Abstract
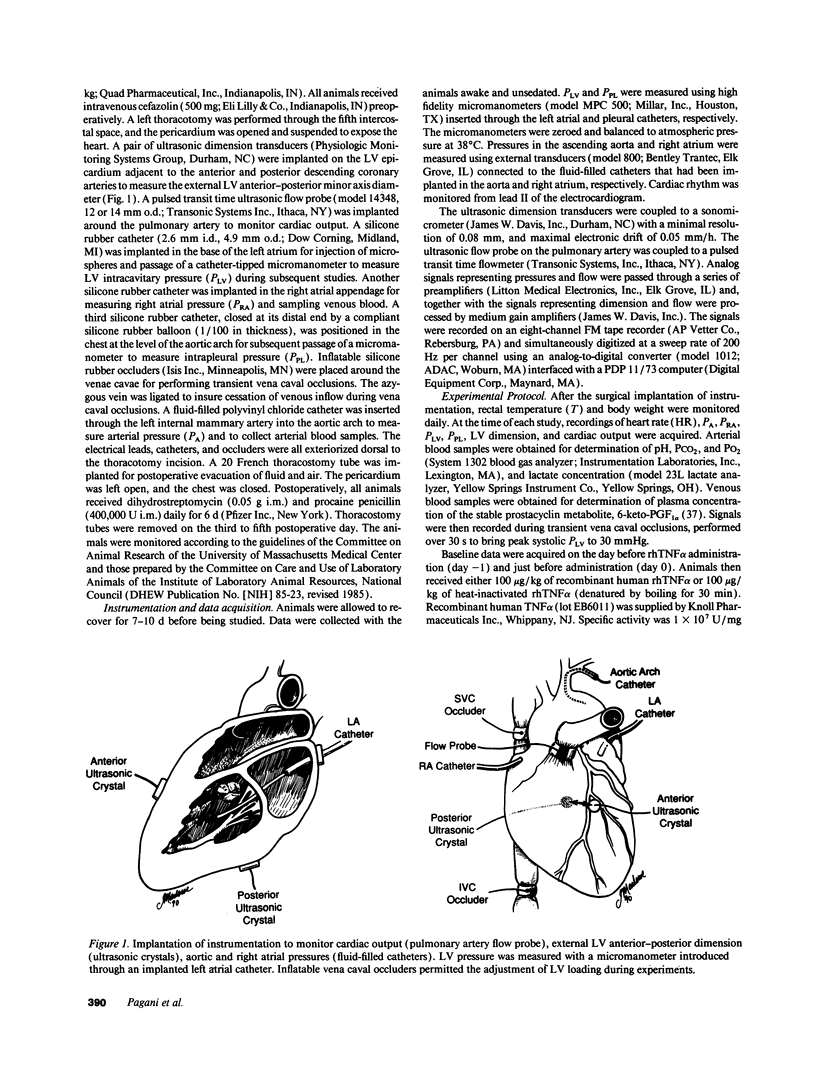
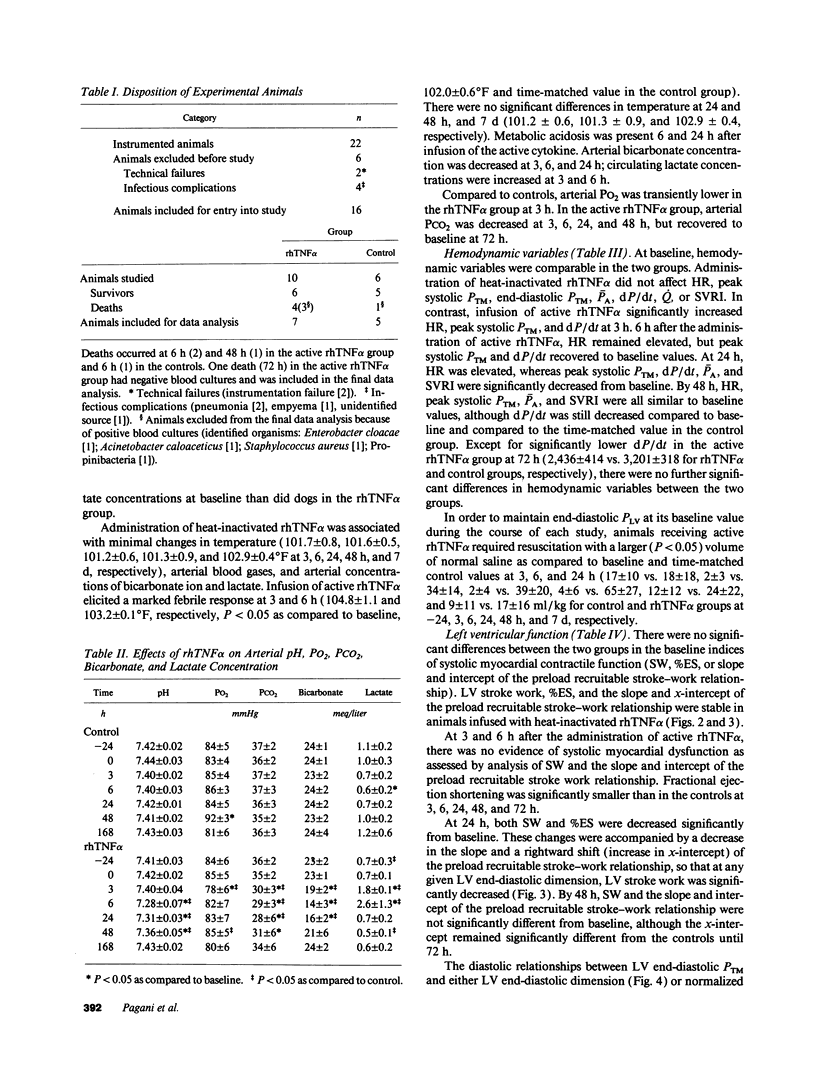
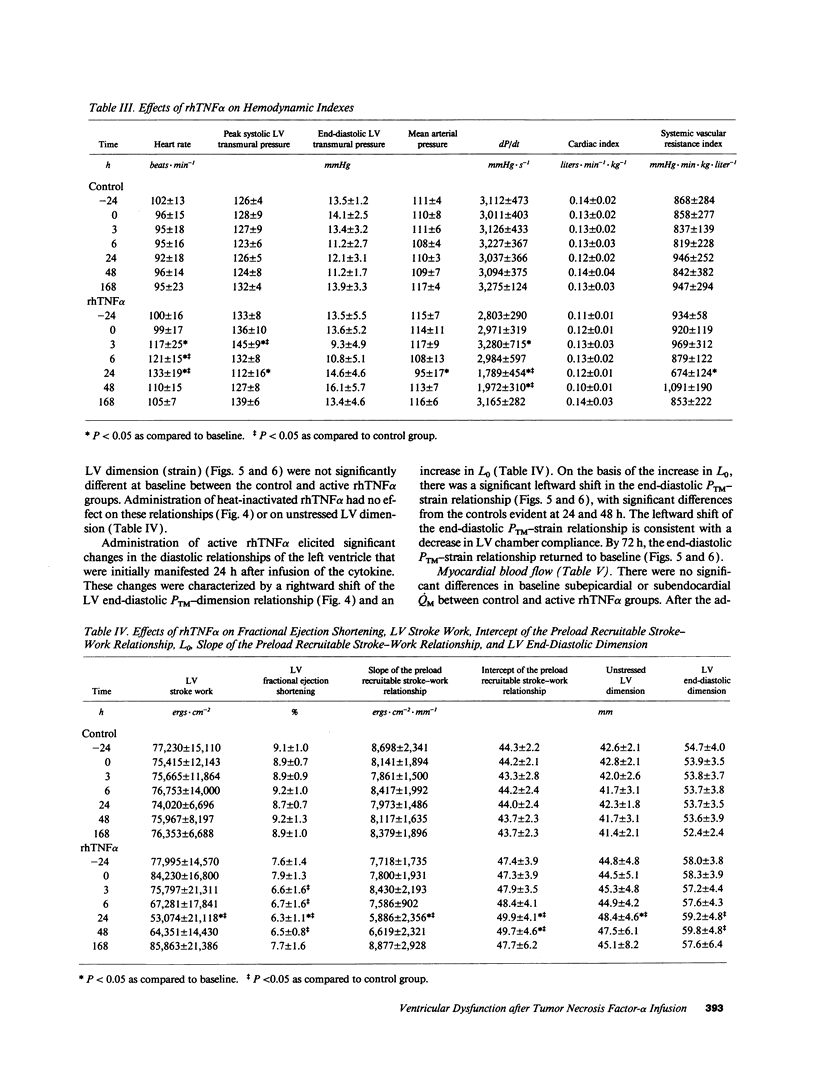
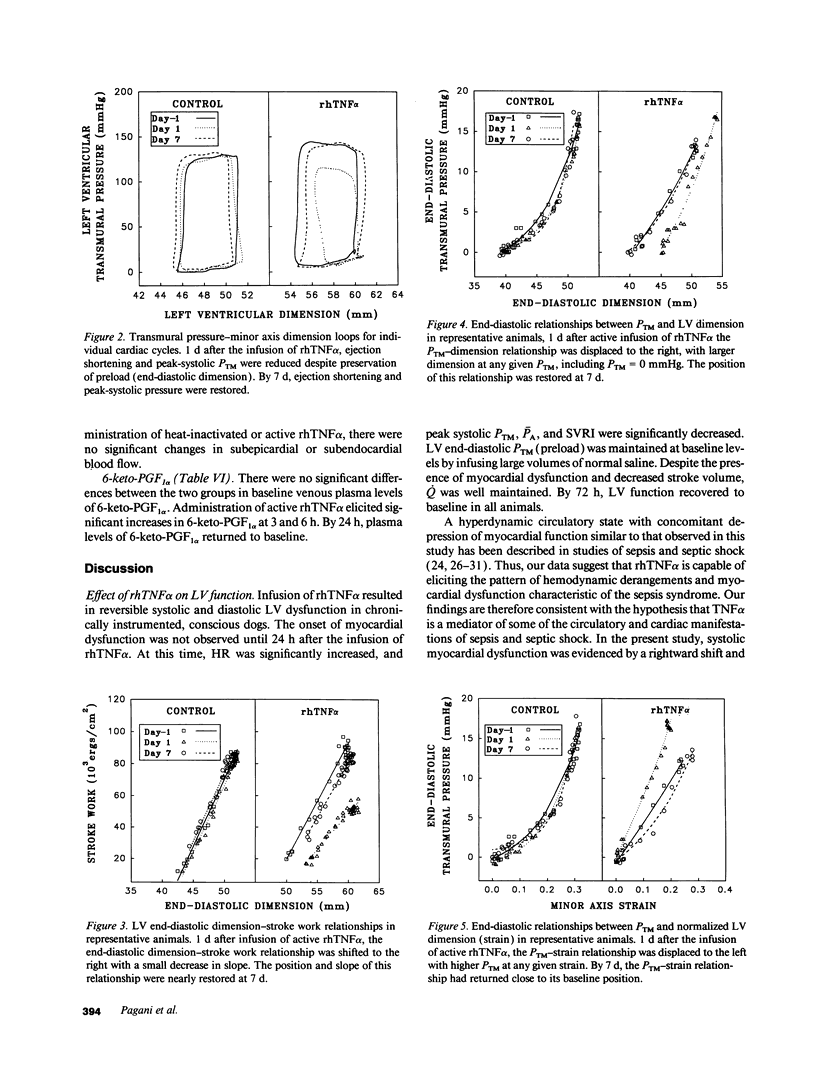
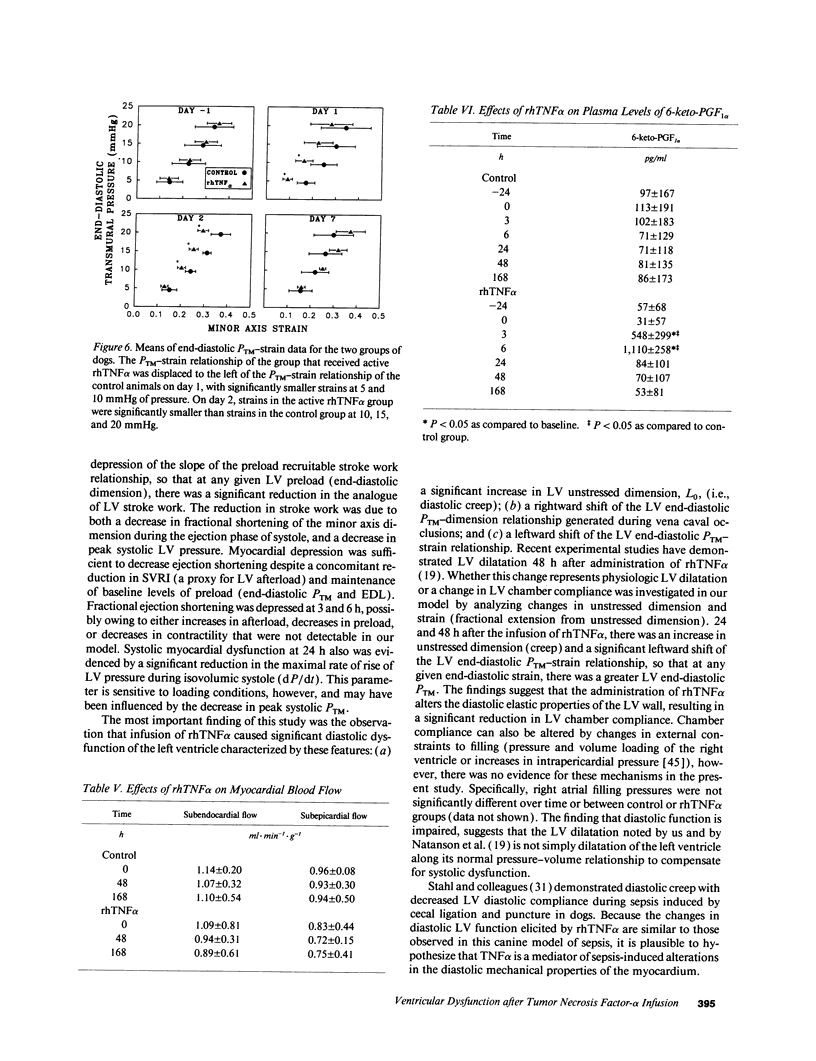
We used a load-insensitive index of systolic left ventricular (LV) function and an analysis of diastolic pressure-dimension relationships to test the hypothesis that recombinant human (rh) tumor necrosis factor-alpha (TNF alpha) impairs LV function in dogs. Animals were studied 7-10 d after aseptic implantation of instrumentation to monitor cardiac output, external anterior-posterior LV diameter, and LV and pleural pressures. Data were analyzed from seven dogs that received active rhTNF alpha (100 micrograms/kg over 60 min) and from five dogs that received heat-inactivated rhTNF alpha. At 24 h after infusion of active rhTNF alpha, the slope of the LV end-diastolic dimension-stroke work relationship decreased significantly, indicating a decrement in LV systolic contractility. Simultaneously, LV unstressed dimension increased significantly, suggesting diastolic myocardial creep. The end-diastolic relationship between LV transmural pressure and normalized LV dimension (strain) was markedly displaced to the left, suggesting increased diastolic elastic stiffness. Despite these changes in LV performance, cardiac index was maintained by tachycardia. The abnormalities in LV function were resolved by 72 h. We conclude that rhTNF alpha reversibly impairs LV systolic and diastolic function in unanesthetized dogs. Because dysfunction occurs greater than 6 h after the infusion of rhTNF alpha and persists for 24-48 h, the mechanism underlying this phenomenon may involve secondary mediators or a change in myocardial gene expression.