distribution of the non-polymorphic major histocompatibility complex class I-like molecule, CD1d. Immunology. 1993;80:561–5. [PMC free article] [PubMed] [Google Scholar]
- 94.Fais F, Morabito F, Stelitano C, Callea V, Zanardi S, Scudeletti M, et al. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates alpha-galactosylceramide presentation to natural killer T lymphocytes. Int J Cancer. 2004;109:402–11. doi: 10.1002/ijc.11723. [DOI] [PubMed] [Google Scholar]
- 95.Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–77. doi: 10.1038/sj.leu.2402943. [DOI] [PubMed] [Google Scholar]
- 96.Chong TW, Goh FY, Sim MY, Huang HH, Thike DAA, Lim WK, et al. CD1d expression in renal cell carcinoma is associated with higher relapse rates, poorer cancer-specific and overall survival. J Clin Pathol. 2014;68:200–5. doi: 10.1136/jclinpath-2014-202735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–9. doi: 10.1002/ijc.20050. [DOI] [PubMed] [Google Scholar]
- 98.Liu D, Song L, Brawley VS, Robison N, Wei J, Gao X, et al. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin Immunol. 2013;149:55–64. doi: 10.1016/j.clim.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008. [DOI] [PubMed] [Google Scholar]
- 100.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and DAMPs in cancer therapy. Nat Rev Cancer. 2012;12:860–75. doi: 10.1038/nrc3380. [DOI] [PubMed] [Google Scholar]
- 101.Brennan PJ, Tatituri RVV, Brigl M, Kim EY, Tuli A, Sanderson JP, et al. Invariant natural killer T cells recognize lipid self antigen induced by microbial danger signals. Nat Immunol. 2011;12:1202–11. doi: 10.1038/ni.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang S, Chen Z, Lam V, Han J, Hassler J, Finck BN, et al. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 2012;16:473–86. doi: 10.1016/j.cmet.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brozovic S, Nagaishi T, Yoshida M, Betz S, Salas A, Chen D, et al. CD1d function is regulated by microsomal triglyceride transfer protein. Nat Med. 2004;10:535–9. doi: 10.1038/nm1043. [DOI] [PubMed] [Google Scholar]
- 104.Zeissig S, Dougan SK, Barral DC, Junker Y, Chen Z, Kaser A, et al. Primary deficiency of microsomal triglyceride transfer protein in human abetalipoproteinemia is associated with loss of CD1 function. J Clin Invest. 2010;120:2889–99. doi: 10.1172/JCI42703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hetz C, Martinon F, Rodriguez D, Glimcher LH. The unfolded protein response: integrating stress signals through the stress sensor IRE1alpha. Physiol Rev. 2011;91:1219–43. doi: 10.1152/physrev.00001.2011. [DOI] [PubMed] [Google Scholar]
- 106.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–6. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahadevan NR, Rodvold J, Sepulveda H, Rossi S, Drew AF, Zanetti M. Transmission of endoplasmic reticulum stress and pro-inflammation from tumor cells to myeloid cells. Proc Natl Acad Sci U S A. 2011;108:6561–6. doi: 10.1073/pnas.1008942108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L, Jhaveri R, Huang J, Qi Y, Diehl AM. Endoplasmic reticulum stress, hepatocyte CD1d and NKT cell abnormalities in murine fatty livers. Lab Invest. 2007;87:927–37. doi: 10.1038/labinvest.3700603. [DOI] [PubMed] [Google Scholar]
- 109.Kawano T, Cui JQ, Koezuka Y, Toura I, Kaneko Y, Sato H, et al. Natural killer-like nonspecific tumor cell lysis mediated by specific ligand-activated Vα14 NKT cells. Proc Natl Acad Sci U S A. 1998;95:5690–3. doi: 10.1073/pnas.95.10.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hayakawa Y, Takeda K, Yagita H, Kakuta S, Iwakura Y, Van Kaer L, et al. Critical contribution of IFN-γ and NK cells, but not perforin-mediated cytotoxicity, to anti-metastatic effect of α-galactosylceramide. Eur J Immunol. 2001;31:1720–7. [PubMed] [Google Scholar]
