Abstract
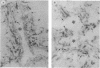
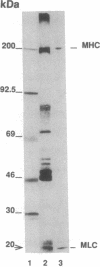
Cytoskeleton-rich canalicular membranes (CCMs) with preserved cytoskeleton and demembranated CCMs, consisting only of cytoskeletal elements, were used to examine the relationship of pericanalicular microfilaments, myosin II phosphorylation, and canalicular contraction. The components of CCMs were visualized by fluorescence microscopy using the filamentous actin probe rhodamine-phalloidin and by electron microscopy, before and after incubation in 1 microM Ca2+/1 mM ATP (contraction solution). Canalicular contraction (luminal closure) was evaluated by morphometric analysis. Myosin II was extracted from CCMs, purified by immunoprecipitation, and analyzed on Western blots. In sequential experiments, autoradiographs of gels from [gamma-32P]-ATP-treated CCMs in the presence or absence of Ca2+ were examined after 0.25, 0.50, 1, 2, 3, 5, and 10 min, and the effects of W7 (a calmodulin antagonist) and ML9 (a myosin light chain kinase inhibitor) were evaluated. The results showed that phosphorylation of the 20-kDa protein was low in controls but enhanced beginning 0.25-0.50 min after addition of contraction solution. Both W7 and ML9 significantly inhibited this reaction and inhibited canalicular contraction. The results indicate that phosphorylation of the regulatory 20-kDa myosin light chain of canaliculus-associated myosin II coincides with or precedes contraction of the canaliculus. We conclude that the canalicular contractile apparatus is composed of actin filaments and a myosin II motor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M., Che M., Gatmaitan Z., Leveille C., Nishida T., St Pierre M. The biology of the bile canaliculus, 1993. Hepatology. 1993 Feb;17(2):318–329. [PubMed] [Google Scholar]
- Burgess W. H., Jemiolo D. K., Kretsinger R. H. Interaction of calcium and calmodulin in the presence of sodium dodecyl sulfate. Biochim Biophys Acta. 1980 Jun 26;623(2):257–270. doi: 10.1016/0005-2795(80)90254-8. [DOI] [PubMed] [Google Scholar]
- Cheney R. E., Riley M. A., Mooseker M. S. Phylogenetic analysis of the myosin superfamily. Cell Motil Cytoskeleton. 1993;24(4):215–223. doi: 10.1002/cm.970240402. [DOI] [PubMed] [Google Scholar]
- Citi S., Kendrick-Jones J. Regulation in vitro of brush border myosin by light chain phosphorylation. J Mol Biol. 1986 Apr 5;188(3):369–382. doi: 10.1016/0022-2836(86)90161-0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fisher M. M., Bloxam D. L., Oda M., Phillips M. J., Yousef I. M. Characterization of rat liver cell plasma membranes. Proc Soc Exp Biol Med. 1975 Oct;150(1):177–184. doi: 10.3181/00379727-150-38998. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Tanaka T. Naphthalenesulfonamides as calmodulin antagonists. Methods Enzymol. 1983;102:185–194. doi: 10.1016/s0076-6879(83)02019-4. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Tanaka T. Transmembrane Ca2+ signaling and a new class of inhibitors. Methods Enzymol. 1987;139:570–582. doi: 10.1016/0076-6879(87)39113-x. [DOI] [PubMed] [Google Scholar]
- Ito M., Tanaka T., Nunoki K., Hidaka H., Suzuki K. The Ca2+ -activated protease (calpain) modulates Ca2+/calmodulin dependent activity of smooth muscle myosin light chain kinase. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1321–1328. doi: 10.1016/0006-291x(87)91582-8. [DOI] [PubMed] [Google Scholar]
- Kamm K. E., Stull J. T. Regulation of smooth muscle contractile elements by second messengers. Annu Rev Physiol. 1989;51:299–313. doi: 10.1146/annurev.ph.51.030189.001503. [DOI] [PubMed] [Google Scholar]
- Kawahara H., French S. W. Role of cytoskeleton in canalicular contraction in cultured differentiated hepatocytes. Am J Pathol. 1990 Mar;136(3):521–532. [PMC free article] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Conzelman K. A., Chasan R., Mooseker M. S. Role of myosin in terminal web contraction in isolated intestinal epithelial brush borders. J Cell Biol. 1985 May;100(5):1647–1655. doi: 10.1083/jcb.100.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller T. C., 3rd, Mooseker M. S. Ca++-calmodulin-dependent phosphorylation of myosin, and its role in brush border contraction in vitro. J Cell Biol. 1982 Dec;95(3):943–959. doi: 10.1083/jcb.95.3.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura T., Brauneis U., Gatmaitan Z., Arias I. M. Extracellular ATP, intracellular calcium and canalicular contraction in rat hepatocyte doublets. Hepatology. 1991 Oct;14(4 Pt 1):640–647. doi: 10.1016/0270-9139(91)90051-v. [DOI] [PubMed] [Google Scholar]