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abel F. L. Maximal negative dP/dt as an indicator of end of systole. Am J Physiol. 1981 Apr;240(4):H676–H679. doi: 10.1152/ajpheart.1981.240.4.H676. [DOI] [PubMed] [Google Scholar]
- Bachwich P. R., Chensue S. W., Larrick J. W., Kunkel S. L. Tumor necrosis factor stimulates interleukin-1 and prostaglandin E2 production in resting macrophages. Biochem Biophys Res Commun. 1986 Apr 14;136(1):94–101. doi: 10.1016/0006-291x(86)90881-8. [DOI] [PubMed] [Google Scholar]
- Beutler B., Milsark I. W., Cerami A. C. Passive immunization against cachectin/tumor necrosis factor protects mice from lethal effect of endotoxin. Science. 1985 Aug 30;229(4716):869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- Blick M., Sherwin S. A., Rosenblum M., Gutterman J. Phase I study of recombinant tumor necrosis factor in cancer patients. Cancer Res. 1987 Jun 1;47(11):2986–2989. [PubMed] [Google Scholar]
- Chung M. K., Gulick T. S., Rotondo R. E., Schreiner G. F., Lange L. G. Mechanism of cytokine inhibition of beta-adrenergic agonist stimulation of cyclic AMP in rat cardiac myocytes. Impairment of signal transduction. Circ Res. 1990 Sep;67(3):753–763. doi: 10.1161/01.res.67.3.753. [DOI] [PubMed] [Google Scholar]
- Ellrodt A. G., Riedinger M. S., Kimchi A., Berman D. S., Maddahi J., Swan H. J., Murata G. H. Left ventricular performance in septic shock: reversible segmental and global abnormalities. Am Heart J. 1985 Aug;110(2):402–409. doi: 10.1016/0002-8703(85)90163-2. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Dahlgren M. D., Morris D. D., Peterson M. A., Schmid-Schönbein G. W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am J Physiol. 1986 Aug;251(2 Pt 2):H314–H323. doi: 10.1152/ajpheart.1986.251.2.H314. [DOI] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Engler R., Covell J. W. Granulocytes cause reperfusion ventricular dysfunction after 15-minute ischemia in the dog. Circ Res. 1987 Jul;61(1):20–28. doi: 10.1161/01.res.61.1.20. [DOI] [PubMed] [Google Scholar]
- Entman M. L., Youker K., Shappell S. B., Siegel C., Rothlein R., Dreyer W. J., Schmalstieg F. C., Smith C. W. Neutrophil adherence to isolated adult canine myocytes. Evidence for a CD18-dependent mechanism. J Clin Invest. 1990 May;85(5):1497–1506. doi: 10.1172/JCI114596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. A., Jacobs D. O., Revhaug A., Wilmore D. W. The effects of tumor necrosis factor and their selective inhibition by ibuprofen. Ann Surg. 1989 Mar;209(3):312–321. doi: 10.1097/00000658-198903000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. F., Snyder Y. M., Butler L. D., Zuckerman S. H. Differential expression of interleukin-1 and tumor necrosis factor in murine septic shock models. Circ Shock. 1989 Dec;29(4):279–290. [PubMed] [Google Scholar]
- Ezra D., Boyd L. M., Feuerstein G., Goldstein R. E. Coronary constriction by leukotriene C4, D4, and E4 in the intact pig heart. Am J Cardiol. 1983 May 1;51(8):1451–1454. doi: 10.1016/0002-9149(83)90328-4. [DOI] [PubMed] [Google Scholar]