- 111.Toura I, Kawano T, Akutsu Y, Nakayama T, Ochiai T, Taniguchi M. Cutting edge: Inhibition of experimental tumor metastasis by dendritic cells pulsed with α-galactosylceramide. J Immunol. 1999;163:2387–91. [PubMed] [Google Scholar]
- 112.Nakagawa R, Motoki K, Ueno H, Iijima R, Nakamura H, Kobayashi E, et al. Treatment of hepatic metastasis of the Colon26 adenocarcinoma with an α-Galactosylceramide, KRN7000. Cancer Res. 1998;58:1202–7. [PubMed] [Google Scholar]
- 113.Hayakawa Y, Rovero S, Forni G, Smyth MJ. α-Galactosylceramide (KRN7000) suppression of chemical- and oncogene-dependent carcinogenesis. Proc Natl Acad Sci U S A. 2003;100:9464–9. doi: 10.1073/pnas.1630663100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ambrosino E, Terabe M, Halder RC, Peng J, Takaku S, Miyake S, et al. Cross-regulation between type I and type II NKT cells in regulating tumor immunity: a new immunoregulatory axis. J Immunol. 2007;179:5126–36. doi: 10.4049/jimmunol.179.8.5126. [DOI] [PubMed] [Google Scholar]
- 115.Shimizu K, Goto A, Fukui M, Taniguchi M, Fujii S-I. Tumor cells loaded with α-galactosylceramide induce innate NKT and NK cell-dependent resistance to tumor implantation in mice. J Immunol. 2007;178:2853–61. doi: 10.4049/jimmunol.178.5.2853. [DOI] [PubMed] [Google Scholar]
- 116.Shimizu K, Kurosawa Y, Taniguchi M, Steinman RM, Fujii SI. Cross-presentation of glycolipid from tumor cells loaded with alpha-galactosylceramide leads to potent and long-lived T cell-mediated immunity via dendritic cells. J Exp Med. 2007;204:2641–53. doi: 10.1084/jem.20070458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nakagawa R, Nagafune I, Tazunoki Y, Ehara H, Tomura H, Iijima R, et al. Mechanisms of the antimetastatic effect in the liver and of the hepatocyte injury induced by α-galactosylceramide in mice. J Immunol. 2001;166:6578–84. doi: 10.4049/jimmunol.166.11.6578. [DOI] [PubMed] [Google Scholar]
- 118.Smyth MJ, Crowe NY, Pellicci DG, Kyparissoudis K, Kelly JM, Takeda K, et al. Sequential production of interferon-γ by NK1.1+ T cells and natural killer cells is essential for the antimetastatic effect of α-galactosylceramide. Blood. 2002;99:1259–66. doi: 10.1182/blood.v99.4.1259. [DOI] [PubMed] [Google Scholar]
- 119.Paget C, Chow MT, Duret H, Mattarollo SR, Smyth MJ. Role of gammadelta T cells in alpha-galactosylceramide-mediated immunity. J Immunol. 2012;188:3928–39. doi: 10.4049/jimmunol.1103582. [DOI] [PubMed] [Google Scholar]
- 120.Nishimura T, Kitamura H, Iwakabe K, Yahata T, Ohta A, Sato M, et al. The interface between innate and acquired immunity: glycolipid antigen presentation by CD1d-expressing dendritic cells to NKT cells induces the differentiation of antigen-specific cytotoxic T lymphocytes. Int Immunol. 2000;12:987–94. doi: 10.1093/intimm/12.7.987. [DOI] [PubMed] [Google Scholar]
- 121.Nieda M, Nicol A, Koezuka Y, Kikuchi A, Lapteva N, Tanaka Y, et al. TRAIL expression by activated human CD4+Vα24 NKT cells induces in vitro and in vivo apoptosis of human acute myeloid leukemia cells. Blood. 2001;97:2067–74. doi: 10.1182/blood.v97.7.2067. [DOI] [PubMed] [Google Scholar]
- 122.Kawano T, Nakayama T, Kamada N, Kaneko Y, Harada M, Ogura N, et al. Antitumor cytotoxicity mediated by ligand-activated human Vα24 NKT cells. Cancer Res. 1999;59:5102–5. [PubMed] [Google Scholar]
- 123.Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, et al. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. T J Immunol. 2005;175:3092–101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Silk JD, Salio M, Reddy BG, Shepherd D, Gileadi U, Brown J, et al. Cutting edge: nonglycosidic CD1d lipid ligands activate human and murine invariant NKT cells. J Immunol. 2008;180:6452–6. doi: 10.4049/jimmunol.180.10.6452. [DOI] [PubMed] [Google Scholar]