- Kitanishi-Yumura T., Fukui Y. Actomyosin organization during cytokinesis: reversible translocation and differential redistribution in Dictyostelium. Cell Motil Cytoskeleton. 1989;12(2):78–89. doi: 10.1002/cm.970120203. [DOI] [PubMed] [Google Scholar]
- Korn E. D., Hammer J. A., 3rd Myosins of nonmuscle cells. Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Conti M. A., Adelstein R., Glass D. B., Welch W. J., Feramisco J. R. Regulation of actin microfilament integrity in living nonmuscle cells by the cAMP-dependent protein kinase and the myosin light chain kinase. J Cell Biol. 1988 Jun;106(6):1955–1971. doi: 10.1083/jcb.106.6.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooseker M. S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- Nishikawa M., Sellers J. R., Adelstein R. S., Hidaka H. Protein kinase C modulates in vitro phosphorylation of the smooth muscle heavy meromyosin by myosin light chain kinase. J Biol Chem. 1984 Jul 25;259(14):8808–8814. [PubMed] [Google Scholar]
- Oshio C., Phillips M. J. Contractility of bile canaliculi: implications for liver function. Science. 1981 May 29;212(4498):1041–1042. doi: 10.1126/science.7015506. [DOI] [PubMed] [Google Scholar]
- Owaribe K., Kodama R., Eguchi G. Demonstration of contractility of circumferential actin bundles and its morphogenetic significance in pigmented epithelium in vitro and in vivo. J Cell Biol. 1981 Aug;90(2):507–514. doi: 10.1083/jcb.90.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Thomas S. M., Niederman R. Human platelet myosin. I. Purification by a rapid method applicable to other nonmuscle cells. Anal Biochem. 1974 Jul;60(1):258–266. doi: 10.1016/0003-2697(74)90152-3. [DOI] [PubMed] [Google Scholar]
- Schroeder T. E. Actin in dividing cells: contractile ring filaments bind heavy meromyosin. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1688–1692. doi: 10.1073/pnas.70.6.1688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers J. R. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991 Feb;3(1):98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- Song C. S., Rubin W., Rifkind A. B., Kappas A. Plasma membranes of the rat liver. Isolation and enzymatic characterization of a fraction rich in bile canaliculi. J Cell Biol. 1969 Apr;41(1):124–132. doi: 10.1083/jcb.41.1.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukada N., Phillips M. J. Bile canalicular contraction is coincident with reorganization of pericanalicular filaments and co-localization of actin and myosin-II. J Histochem Cytochem. 1993 Mar;41(3):353–363. doi: 10.1177/41.3.7679126. [DOI] [PubMed] [Google Scholar]
- Watanabe N., Tsukada N., Smith C. R., Edwards V., Phillips M. J. Permeabilized hepatocyte couplets. Adenosine triphosphate-dependent bile canalicular contractions and a circumferential pericanalicular microfilament belt demonstrated. Lab Invest. 1991 Aug;65(2):203–213. [PubMed] [Google Scholar]
- Watanabe N., Tsukada N., Smith C. R., Phillips M. J. Motility of bile canaliculi in the living animal: implications for bile flow. J Cell Biol. 1991 Jun;113(5):1069–1080. doi: 10.1083/jcb.113.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe S., Phillips M. J. Ca2+ causes active contraction of bile canaliculi: direct evidence from microinjection studies. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6164–6168. doi: 10.1073/pnas.81.19.6164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakita Y., Yamashiro S., Matsumura F. In vivo phosphorylation of regulatory light chain of myosin II during mitosis of cultured cells. J Cell Biol. 1994 Jan;124(1-2):129–137. doi: 10.1083/jcb.124.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]