Abstract
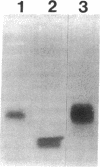
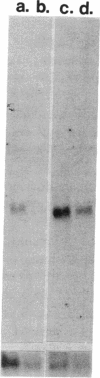
Hepatic lipocytes play a central role in the pathogenesis of liver fibrosis, both via production of extracellular matrix proteins and through secretion of matrix metalloproteinases. In this study, we have characterized lipocyte expression and release of tissue inhibitor of metalloproteinases-1 (TIMP-1), an important inhibitor of metalloproteinase activity, whose role in liver has not previously been examined. TIMP-1 was immunolocalized to human lipocytes, and secretion of TIMP-1 was confirmed by ELISA of culture media; (mean +/- SD) 159 +/- 79 ng of TIMP-1/10(6) cells per 24 h. Evidence for functional inhibitory activity of released TIMP-1 was obtained by (a) reverse zymography that demonstrated a single inhibitor band, M(r) 28 kD, that co-migrated with a TIMP-1-positive control sample; and (b) unmasking of inhibited gelatinase activity in lipocyte medium by separating it from TIMP-1 using gelatin sepharose chromatography; gelatinase activity in chromatographed medium increased more than 20-fold, compared with unfractionated medium, and could be reinhibited by adding back fractions that contained inhibitor. By Northern analysis, freshly isolated human lipocytes exhibited low levels of mRNA expression for TIMP-1, but this increased markedly relative to beta-actin expression with lipocyte activation during cell culture. We conclude that human hepatic lipocytes synthesize TIMP-1, a potent metalloproteinase inhibitor, and that TIMP-1 expression increases with lipocyte activation. These data indicate that hepatic lipocytes can regulate matrix degradation in the liver, and suggest that expression of TIMP-1 by activated lipocytes may contribute to the progression of liver fibrosis.
Full text
PDF
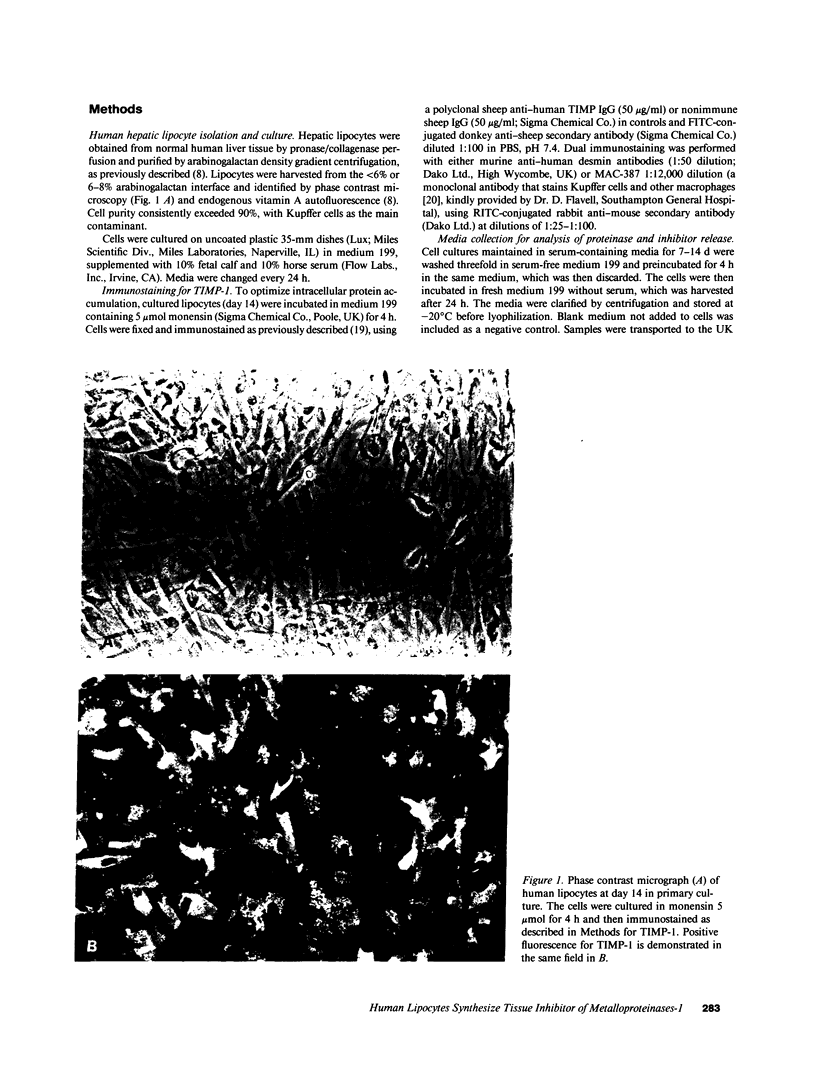




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthur M. J., Friedman S. L., Roll F. J., Bissell D. M. Lipocytes from normal rat liver release a neutral metalloproteinase that degrades basement membrane (type IV) collagen. J Clin Invest. 1989 Oct;84(4):1076–1085. doi: 10.1172/JCI114270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachem M. G., Meyer D., Melchior R., Sell K. M., Gressner A. M. Activation of rat liver perisinusoidal lipocytes by transforming growth factors derived from myofibroblastlike cells. A potential mechanism of self perpetuation in liver fibrogenesis. J Clin Invest. 1992 Jan;89(1):19–27. doi: 10.1172/JCI115561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooksley S., Hipkiss J. B., Tickle S. P., Holmes-Ievers E., Docherty A. J., Murphy G., Lawson A. D. Immunoassays for the detection of human collagenase, stromelysin, tissue inhibitor of metalloproteinases (TIMP) and enzyme-inhibitor complexes. Matrix. 1990 Oct;10(5):285–291. doi: 10.1016/s0934-8832(11)80183-6. [DOI] [PubMed] [Google Scholar]
- Docherty A. J., Lyons A., Smith B. J., Wright E. M., Stephens P. E., Harris T. J., Murphy G., Reynolds J. J. Sequence of human tissue inhibitor of metalloproteinases and its identity to erythroid-potentiating activity. Nature. 1985 Nov 7;318(6041):66–69. doi: 10.1038/318066a0. [DOI] [PubMed] [Google Scholar]