- 126.Hogan AE, O’Reilly V, Dunne MR, Dere RT, Zeng SG, O’Brien C, et al. Activation of human invariant natural killer T cells with a thioglycoside analogue of α-galactosylceramide. Clin Immunol. 2011;140:196–207. doi: 10.1016/j.clim.2011.03.016. [DOI] [PubMed] [Google Scholar]
- 127.Wojno J, Jukes J-P, Ghadbane H, Shepherd D, Besra GS, Cerundolo V, et al. Amide analogues of CD1d agonists modulate inkt-cell-mediated cytokine production. ACS Chem Biol. 2012;7:847–55. doi: 10.1021/cb2005017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Jervis PJ, Polzella P, Wojno J, Jukes JP, Ghadbane H, Garcia Diaz YR, et al. Design, synthesis, and functional activity of labeled CD1d glycolipid agonists. Bioconjug Chem. 2013;24:586–94. doi: 10.1021/bc300556e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, 3rd, et al. Effects of lipid chain lengths in α-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–3. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- 130.Yu KO, Im JS, Molano A, Dutronc Y, Illarionov PA. Modulation of CD1d-restricted NKT cell responses by using N-acyl variants of α-galactosylceramides. Proc Natl Acad Sci USA. 2005;102:3383–8. doi: 10.1073/pnas.0407488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schmieg J, Yang G, Franck RW, Tsuji M. Superior protection against malaria and melanoma metastases by a C-glycoside analogue of the natural killer T cell ligand alpha-Galactosylceramide. J Exp Med. 2003;198:1631–41. doi: 10.1084/jem.20031192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Li X, Fujio M, Imamura M, Wu D, Vasan S, Wong CH, et al. Design of a potent CD1d-binding NKT cell ligand as a vaccine adjuvant. Proc Natl Acad Sci U S A. 2010;107:13010–5. doi: 10.1073/pnas.1006662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tashiro T, Sekine-Kondo E, Shigeura T, Nakagawa R, Inoue S, Omori-Miyake M, et al. Induction of Th1-biased cytokine production by alpha-carba-GalCer, a neoglycolipid ligand for NKT cells. Int Immunol. 2010;22:319–28. doi: 10.1093/intimm/dxq012. [DOI] [PubMed] [Google Scholar]
- 134.Fujii S, Shimizu K, Klimek V, Geller MD, Nimer SD, Dhodapkar MV. Severe and selective deficiency of interferon-γ-producing invariant natural killer T cells in patients with myelodysplastic syndromes. Br J Haematol. 2003;122:617–22. doi: 10.1046/j.1365-2141.2003.04465.x. [DOI] [PubMed] [Google Scholar]
- 135.Molling JW, Kolgen W, van der Vliet HJJ, Boomsma MF, Kruizenga H, Smorenburg CH, et al. Peripheral blood IFN-γ-secreting Vα24+Vβ11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer. 2005;116:87–93. doi: 10.1002/ijc.20998. [DOI] [PubMed] [Google Scholar]
- 136.Najera Chuc A, Cervantes LM, Retiguin F, Ojeda J, Maldonado E. Low number of invariant NKT cells is associated with poor survival in acute myeloid leukemia. J Cancer Res Clin Oncol. 2012;138:1427–32. doi: 10.1007/s00432-012-1251-x. [DOI] [PubMed] [Google Scholar]
- 137.Molling JW, Langius JAE, Langendijk JA, Leemans CR, Bontkes HJ, van der Vliet HJJ, et al. Low levels of circulating invariant natural killer t cells predict poor clinical outcome in patients with head and neck squamous cell carcinoma. J Clin Oncol. 2007;25:862–8. doi: 10.1200/JCO.2006.08.5787. [DOI] [PubMed] [Google Scholar]