- Factor S. M., Flomenbaum M., Zhao M. J., Eng C., Robinson T. F. The effects of acutely increased ventricular cavity pressure on intrinsic myocardial connective tissue. J Am Coll Cardiol. 1988 Dec;12(6):1582–1589. doi: 10.1016/s0735-1097(88)80029-9. [DOI] [PubMed] [Google Scholar]
- Fink M. P., Gardiner W. M., Roethel R., Fletcher J. R. Plasma levels of 6-keto PGF1 alpha but not TxB2 increase in rats with peritonitis due to cecal ligation. Circ Shock. 1985;16(3):297–305. [PubMed] [Google Scholar]
- Gaskill H. V., 3rd Continuous infusion of tumor necrosis factor: mechanisms of toxicity in the rat. J Surg Res. 1988 Jun;44(6):664–671. doi: 10.1016/0022-4804(88)90098-4. [DOI] [PubMed] [Google Scholar]
- Glantz S. A., Parmley W. W. Factors which affect the diastolic pressure-volume curve. Circ Res. 1978 Feb;42(2):171–180. doi: 10.1161/01.res.42.2.171. [DOI] [PubMed] [Google Scholar]
- Glower D. D., Schaper J., Kabas J. S., Hoffmeister H. M., Schaper W., Spratt J. A., Davis J. W., Rankin J. S. Relation between reversal of diastolic creep and recovery of systolic function after ischemic myocardial injury in conscious dogs. Circ Res. 1987 Jun;60(6):850–860. doi: 10.1161/01.res.60.6.850. [DOI] [PubMed] [Google Scholar]
- Glower D. D., Spratt J. A., Kabas J. S., Davis J. W., Rankin J. S. Quantification of regional myocardial dysfunction after acute ischemic injury. Am J Physiol. 1988 Jul;255(1 Pt 2):H85–H93. doi: 10.1152/ajpheart.1988.255.1.H85. [DOI] [PubMed] [Google Scholar]
- Glower D. D., Spratt J. A., Snow N. D., Kabas J. S., Davis J. W., Olsen C. O., Tyson G. S., Sabiston D. C., Jr, Rankin J. S. Linearity of the Frank-Starling relationship in the intact heart: the concept of preload recruitable stroke work. Circulation. 1985 May;71(5):994–1009. doi: 10.1161/01.cir.71.5.994. [DOI] [PubMed] [Google Scholar]
- Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale S. L., Alker K. J., Kloner R. A. Evaluation of nonradioactive, colored microspheres for measurement of regional myocardial blood flow in dogs. Circulation. 1988 Aug;78(2):428–434. doi: 10.1161/01.cir.78.2.428. [DOI] [PubMed] [Google Scholar]
- Hesse D. G., Tracey K. J., Fong Y., Manogue K. R., Palladino M. A., Jr, Cerami A., Shires G. T., Lowry S. F. Cytokine appearance in human endotoxemia and primate bacteremia. Surg Gynecol Obstet. 1988 Feb;166(2):147–153. [PubMed] [Google Scholar]
- Huber M., Beutler B., Keppler D. Tumor necrosis factor alpha stimulates leukotriene production in vivo. Eur J Immunol. 1988 Dec;18(12):2085–2088. doi: 10.1002/eji.1830181233. [DOI] [PubMed] [Google Scholar]
- Johnson J., Meyrick B., Jesmok G., Brigham K. L. Human recombinant tumor necrosis factor alpha infusion mimics endotoxemia in awake sheep. J Appl Physiol (1985) 1989 Mar;66(3):1448–1454. doi: 10.1152/jappl.1989.66.3.1448. [DOI] [PubMed] [Google Scholar]
- Kettelhut I. C., Fiers W., Goldberg A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4273–4277. doi: 10.1073/pnas.84.12.4273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiener P. A., Marek F., Rodgers G., Lin P. F., Warr G., Desiderio J. Induction of tumor necrosis factor, IFN-gamma, and acute lethality in mice by toxic and non-toxic forms of lipid A. J Immunol. 1988 Aug 1;141(3):870–874. [PubMed] [Google Scholar]
