Abstract
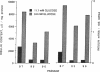
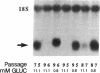
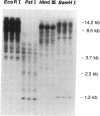
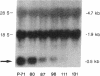
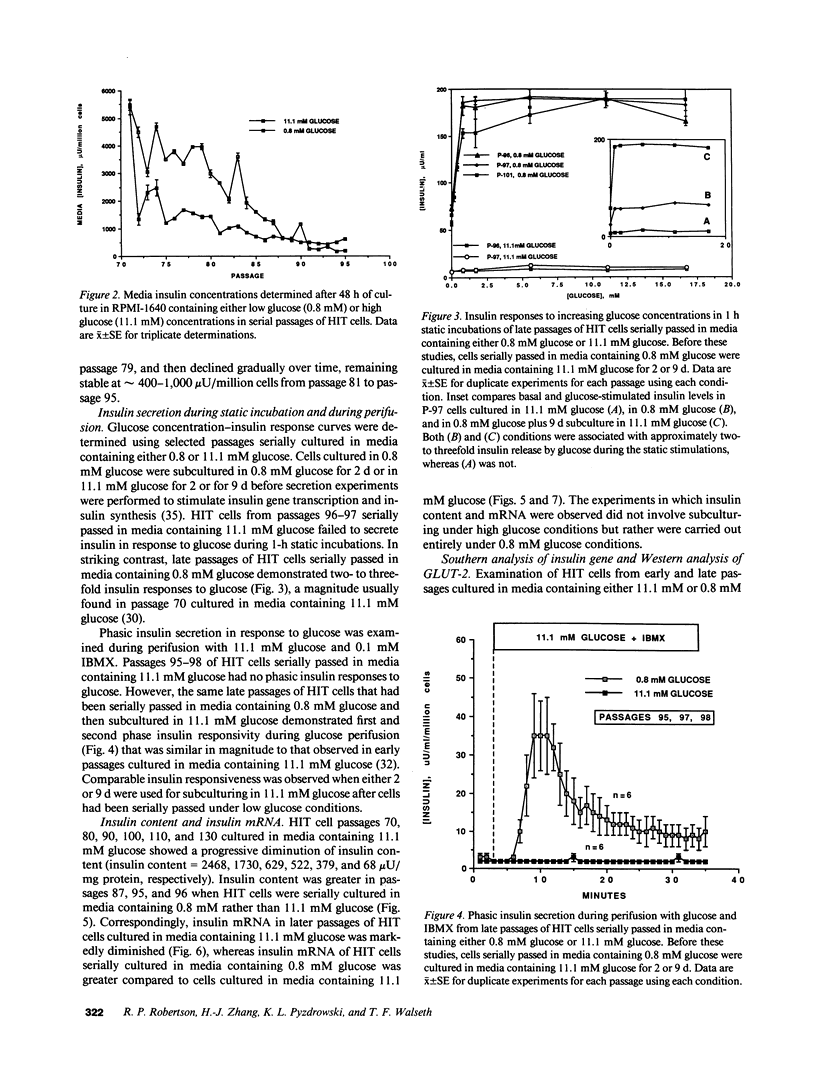
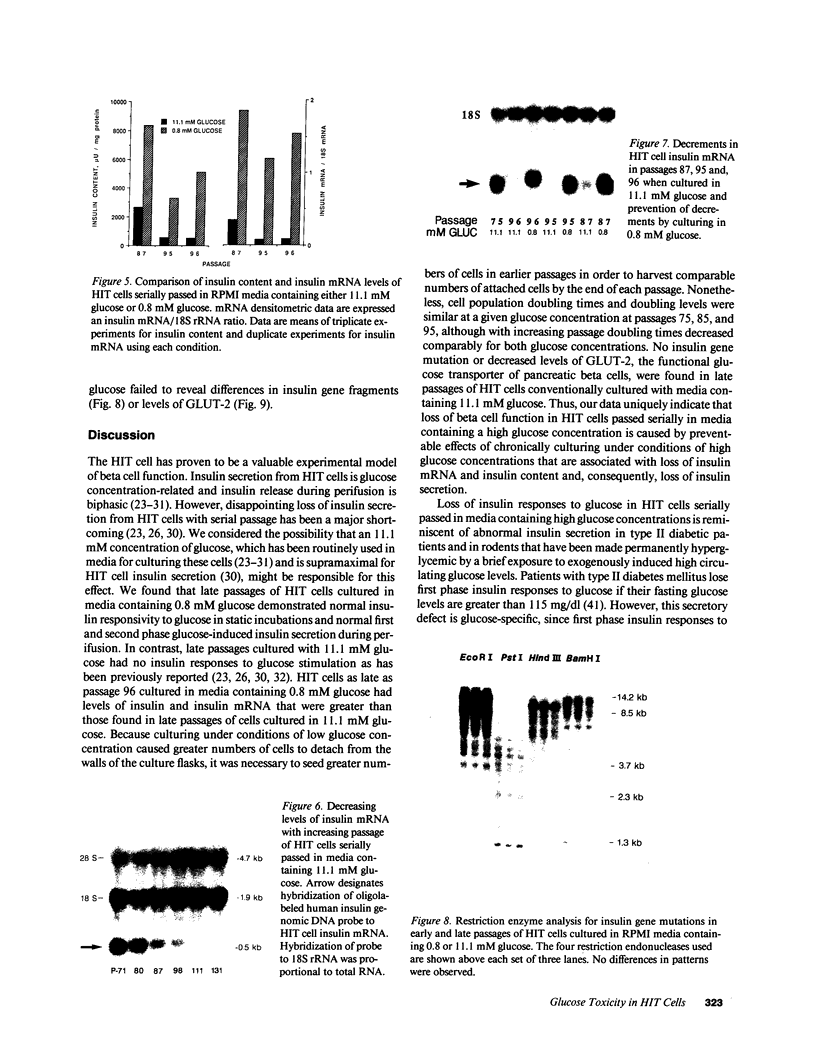
Glucose toxicity of the pancreatic beta cell is considered to play a secondary role in the pathogenesis of type II diabetes mellitus. To gain insights into possible mechanisms of action of glucose toxicity, we designed studies to assess whether the loss of insulin secretion associated with serial passages of HIT-T15 cells might be caused by chronic exposure to high glucose levels since these cells are routinely cultured in media containing supramaximal stimulatory concentrations of glucose. We found that late passages of HIT cells serially cultured in media containing 11.1 mM glucose lost insulin responsivity and had greatly diminished levels of insulin content and insulin mRNA. In marked contrast, late passages of HIT cells cultured serially in media containing 0.8 mM glucose retained insulin mRNA, insulin content, and insulin responsivity to glucose in static incubations and during perifusion with glucose. No insulin gene mutation or alteration of levels of GLUT-2 were found in late passages of HIT cells cultured with media containing 11.1 mM glucose. These data uniquely indicate that loss of beta cell function in HIT cells passed serially under high glucose conditions is caused by loss of insulin mRNA, insulin content, and insulin secretion and is preventable by culturing HIT cells under low glucose conditions. This strongly suggests potential genetic mechanisms of action for glucose toxicity of beta cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashcroft S. J., Hammonds P., Harrison D. E. Insulin secretory responses of a clonal cell line of simian virus 40-transformed B cells. Diabetologia. 1986 Oct;29(10):727–733. doi: 10.1007/BF00870283. [DOI] [PubMed] [Google Scholar]
- Bonner-Weir S., Trent D. F., Weir G. C. Partial pancreatectomy in the rat and subsequent defect in glucose-induced insulin release. J Clin Invest. 1983 Jun;71(6):1544–1553. doi: 10.1172/JCI110910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunzell J. D., Robertson R. P., Lerner R. L., Hazzard W. R., Ensinck J. W., Bierman E. L., Porte D., Jr Relationships between fasting plasma glucose levels and insulin secretion during intravenous glucose tolerance tests. J Clin Endocrinol Metab. 1976 Feb;42(2):222–229. doi: 10.1210/jcem-42-2-222. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Crockford P. M., Hazzard W. R., Williams R. H. Insulin response to glucagon. The opposing effects of diabetes and obesity. Diabetes. 1969 Apr;18(4):216–224. doi: 10.2337/diab.18.4.216. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- German M. S., Moss L. G., Rutter W. J. Regulation of insulin gene expression by glucose and calcium in transfected primary islet cultures. J Biol Chem. 1990 Dec 25;265(36):22063–22066. [PubMed] [Google Scholar]
- Giroix M. H., Serradas P., Portha B. The desensitization of normal B-cells to glucose in vitro is transient and not related to high glucose levels. Endocrinology. 1989 Oct;125(4):1999–2007. doi: 10.1210/endo-125-4-1999. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Ceriello A., di Pinto P., Saccomanno F., Gentile S., Cappiapuoti F. Impaired insulin secretion in human diabetes mellitus. The effect of naloxone-induced opiate receptor blockade. Diabetes. 1982 Apr;31(4 Pt 1):367–370. doi: 10.2337/diab.31.4.367. [DOI] [PubMed] [Google Scholar]
- Giugliano D., Di Pinto P., Ceriello A., Paolisso G., Saccomanno F., Torella R., D'Onofrio F. Glycemic control with an artificial pancreas improves insulin responses to both oral and glucose in nonobese noninsulin-dependent diabetic subjects. Acta Diabetol Lat. 1985 Jul-Sep;22(3):203–213. doi: 10.1007/BF02590771. [DOI] [PubMed] [Google Scholar]
- Glaser B., Leibovich G., Nesher R., Hartling S., Binder C., Cerasi E. Improved beta-cell function after intensive insulin treatment in severe non-insulin-dependent diabetes. Acta Endocrinol (Copenh) 1988 Jul;118(3):365–373. doi: 10.1530/acta.0.1180365. [DOI] [PubMed] [Google Scholar]
- Grodsky G. M. A new phase of insulin secretion. How will it contribute to our understanding of beta-cell function? Diabetes. 1989 Jun;38(6):673–678. doi: 10.2337/diab.38.6.673. [DOI] [PubMed] [Google Scholar]
- Hammonds P., Schofield P. N., Ashcroft S. J. Glucose regulates preproinsulin messenger RNA levels in a clonal cell line of simian virus 40-transformed B cells. FEBS Lett. 1987 Mar 9;213(1):149–154. doi: 10.1016/0014-5793(87)81481-3. [DOI] [PubMed] [Google Scholar]
- Harris B. A., Robishaw J. D., Mumby S. M., Gilman A. G. Molecular cloning of complementary DNA for the alpha subunit of the G protein that stimulates adenylate cyclase. Science. 1985 Sep 20;229(4719):1274–1277. doi: 10.1126/science.3839937. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Nagulesparan M., Klimes I., Clark R., Sasaki H., Aronoff S. L., Vasquez B., Rubenstein A. H., Unger R. H. Improvement of insulin secretion but not insulin resistance after short term control of plasma glucose in obese type II diabetics. J Clin Endocrinol Metab. 1982 Feb;54(2):217–222. doi: 10.1210/jcem-54-2-217. [DOI] [PubMed] [Google Scholar]