- Edwards D. R., Murphy G., Reynolds J. J., Whitham S. E., Docherty A. J., Angel P., Heath J. K. Transforming growth factor beta modulates the expression of collagenase and metalloproteinase inhibitor. EMBO J. 1987 Jul;6(7):1899–1904. doi: 10.1002/j.1460-2075.1987.tb02449.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emonard H., Guillouzo A., Lapiere C. M., Grimaud J. A. Human liver fibroblast capacity for synthesizing interstitial collagenase in vitro. Cell Mol Biol. 1990;36(4):461–467. [PubMed] [Google Scholar]
- Flavell D. J., Jones D. B., Wright D. H. Identification of tissue histiocytes on paraffin sections by a new monoclonal antibody. J Histochem Cytochem. 1987 Nov;35(11):1217–1226. doi: 10.1177/35.11.3309045. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Arthur M. J. Activation of cultured rat hepatic lipocytes by Kupffer cell conditioned medium. Direct enhancement of matrix synthesis and stimulation of cell proliferation via induction of platelet-derived growth factor receptors. J Clin Invest. 1989 Dec;84(6):1780–1785. doi: 10.1172/JCI114362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman S. L., Rockey D. C., McGuire R. F., Maher J. J., Boyles J. K., Yamasaki G. Isolated hepatic lipocytes and Kupffer cells from normal human liver: morphological and functional characteristics in primary culture. Hepatology. 1992 Feb;15(2):234–243. doi: 10.1002/hep.1840150211. [DOI] [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Arenson D. M., Bissell D. M. Maintenance of differentiated phenotype of cultured rat hepatic lipocytes by basement membrane matrix. J Biol Chem. 1989 Jun 25;264(18):10756–10762. [PubMed] [Google Scholar]
- Friedman S. L., Roll F. J., Boyles J., Bissell D. M. Hepatic lipocytes: the principal collagen-producing cells of normal rat liver. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8681–8685. doi: 10.1073/pnas.82.24.8681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts A., Vrijsen R., Rauterberg J., Burt A., Schellinck P., Wisse E. In vitro differentiation of fat-storing cells parallels marked increase of collagen synthesis and secretion. J Hepatol. 1989 Jul;9(1):59–68. doi: 10.1016/0168-8278(89)90076-7. [DOI] [PubMed] [Google Scholar]
- Gressner A. M. Time-related distribution profiles of sulfated glycosaminoglycans in cells, cell surfaces, and media of cultured rat liver fat-storing cells. Proc Soc Exp Biol Med. 1991 Mar;196(3):307–315. doi: 10.3181/00379727-196-43193. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Murphy G., Reynolds J. J. Immunolocalization of tissue inhibitor of metalloproteinases (TIMP) in human cells. Characterization and use of a specific antiserum. J Cell Sci. 1985 Feb;73:105–119. doi: 10.1242/jcs.73.1.105. [DOI] [PubMed] [Google Scholar]
- Herbst H., Heinrichs O., Schuppan D., Milani S., Stein H. Temporal and spatial patterns of transin/stromelysin RNA expression following toxic injury in rat liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(5):295–300. doi: 10.1007/BF02899560. [DOI] [PubMed] [Google Scholar]
- Maher J. J., Friedman S. L., Roll F. J., Bissell D. M. Immunolocalization of laminin in normal rat liver and biosynthesis of laminin by hepatic lipocytes in primary culture. Gastroenterology. 1988 Apr;94(4):1053–1062. doi: 10.1016/0016-5085(88)90566-5. [DOI] [PubMed] [Google Scholar]
- Mak K. M., Leo M. A., Lieber C. S. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984 Jul;87(1):188–200. [PubMed] [Google Scholar]