- Kreil E. A., Greene E., Fitzgibbon C., Robinson D. R., Zapol W. M. Effects of recombinant human tumor necrosis factor alpha, lymphotoxin, and Escherichia coli lipopolysaccharide on hemodynamics, lung microvascular permeability, and eicosanoid synthesis in anesthetized sheep. Circ Res. 1989 Aug;65(2):502–514. doi: 10.1161/01.res.65.2.502. [DOI] [PubMed] [Google Scholar]
- Laine G. A., Allen S. J. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ Res. 1991 Jun;68(6):1713–1721. doi: 10.1161/01.res.68.6.1713. [DOI] [PubMed] [Google Scholar]
- MacLean L. D., Mulligan W. G., McLean A. P., Duff J. H. Patterns of septic shock in man--a detailed study of 56 patients. Ann Surg. 1967 Oct;166(4):543–562. doi: 10.1097/00000658-196710000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathison J. C., Wolfson E., Ulevitch R. J. Participation of tumor necrosis factor in the mediation of gram negative bacterial lipopolysaccharide-induced injury in rabbits. J Clin Invest. 1988 Jun;81(6):1925–1937. doi: 10.1172/JCI113540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J. D., Yurt R. W., Duhaney R., Hesse D. G., Tracey K. J., Fong Y. M., Verma M., Shires G. T., Dineen P., Lowry S. F. Tumor necrosis factor-enhanced leukotriene B4 generation and chemotaxis in human neutrophils. Arch Surg. 1988 Dec;123(12):1454–1458. doi: 10.1001/archsurg.1988.01400360024002. [DOI] [PubMed] [Google Scholar]
- Michie H. R., Manogue K. R., Spriggs D. R., Revhaug A., O'Dwyer S., Dinarello C. A., Cerami A., Wolff S. M., Wilmore D. W. Detection of circulating tumor necrosis factor after endotoxin administration. N Engl J Med. 1988 Jun 9;318(23):1481–1486. doi: 10.1056/NEJM198806093182301. [DOI] [PubMed] [Google Scholar]