- Hill R. S., Boyd A. E., 3rd Perifusion of a clonal cell line of Simian virus 40-transformed beta cells. Insulin secretory dynamics in response to glucose, 3-isobutyl-1-methylxanthine, and potassium. Diabetes. 1985 Feb;34(2):115–120. doi: 10.2337/diab.34.2.115. [DOI] [PubMed] [Google Scholar]
- Imamura T., Koffler M., Helderman J. H., Prince D., Thirlby R., Inman L., Unger R. H. Severe diabetes induced in subtotally depancreatized dogs by sustained hyperglycemia. Diabetes. 1988 May;37(5):600–609. doi: 10.2337/diab.37.5.600. [DOI] [PubMed] [Google Scholar]
- Korsgren O., Jansson L., Sandler S., Andersson A. Hyperglycemia-induced B cell toxicity. The fate of pancreatic islets transplanted into diabetic mice is dependent on their genetic background. J Clin Invest. 1990 Dec;86(6):2161–2168. doi: 10.1172/JCI114955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka K., Kuzuya T., Akanuma Y., Hagura R. Increase in insulin response after treatment of overt maturity-onset diabetes is independent of the mode of treatment. Diabetologia. 1980 Jan;18(1):23–28. doi: 10.1007/BF01228297. [DOI] [PubMed] [Google Scholar]
- Lambert D. G., Hughes K., Atkins T. W. Insulin release from a cloned hamster B-cell line (HIT-T15). The effects of glucose, amino acids, sulphonylureas and colchicine. Biochem Biophys Res Commun. 1986 Oct 30;140(2):616–625. doi: 10.1016/0006-291x(86)90776-x. [DOI] [PubMed] [Google Scholar]
- Leahy J. L., Bonner-Weir S., Weir G. C. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest. 1988 May;81(5):1407–1414. doi: 10.1172/JCI113470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner R. L. Augmented insulin responses to glucose after secretin priming in diabetic subjects. J Clin Endocrinol Metab. 1979 Mar;48(3):462–466. doi: 10.1210/jcem-48-3-462. [DOI] [PubMed] [Google Scholar]
- Meglasson M. D., Manning C. D., Najafi H., Matschinsky F. M. Fuel-stimulated insulin secretion by clonal hamster beta-cell line HIT T-15. Diabetes. 1987 Apr;36(4):477–484. doi: 10.2337/diab.36.4.477. [DOI] [PubMed] [Google Scholar]