- Mak K. M., Lieber C. S. Lipocytes and transitional cells in alcoholic liver disease: a morphometric study. Hepatology. 1988 Sep-Oct;8(5):1027–1033. doi: 10.1002/hep.1840080508. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Hahn E. G., Stein H. In situ hybridization for procollagen types I, III and IV mRNA in normal and fibrotic rat liver: evidence for predominant expression in nonparenchymal liver cells. Hepatology. 1989 Jul;10(1):84–92. doi: 10.1002/hep.1840100117. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Kim K. Y., Riecken E. O., Stein H. Procollagen expression by nonparenchymal rat liver cells in experimental biliary fibrosis. Gastroenterology. 1990 Jan;98(1):175–184. doi: 10.1016/0016-5085(90)91307-r. [DOI] [PubMed] [Google Scholar]
- Milani S., Herbst H., Schuppan D., Surrenti C., Riecken E. O., Stein H. Cellular localization of type I III and IV procollagen gene transcripts in normal and fibrotic human liver. Am J Pathol. 1990 Jul;137(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Montfort I., Pérez-Tamayo R., Alvizouri A. M., Tello E. Collagenase of hepatocytes and sinusoidal liver cells in the reversibility of experimental cirrhosis of the liver. Virchows Arch B Cell Pathol Incl Mol Pathol. 1990;59(5):281–289. doi: 10.1007/BF02899415. [DOI] [PubMed] [Google Scholar]
- Montfort I., Pérez-Tamayo R. Collagenase in experimental carbon tetrachloride cirrhosis of the liver. Am J Pathol. 1978 Aug;92(2):411–420. [PMC free article] [PubMed] [Google Scholar]
- Murphy G., Cartwright E. C., Sellers A., Reynolds J. J. The detection and characterisation of collagenase inhibitors from rabbit tissues in culture. Biochim Biophys Acta. 1977 Aug 11;483(2):493–498. doi: 10.1016/0005-2744(77)90080-8. [DOI] [PubMed] [Google Scholar]
- Murphy G., Hembry R. M., Hughes C. E., Fosang A. J., Hardingham T. E. Role and regulation of metalloproteinases in connective tissue turnover. Biochem Soc Trans. 1990 Oct;18(5):812–815. doi: 10.1042/bst0180812. [DOI] [PubMed] [Google Scholar]
- Overall C. M., Wrana J. L., Sodek J. Independent regulation of collagenase, 72-kDa progelatinase, and metalloendoproteinase inhibitor expression in human fibroblasts by transforming growth factor-beta. J Biol Chem. 1989 Jan 25;264(3):1860–1869. [PubMed] [Google Scholar]
- Perez-Tamayo R., Montfort I., Gonzalez E. Collagenolytic activity in experimental cirrhosis of the liver. Exp Mol Pathol. 1987 Dec;47(3):300–308. doi: 10.1016/0014-4800(87)90015-3. [DOI] [PubMed] [Google Scholar]
- Ramadori G., Rieder H., Knittel T., Dienes H. P., Meyer zum Büschenfelde K. H. Fat storing cells (FSC) of rat liver synthesize and secrete fibronectin. Comparison with hepatocytes. J Hepatol. 1987 Apr;4(2):190–197. doi: 10.1016/s0168-8278(87)80079-x. [DOI] [PubMed] [Google Scholar]
- Rockey D. C., Boyles J. K., Gabbiani G., Friedman S. L. Rat hepatic lipocytes express smooth muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992 Apr;24(2):193–203. [PubMed] [Google Scholar]
- Ward R. V., Hembry R. M., Reynolds J. J., Murphy G. The purification of tissue inhibitor of metalloproteinases-2 from its 72 kDa progelatinase complex. Demonstration of the biochemical similarities of tissue inhibitor of metalloproteinases-2 and tissue inhibitor of metalloproteinases-1. Biochem J. 1991 Aug 15;278(Pt 1):179–187. doi: 10.1042/bj2780179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welgus H. G., Stricklin G. P., Eisen A. Z., Bauer E. A., Cooney R. V., Jeffrey J. J. A specific inhibitor of vertebrate collagenase produced by human skin fibroblasts. J Biol Chem. 1979 Mar 25;254(6):1938–1943. [PubMed] [Google Scholar]