- 138.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Vα24-positive natural killer t cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 139.Shaulov A, Yue S, Wang R, Joyce RM, Balk SP, Kim HT, et al. Peripheral blood progenitor cell product contains Th1-biased noninvariant CD1d-reactive natural killer T cells: Implications for posttransplant survival. Exp Hematol. 2008;36:464–72. doi: 10.1016/j.exphem.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Metelitsa LS, Wu H-W, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. The J Exp Med. 2004;199:1213–21. doi: 10.1084/jem.20031462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Berzins SP, Kyparissoudis K, Pellicci DG, Hammond KJ, Sidobre S, Baxter A, et al. Systemic NKT cell deficiency in NOD mice is not detected in peripheral blood: implications for human studies. Immunol Cell Biol. 2004;82:247–52. doi: 10.1046/j.1440-1711.2004.01238.x. [DOI] [PubMed] [Google Scholar]
- 142.Song L, Ara T, Wu H-W, Woo C-W, Reynolds CP, Seeger RC, et al. Oncogene MYCN regulates localization of NKT cells to the site of disease in neuroblastoma. J ClinInvest. 2007;117(9):2702–12. doi: 10.1172/JCI30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bricard G, Cesson V, Devevre E, Bouzourene H, Barbey C, Rufer N, et al. Enrichment of human CD4+ vα24/vβ11 invariant NKT cells in intrahepatic malignant tumors. J Immunol. 2009;182:5140–51. doi: 10.4049/jimmunol.0711086. [DOI] [PubMed] [Google Scholar]
- 144.Motohashi S, Kobayashi S, Ito T, Magara KK, Mikuni O, Kamada N, et al. Preserved IFN-α production of circulating Vα24 NKT cells in primary lung cancer patients. Int J Cancer. 2002;102:159–65. doi: 10.1002/ijc.10678. [DOI] [PubMed] [Google Scholar]
- 145.Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, et al. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–8. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 146.Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Vα24+ natural killer T cells: A prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7. doi: 10.1158/1078-0432.CCR-05-0877. [DOI] [PubMed] [Google Scholar]
- 147.Terabe M, Berzofsky JA. The role of NKT cells in tumor immunity. Adv Cancer Res. 2008;101:277–348. doi: 10.1016/S0065-230X(08)00408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Giaccone G, Punt CJA, Ando Y, Ruijter R, Nishi N, Peters M, et al. A phase I study of the natural killer T-cell ligand α-galactosylceramide (KRN7000) in patients with solid tumors. Clin Cancer Res. 2002;8:3702–9. [PubMed] [Google Scholar]
- 149.Crowe NY, Uldrich AP, Kyparissoudis K, Hammond KJ, Hayakawa Y, Sidobre S, et al. Glycolipid antigen drives rapid expansion and sustained cytokine production by NK T cells. J Immunol. 2003;71:4020–7. doi: 10.4049/jimmunol.171.8.4020. [DOI] [PubMed] [Google Scholar]
- 150.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182:2816–26. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ishikawa A, Motohashi S, Ishikawa E, Fuchida H, Higashino K, Otsuji M, et al. A phase I study of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2005;11:1910–7. doi: 10.1158/1078-0432.CCR-04-1453. [DOI] [PubMed] [Google Scholar]
- 152.Fujii S, Shimizu K, Kronenberg M, Steinman RM. Prolonged IFN-γ-producing NKT response induced with α-galactosylceramide-loaded DCs. Nat Immunol. 2002;3:867–74. doi: 10.1038/ni827. [DOI] [PubMed] [Google Scholar]
- 153.Bezbradica JS, Stanic AK, Matsuki N, Bour-Jordan H, Bluestone JA. Distinct roles of dendritic cells and B cells in Vα14Jα18 natural T cell activation in vivo. J. Immunol. 2005;174:4696–705. doi: 10.4049/jimmunol.174.8.4696. [DOI] [PubMed] [Google Scholar]
- 154.Nieda M, Okai M, Tazbirkova A, Lin H, Yamaura A, Ide K, et al. Therapeutic activation of Valpha24+Vbeta11+ NKT cells in human subjects results in highly coordinated secondary activation of acquired and innate immunity. Blood. 2004;103:383–9. doi: 10.1182/blood-2003-04-1155. [DOI] [PubMed] [Google Scholar]
