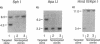Abstract
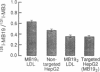
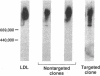
The HepG2 cell line has been used extensively to study the synthesis and secretion of apolipoprotein (apo) B. In this study, we tested whether gene-targeting techniques can be used to inactivate one of the apo B alleles in HepG2 cells by homologous recombination using a transfected gene-targeting vector. Our vector contained exons 1-7 of the apo B gene, in which exon 2 was interrupted by a promoterless neomycin resistance (neo(r)) gene. The recombination of this vector with the cognate gene would inactivate an apo B allele and enable the apo B promoter to activate the transcription of the neo(r) gene. To detect the rare homologous recombinant clone, we developed a novel solid phase RIA that uses the apo B-specific monoclonal antibody MB19 to analyze the apo B secreted by G418-resistant (G418r) clones. Antibody MB19 detects a two-allele genetic polymorphism in apo B by binding to the apo B allotypes MB19(1) and MB19(2) with high and low affinity, respectively. HepG2 cells normally secrete both the apo B MB19 allotypes. Using the MB19 immunoassay, we identified a G418r HepG2 clone that had lost the ability to secrete the MB19(1) allotype. The inactivation of an apo B allele of this clone was confirmed by the polymerase chain reaction amplification of an 865-bp fragment unique to the targeted apo B allele and by Southern blotting of genomic DNA. This study demonstrates that gene-targeting techniques can be used to modify the apo B gene in HepG2 cells and demonstrates the usefulness of a novel solid phase RIA system for detecting apo B gene targeting events in this cell line.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baserga S. J., Benz E. J., Jr Nonsense mutations in the human beta-globin gene affect mRNA metabolism. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2056–2060. doi: 10.1073/pnas.85.7.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackhart B. D., Ludwig E. M., Pierotti V. R., Caiati L., Onasch M. A., Wallis S. C., Powell L., Pease R., Knott T. J., Chu M. L. Structure of the human apolipoprotein B gene. J Biol Chem. 1986 Nov 25;261(33):15364–15367. [PubMed] [Google Scholar]
- Blackhart B. D., Yao Z. M., McCarthy B. J. An expression system for human apolipoprotein B100 in a rat hepatoma cell line. J Biol Chem. 1990 May 25;265(15):8358–8360. [PubMed] [Google Scholar]
- Boerwinkle E., Chan L. A three codon insertion/deletion polymorphism in the signal peptide region of the human apolipoprotein B (APOB) gene directly typed by the polymerase chain reaction. Nucleic Acids Res. 1989 May 25;17(10):4003–4003. doi: 10.1093/nar/17.10.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capecchi M. R. Altering the genome by homologous recombination. Science. 1989 Jun 16;244(4910):1288–1292. doi: 10.1126/science.2660260. [DOI] [PubMed] [Google Scholar]
- Charron J., Malynn B. A., Robertson E. J., Goff S. P., Alt F. W. High-frequency disruption of the N-myc gene in embryonic stem and pre-B cell lines by homologous recombination. Mol Cell Biol. 1990 Apr;10(4):1799–1804. doi: 10.1128/mcb.10.4.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Dixon J. L., Furukawa S., Ginsberg H. N. Oleate stimulates secretion of apolipoprotein B-containing lipoproteins from Hep G2 cells by inhibiting early intracellular degradation of apolipoprotein B. J Biol Chem. 1991 Mar 15;266(8):5080–5086. [PubMed] [Google Scholar]
- Hamilton R. L., Moorehouse A., Havel R. J. Isolation and properties of nascent lipoproteins from highly purified rat hepatocytic Golgi fractions. J Lipid Res. 1991 Mar;32(3):529–543. [PubMed] [Google Scholar]
- Hasty P., Ramírez-Solis R., Krumlauf R., Bradley A. Introduction of a subtle mutation into the Hox-2.6 locus in embryonic stem cells. Nature. 1991 Mar 21;350(6315):243–246. doi: 10.1038/350243a0. [DOI] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Bradley A. The length of homology required for gene targeting in embryonic stem cells. Mol Cell Biol. 1991 Nov;11(11):5586–5591. doi: 10.1128/mcb.11.11.5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasty P., Rivera-Pérez J., Chang C., Bradley A. Target frequency and integration pattern for insertion and replacement vectors in embryonic stem cells. Mol Cell Biol. 1991 Sep;11(9):4509–4517. doi: 10.1128/mcb.11.9.4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itzhaki J. E., Porter A. C. Targeted disruption of a human interferon-inducible gene detected by secretion of human growth hormone. Nucleic Acids Res. 1991 Jul 25;19(14):3835–3842. doi: 10.1093/nar/19.14.3835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H. S., Popovich B. W., Shehee W. R., Shesely E. G., Smithies O. Problems encountered in detecting a targeted gene by the polymerase chain reaction. Gene. 1991 Jul 22;103(2):227–233. [PubMed] [Google Scholar]
- Levy-Wilson B., Soria L., Ludwig E. H., Argyres M., Brooks A. R., Blackhart B. D., Friedl W., McCarthy B. J. A polymorphism in a region with enhancer activity in the second intron of the human apolipoprotein B gene. J Lipid Res. 1991 Jan;32(1):137–145. [PubMed] [Google Scholar]
