Abstract
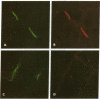
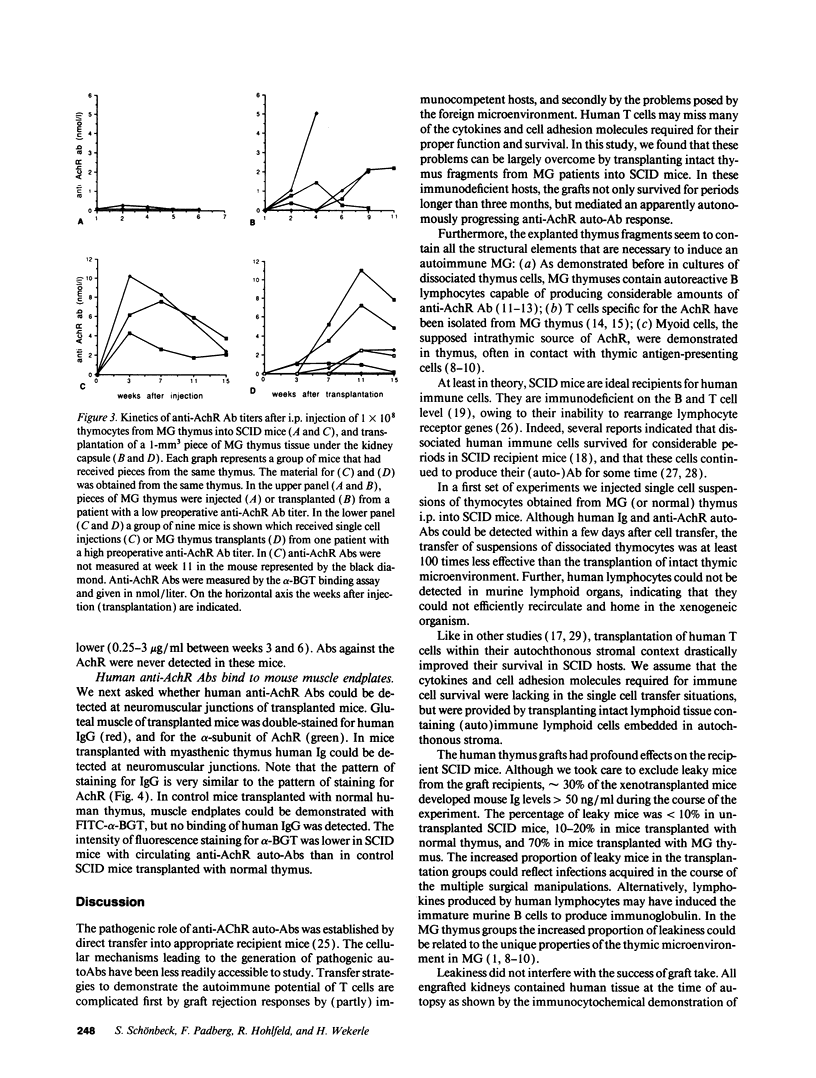
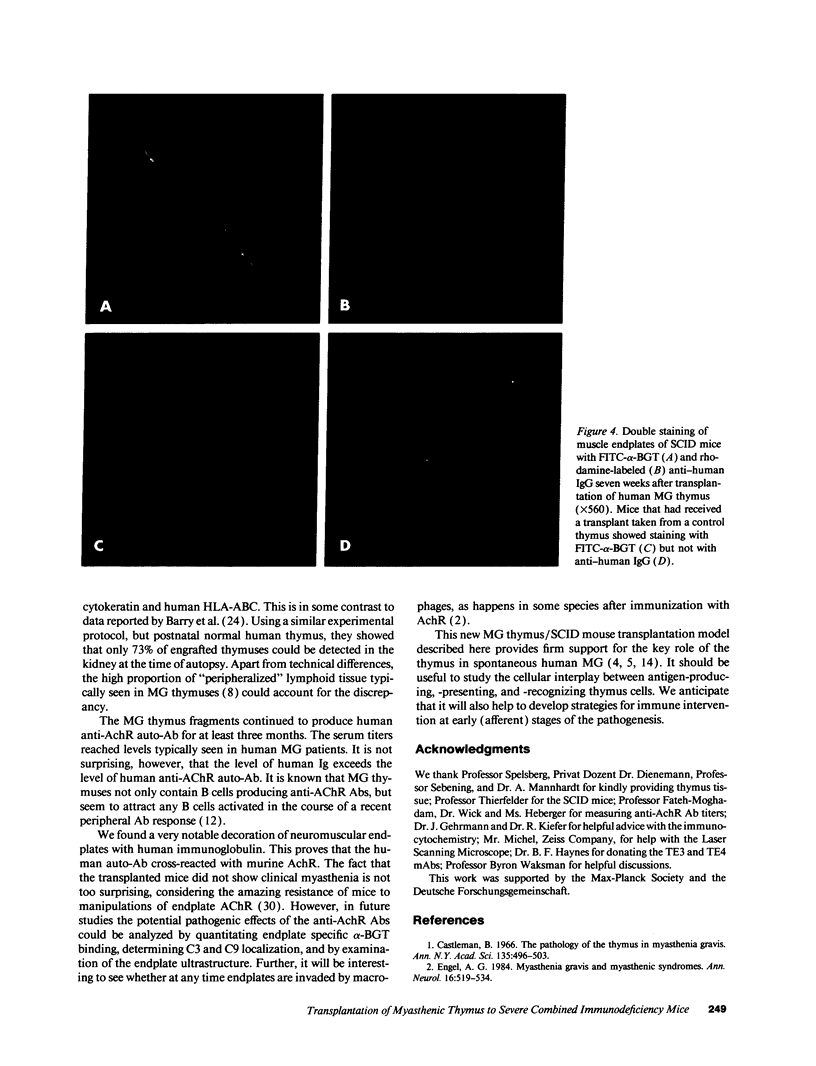
To study the role of the thymus in the cellular pathogenesis of myasthenia gravis (MG) we transplanted thymus tissue fragments from MG thymuses beneath the kidney capsule of severe combined immunodeficiency (SCID) mice. Immunocytochemical studies documented that the human thymus tissues are accepted as long-term grafts in the host SCID mice, with human lymphocytes, thymic stroma, and thymic myoid cells demonstrable in transplanted thymus for at least 15 weeks after transplantation. Human anti-acetylcholine receptor antibodies became detectable 1 to 2 weeks after transplantation, and in most chimeras the titers increased over at least 11 weeks to reach levels typically found in severe human MG. Human Ig deposits were detected at skeletal muscle end-plates, demonstrating that the human (auto)antibodies bound to murine acetylcholine receptor. In contrast, transfers of dissociated thymus cells only lead to a transient increase of anti-acetylcholine receptor antibodies. Our data prove that myasthenia gravis thymus is able to induce and maintain autoantibody production in immunodeprived host animals, and that this tissue contains all cellular components required for autoantibody production. Transplantation of solid thymus tissue seems to transfer an autoimmune microenvironment, which will allow direct studies of the mechanism of autosensitization inside the thymus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barry T. S., Jones D. M., Richter C. B., Haynes B. F. Successful engraftment of human postnatal thymus in severe combined immune deficient (SCID) mice: differential engraftment of thymic components with irradiation versus anti-asialo GM-1 immunosuppressive regimens. J Exp Med. 1991 Jan 1;173(1):167–180. doi: 10.1084/jem.173.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman P. W., Patrick J. Experimental myasthenia gravis. A murine system. J Exp Med. 1980 Jan 1;151(1):204–223. doi: 10.1084/jem.151.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosma G. C., Custer R. P., Bosma M. J. A severe combined immunodeficiency mutation in the mouse. Nature. 1983 Feb 10;301(5900):527–530. doi: 10.1038/301527a0. [DOI] [PubMed] [Google Scholar]