- 155.Chang DH, Osman K, Connolly J, Kukreja A, Krasovsky J. Sustained expansion of NKT cells and antigen-specific T cells after injection of α-galactosyl-ceramide loaded mature dendritic cells in cancer patients. J Exp Med. 2005;201:1503–1517. doi: 10.1084/jem.20042592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Uchida T, Horiguchi S, Tanaka Y, Yamamoto H, Kunii N, Motohashi S, et al. Phase I study of alpha-galactosylceramide-pulsed antigen presenting cells administration to the nasal submucosa in unresectable or recurrent head and neck cancer. Cancer Immunol Immunother. 2008;57:37–45. doi: 10.1007/s00262-007-0373-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kurosaki M, Horiguchi S, Yamasaki K, Uchida Y, Motohashi S, Nakayama T, et al. Migration and immunological reaction after the administration of alphaGalCer-pulsed antigen-presenting cells into the submucosa of patients with head and neck cancer. Cancer Immunol Immunother. 2011;60:207–15. doi: 10.1007/s00262-010-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nicol AJ, Tazbirkova A, Nieda M. Comparison of clinical and immunological effects of intravenous and intradermal administration of alpha-galactosylceramide (KRN7000)-pulsed dendritic cells. Clin Cancer Res. 2011;17:5140–51. doi: 10.1158/1078-0432.CCR-10-3105. [DOI] [PubMed] [Google Scholar]
- 159.Nagato K, Motohashi S, Ishibashi F, Okita K, Yamasaki K, Moriya Y, et al. Accumulation of activated invariant natural killer T cells in the tumor microenvironment after α-galactosylceramide-pulsed antigen presenting cells. J Clin Immunol. 2012;32:1071–81. doi: 10.1007/s10875-012-9697-9. [DOI] [PubMed] [Google Scholar]
- 160.Richter J, Neparidze N, Zhang L, Nair S, Monesmith T, Sundaram R, et al. Clinical regressions and broad immune activation following combination therapy targeting human NKT cells in myeloma. Blood. 2013;121:423–30. doi: 10.1182/blood-2012-06-435503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Motohashi S, Nagato K, Kunii N, Yamamoto H, Yamasaki K, Okita K, et al. A phase I-II study of α-galactosylceramide-pulsed IL-2/GM-CSF-cultured peripheral blood mononuclear cells in patients with advanced and recurrent non-small cell lung cancer. J Immunol. 2009;182:2492–501. doi: 10.4049/jimmunol.0800126. [DOI] [PubMed] [Google Scholar]
- 162.Chang DH, Liu N, Klimek V, Hassoun H, Mazumder A, Nimer SD, et al. Enhancement of ligand-dependent activation of human natural killer T cells by lenalidomide: therapeutic implications. Blood. 2006;108:618–21. doi: 10.1182/blood-2005-10-4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Motohashi S, Ishikawa A, Ishikawa E, Otsuji M, Iizasa T, Hanaoka H, et al. A phase I study of in vitro expanded natural killer T cells in patients with advanced and recurrent non-small cell lung cancer. Clin Cancer Res. 2006;12:6079–86. doi: 10.1158/1078-0432.CCR-06-0114. [DOI] [PubMed] [Google Scholar]
- 164.Yamasaki K, Horiguchi S, Kurosaki M, Kunii N, Nagato K, Hanaoka H, et al. Induction of NKT cell-specific immune responses in cancer tissues after NKT cell-targeted adoptive immunotherapy. Clin Immunol. 2011;138:255–65. doi: 10.1016/j.clim.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 165.Kunii N, Horiguchi S, Motohashi S, Yamamoto H, Ueno N, Yamamoto S, et al. Combination therapy of in vitro-expanded natural killer T cells and alpha-galactosylceramide-pulsed antigen-presenting cells in patients with recurrent head and neck carcinoma. Cancer Sci. 2009;100:1092–8. doi: 10.1111/j.1349-7006.2009.01135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Watarai H, Fujii S, Yamada D, Rybouchkin A, Sakata S, Nagata Y, et al. Murine induced pluripotent stem cells can be derived from and differentiate into natural killer T cells. J Clin Invest. 2010;120:2610–8. doi: 10.1172/JCI42027. [DOI] [PMC free article] [PubMed] [Google Scholar]