- Palmer J. P., Benson J. W., Walter R. M., Ensinck J. W. Arginine-stimulated acute phase of insulin and glucagon secretion in diabetic subjects. J Clin Invest. 1976 Sep;58(3):565–570. doi: 10.1172/JCI108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Chen M. A role for prostaglandin E in defective insulin secretion and carbohydrate intolerance in diabetes mellitus. J Clin Invest. 1977 Sep;60(3):747–753. doi: 10.1172/JCI108827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Halter J. B., Porte D., Jr A role for alpha-adrenergic receptors in abnormal insulin secretion in diabetes mellitus. J Clin Invest. 1976 Mar;57(3):791–795. doi: 10.1172/JCI108338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Porte D., Jr The glucose receptor. A defective mechanism in diabetes mellitus distinct from the beta adrenergic receptor. J Clin Invest. 1973 Apr;52(4):870–876. doi: 10.1172/JCI107251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson R. P., Tsai P., Little S. A., Zhang H. J., Walseth T. F. Receptor-mediated adenylate cyclase-coupled mechanism for PGE2 inhibition of insulin secretion in HIT cells. Diabetes. 1987 Sep;36(9):1047–1053. doi: 10.2337/diab.36.9.1047. [DOI] [PubMed] [Google Scholar]
- Robertson R. P. Type II diabetes, glucose "non-sense," and islet desensitization. Diabetes. 1989 Dec;38(12):1501–1505. doi: 10.2337/diab.38.12.1501. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Giaccari A., DeFronzo R. A. Glucose toxicity. Diabetes Care. 1990 Jun;13(6):610–630. doi: 10.2337/diacare.13.6.610. [DOI] [PubMed] [Google Scholar]
- Rossetti L., Shulman G. I., Zawalich W., DeFronzo R. A. Effect of chronic hyperglycemia on in vivo insulin secretion in partially pancreatectomized rats. J Clin Invest. 1987 Oct;80(4):1037–1044. doi: 10.1172/JCI113157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sako Y., Grill V. E. Coupling of beta-cell desensitization by hyperglycemia to excessive stimulation and circulating insulin in glucose-infused rats. Diabetes. 1990 Dec;39(12):1580–1583. doi: 10.2337/diab.39.12.1580. [DOI] [PubMed] [Google Scholar]
- Santerre R. F., Cook R. A., Crisel R. M., Sharp J. D., Schmidt R. J., Williams D. C., Wilson C. P. Insulin synthesis in a clonal cell line of simian virus 40-transformed hamster pancreatic beta cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4339–4343. doi: 10.1073/pnas.78.7.4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmeltz R., Wendorff H. J., Field J. B. Effect of control of blood glucose on plasma insulin responses to various stimuli in secondary failures to oral hypoglycemic agents and in newly diagnosed, maturity onset, ketosis-resistant diabetics. J Clin Endocrinol Metab. 1978 Apr;46(4):519–527. doi: 10.1210/jcem-46-4-519. [DOI] [PubMed] [Google Scholar]