- Bosma G. C., Fried M., Custer R. P., Carroll A., Gibson D. M., Bosma M. J. Evidence of functional lymphocytes in some (leaky) scid mice. J Exp Med. 1988 Mar 1;167(3):1016–1033. doi: 10.1084/jem.167.3.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castleman B. The pathology of the thymus gland in myasthenia gravis. Ann N Y Acad Sci. 1966 Jan 26;135(1):496–505. doi: 10.1111/j.1749-6632.1966.tb45497.x. [DOI] [PubMed] [Google Scholar]
- Duchosal M. A., McConahey P. J., Robinson C. A., Dixon F. J. Transfer of human systemic lupus erythematosus in severe combined immunodeficient (SCID) mice. J Exp Med. 1990 Sep 1;172(3):985–988. doi: 10.1084/jem.172.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G. Myasthenia gravis and myasthenic syndromes. Ann Neurol. 1984 Nov;16(5):519–534. doi: 10.1002/ana.410160502. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Scearce R. M., Lobach D. F., Hensley L. L. Phenotypic characterization and ontogeny of mesodermal-derived and endocrine epithelial components of the human thymic microenvironment. J Exp Med. 1984 Apr 1;159(4):1149–1168. doi: 10.1084/jem.159.4.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao I., Drachman D. B. Thymic muscle cells bear acetylcholine receptors: possible relation to myasthenia gravis. Science. 1977 Jan 7;195(4273):74–75. doi: 10.1126/science.831257. [DOI] [PubMed] [Google Scholar]
- Kirchner T., Hoppe F., Schalke B., Müller-Hermelink H. K. Microenvironment of thymic myoid cells in myasthenia gravis. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;54(5):295–302. doi: 10.1007/BF02899226. [DOI] [PubMed] [Google Scholar]
- Kirchner T., Tzartos S., Hoppe F., Schalke B., Wekerle H., Müller-Hermelink H. K. Pathogenesis of myasthenia gravis. Acetylcholine receptor-related antigenic determinants in tumor-free thymuses and thymic epithelial tumors. Am J Pathol. 1988 Feb;130(2):268–280. [PMC free article] [PubMed] [Google Scholar]
- Levy R., Czernobilsky B., Geiger B. Subtyping of epithelial cells of normal and metaplastic human uterine cervix, using polypeptide-specific cytokeratin antibodies. Differentiation. 1988 Dec;39(3):185–196. doi: 10.1111/j.1432-0436.1988.