- Milne R., Théolis R., Jr, Maurice R., Pease R. J., Weech P. K., Rassart E., Fruchart J. C., Scott J., Marcel Y. L. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J Biol Chem. 1989 Nov 25;264(33):19754–19760. [PubMed] [Google Scholar]
- Peabody D. S., Berg P. Termination-reinitiation occurs in the translation of mammalian cell mRNAs. Mol Cell Biol. 1986 Jul;6(7):2695–2703. doi: 10.1128/mcb.6.7.2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pease R. J., Milne R. W., Jessup W. K., Law A., Provost P., Fruchart J. C., Dean R. T., Marcel Y. L., Scott J. Use of bacterial expression cloning to localize the epitopes for a series of monoclonal antibodies against apolipoprotein B100. J Biol Chem. 1990 Jan 5;265(1):553–568. [PubMed] [Google Scholar]
- Pullinger C. R., North J. D., Teng B. B., Rifici V. A., Ronhild de Brito A. E., Scott J. The apolipoprotein B gene is constitutively expressed in HepG2 cells: regulation of secretion by oleic acid, albumin, and insulin, and measurement of the mRNA half-life. J Lipid Res. 1989 Jul;30(7):1065–1077. [PubMed] [Google Scholar]
- Ritchie H. H., Hughes M. R., Thompson E. T., Malloy P. J., Hochberg Z., Feldman D., Pike J. W., O'Malley B. W. An ochre mutation in the vitamin D receptor gene causes hereditary 1,25-dihydroxyvitamin D3-resistant rickets in three families. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9783–9787. doi: 10.1073/pnas.86.24.9783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon D., Aden D. P., Knowles B. B. Chromosomes of human hepatoma cell lines. Int J Cancer. 1982 Jul 15;30(1):27–33. doi: 10.1002/ijc.2910300106. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Sniderman A., Shapiro S., Marpole D., Skinner B., Teng B., Kwiterovich P. O., Jr Association of coronary atherosclerosis with hyperapobetalipoproteinemia [increased protein but normal cholesterol levels in human plasma low density (beta) lipoproteins]. Proc Natl Acad Sci U S A. 1980 Jan;77(1):604–608. doi: 10.1073/pnas.77.1.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrift R. N., Forte T. M., Cahoon B. E., Shore V. G. Characterization of lipoproteins produced by the human liver cell line, Hep G2, under defined conditions. J Lipid Res. 1986 Mar;27(3):236–250. [PubMed] [Google Scholar]
- Valancius V., Smithies O. Testing an "in-out" targeting procedure for making subtle genomic modifications in mouse embryonic stem cells. Mol Cell Biol. 1991 Mar;11(3):1402–1408. doi: 10.1128/mcb.11.3.1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W. J., Zsigmond E., Gotto A. M., Jr, Lei K. Y., Chan L. Locating a low density lipoprotein-targeting domain of human apolipoprotein B-100 by expressing a minigene construct in transgenic mice. J Biol Chem. 1991 Nov 5;266(31):20893–20898. [PubMed] [Google Scholar]
- Yao Z. M., Blackhart B. D., Linton M. F., Taylor S. M., Young S. G., McCarthy B. J. Expression of carboxyl-terminally truncated forms of human apolipoprotein B in rat hepatoma cells. Evidence that the length of apolipoprotein B has a major effect on the buoyant density of the secreted lipoproteins. J Biol Chem. 1991 Feb 15;266(5):3300–3308. [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Casal D. C., Witztum J. L. Monoclonal antibody MB19 detects genetic polymorphism in human apolipoprotein B. Proc Natl Acad Sci U S A. 1986 Feb;83(4):1101–1105. doi: 10.1073/pnas.83.4.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Bertics S. J., Curtiss L. K., Witztum J. L. Characterization of an abnormal species of apolipoprotein B, apolipoprotein B-37, associated with familial hypobetalipoproteinemia. J Clin Invest. 1987 Jun;79(6):1831–1841. doi: 10.1172/JCI113025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Hubl S. T. An ApaLI restriction site polymorphism is associated with the MB19 polymorphism in apolipoprotein B. J Lipid Res. 1989 Mar;30(3):443–449. [PubMed] [Google Scholar]
- Young S. G., Hubl S. T., Smith R. S., Snyder S. M., Terdiman J. F. Familial hypobetalipoproteinemia caused by a mutation in the apolipoprotein B gene that results in a truncated species of apolipoprotein B (B-31). A unique mutation that helps to define the portion of the apolipoprotein B molecule required for the formation of buoyant, triglyceride-rich lipoproteins. J Clin Invest. 1990 Mar;85(3):933–942. doi: 10.1172/JCI114522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young S. G., Peralta F. P., Dubois B. W., Curtiss L. K., Boyles J. K., Witztum J. L. Lipoprotein B37, a naturally occurring lipoprotein containing the amino-terminal portion of apolipoprotein B100, does not bind to the apolipoprotein B,E (low density lipoprotein) receptor. J Biol Chem. 1987 Dec 5;262(34):16604–16611. [PubMed] [Google Scholar]
- Zheng H., Hasty P., Brenneman M. A., Grompe M., Gibbs R. A., Wilson J. H., Bradley A. Fidelity of targeted recombination in human fibroblasts and murine embryonic stem cells. Proc Natl Acad Sci U S A. 1991 Sep 15;88(18):8067–8071. doi: 10.1073/pnas.88.18.8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- te Riele H., Maandag E. R., Clarke A., Hooper M., Berns A. Consecutive inactivation of both alleles of the pim-1 proto-oncogene by homologous recombination in embryonic stem cells. Nature. 1990 Dec 13;348(6302):649–651. doi: 10.1038/348649a0. [DOI] [PubMed] [Google Scholar]