- Seaquist E. R., Walseth T. F., Nelson D. M., Robertson R. P. Pertussis toxin-sensitive G protein mediation of PGE2 inhibition of cAMP metabolism and phasic glucose-induced insulin secretion in HIT cells. Diabetes. 1989 Nov;38(11):1439–1445. doi: 10.2337/diab.38.11.1439. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swope S. L., Schonbrunn A. Bombesin stimulates insulin secretion by a pancreatic islet cell line. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1822–1826. doi: 10.1073/pnas.81.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. C., McCarthy S. T., Holman R. R., Harris E. Beta-cell function improved by supplementing basal insulin secretion in mild diabetes. Br Med J. 1976 May 22;1(6020):1252–1254. doi: 10.1136/bmj.1.6020.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich S., Prentki M., Wollheim C. B. Somatostatin inhibition of Ca2(+)-induced insulin secretion in permeabilized HIT-T15 cells. Biochem J. 1990 Aug 15;270(1):273–276. doi: 10.1042/bj2700273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Grundy S. Hyperglycaemia as an inducer as well as a consequence of impaired islet cell function and insulin resistance: implications for the management of diabetes. Diabetologia. 1985 Mar;28(3):119–121. doi: 10.1007/BF00273856. [DOI] [PubMed] [Google Scholar]
- Vague P., Moulin J. P. The defective glucose sensitivity of the B cell in non insulin dependent diabetes. Improvement after twenty hours of normoglycaemia. Metabolism. 1982 Feb;31(2):139–142. doi: 10.1016/0026-0495(82)90125-1. [DOI] [PubMed] [Google Scholar]
- Walseth T. F., Zhang H. J., Olson L. K., Schroeder W. A., Robertson R. P. Increase in Gs and cyclic AMP generation in HIT cells. Evidence that the 45-kDa alpha-subunit of Gs has greater functional activity than the 52-kDa alpha-subunit. J Biol Chem. 1989 Dec 15;264(35):21106–21111. [PubMed] [Google Scholar]
- Zhang H. J., Walseth T. F., Robertson R. P. Insulin secretion and cAMP metabolism in HIT cells. Reciprocal and serial passage-dependent relationships. Diabetes. 1989 Jan;38(1):44–48. doi: 10.2337/diab.38.1.44. [DOI] [PubMed] [Google Scholar]
- de Leeuw W. J., Slagboom P. E., Vijg J. Quantitative comparison of mRNA levels in mammalian tissues: 28S ribosomal RNA level as an accurate internal control. Nucleic Acids Res. 1989 Dec 11;17(23):10137–10138. doi: 10.1093/nar/17.23.10137. [DOI] [PMC free article] [PubMed] [Google Scholar]