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Lewis S., Bosma G. C., Rosenberg N., Mizuuchi K., Bosma M. J., Gellert M. The defect in murine severe combined immune deficiency: joining of signal sequences but not coding segments in V(D)J recombination. Cell. 1988 Oct 7;55(1):7–16. doi: 10.1016/0092-8674(88)90004-9. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. M., Seybold M. E., Lennon V. A., Whittingham S., Duane D. D. Antibody to acetylcholine receptor in myasthenia gravis. Prevalence, clinical correlates, and diagnostic value. Neurology. 1976 Nov;26(11):1054–1059. doi: 10.1212/wnl.26.11.1054. [DOI] [PubMed] [Google Scholar]
- Lisak R. P., Levinson A. I., Zweiman B., Kornstein M. J. Antibodies to acetylcholine receptor and tetanus toxoid: in vitro synthesis by thymic lymphocytes. J Immunol. 1986 Aug 15;137(4):1221–1225. [PubMed] [Google Scholar]
- Macht L., Fukuma N., Leader K., Sarsero D., Pegg C. A., Phillips D. I., Yates P., McLachlan S. M., Elson C., Rees Smith B. Severe combined immunodeficient (SCID) mice: a model for investigating human thyroid autoantibody synthesis. Clin Exp Immunol. 1991 Apr;84(1):34–42. doi: 10.1111/j.1365-2249.1991.tb08120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Namikawa R., Kaneshima H., Shultz L. D., Lieberman M., Weissman I. L. The SCID-hu mouse: murine model for the analysis of human hematolymphoid differentiation and function. Science. 1988 Sep 23;241(4873):1632–1639. doi: 10.1126/science.241.4873.1632. [DOI] [PubMed] [Google Scholar]
- McFarland E. J., Scearce R. M., Haynes B. F. The human thymic microenvironment: cortical thymic epithelium is an antigenically distinct region of the thymic microenvironment. J Immunol. 1984 Sep;133(3):1241–1249. [PubMed] [Google Scholar]
- Melms A., Schalke B. C., Kirchner T., Müller-Hermelink H. K., Albert E., Wekerle H. Thymus in myasthenia gravis. Isolation of T-lymphocyte lines specific for the nicotinic acetylcholine receptor from thymuses of myasthenic patients. J Clin Invest. 1988 Mar;81(3):902–908. doi: 10.1172/JCI113401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., Baird S. M., Wilson D. B. Transfer of a functional human immune system to mice with severe combined immunodeficiency. Nature. 1988 Sep 15;335(6187):256–259. doi: 10.1038/335256a0. [DOI] [PubMed] [Google Scholar]
- Mulder D. G., Graves M., Herrmann C. Thymectomy for myasthenia gravis: recent observations and comparisons with past experience. Ann Thorac Surg. 1989 Oct;48(4):551–555. doi: 10.1016/s0003-4975(10)66861-0. [DOI] [PubMed] [Google Scholar]
- Newsom-Davis J., Willcox N., Calder L. Thymus cells in myasthenia gravis selectively enhance production of anti-acetylcholine-receptor antibody by autologous blood lymphocytes. N Engl J Med. 1981 Nov 26;305(22):1313–1318. doi: 10.1056/NEJM198111263052203. [DOI] [PubMed] [Google Scholar]
- Schluep M., Willcox N., Vincent A., Dhoot G. K., Newsom-Davis J. Acetylcholine receptors in human thymic myoid cells in situ: an immunohistological study. Ann Neurol. 1987 Aug;22(2):212–222. doi: 10.1002/ana.410220205. [DOI] [PubMed] [Google Scholar]
- Sommer N., Willcox N., Harcourt G. C., Newsom-Davis J. Myasthenic thymus and thymoma are selectively enriched in acetylcholine receptor-reactive T cells. Ann Neurol. 1990 Sep;28(3):312–319. doi: 10.1002/ana.410280303. [DOI] [PubMed] [Google Scholar]
- Toyka K. V., Brachman D. B., Pestronk A., Kao I. Myasthenia gravis: passive transfer from man to mouse. Science. 1975 Oct 24;190(4212):397–399. doi: 10.1126/science.1179220. [DOI] [PubMed] [Google Scholar]
- Vandekerckhove B. A., Krowka J. F., McCune J. M., de Vries J. E., Spits H., Roncarolo M. G. Clonal analysis of the peripheral T cell compartment of the SCID-hu mouse. J Immunol. 1991 Jun 15;146(12):4173–4179. [PubMed] [Google Scholar]
- Vincent A., Scadding G. K., Thomas H. C., Newsom-Davis J. In-vitro synthesis of anti-acetylcholine-receptor antibody by thymic lymphocytes in myasthenia gravis. Lancet. 1978 Feb 11;1(8059):305–307. doi: 10.1016/s0140-6736(78)90073-9. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Hohlfeld R., Ketelsen U. P., Kalden J. R., Kalies I. Thymic myogenesis, T-lymphocytes and the pathogenesis of myasthenia gravis. Ann N Y Acad Sci. 1981;377:455–476. doi: 10.1111/j.1749-6632.1981.tb33753.x. [DOI] [PubMed] [Google Scholar]
- Wekerle H., Ketelsen U. P. Intrathymic pathogenesis and dual genetic control of myasthenia gravis. Lancet. 1977 Mar 26;1(8013):678–680. doi: 10.1016/s0140-6736(77)92118-3. [DOI] [PubMed] [Google Scholar]
- Wekerle T. H., paterson B., Ketelsen U., Feldman M. Striated muscle fibres differentiate in monolayer cultures of adult thymus reticulum. Nature. 1975 Aug 7;256(5517):493–494. doi: 10.1038/256493a0. [DOI] [PubMed] [Google Scholar]