- Moser R., Schleiffenbaum B., Groscurth P., Fehr J. Interleukin 1 and tumor necrosis factor stimulate human vascular endothelial cells to promote transendothelial neutrophil passage. J Clin Invest. 1989 Feb;83(2):444–455. doi: 10.1172/JCI113903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullane K. M., Fornabaio D. Thromboxane synthetase inhibitors reduce infarct size by a platelet-dependent, aspirin-sensitive mechanism. Circ Res. 1988 Apr;62(4):668–678. doi: 10.1161/01.res.62.4.668. [DOI] [PubMed] [Google Scholar]
- Mullane K. M., Read N., Salmon J. A., Moncada S. Role of leukocytes in acute myocardial infarction in anesthetized dogs: relationship to myocardial salvage by anti-inflammatory drugs. J Pharmacol Exp Ther. 1984 Feb;228(2):510–522. [PubMed] [Google Scholar]
- Mullane K., Hatala M. A., Kraemer R., Sessa W., Westlin W. Myocardial salvage induced by REV-5901: an inhibitor and antagonist of the leukotrienes. J Cardiovasc Pharmacol. 1987 Oct;10(4):398–406. doi: 10.1097/00005344-198710000-00004. [DOI] [PubMed] [Google Scholar]
- Natanson C., Danner R. L., Elin R. J., Hosseini J. M., Peart K. W., Banks S. M., MacVittie T. J., Walker R. I., Parrillo J. E. Role of endotoxemia in cardiovascular dysfunction and mortality. Escherichia coli and Staphylococcus aureus challenges in a canine model of human septic shock. J Clin Invest. 1989 Jan;83(1):243–251. doi: 10.1172/JCI113866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C., Danner R. L., Fink M. P., MacVittie T. J., Walker R. I., Conklin J. J., Parrillo J. E. Cardiovascular performance with E. coli challenges in a canine model of human sepsis. Am J Physiol. 1988 Mar;254(3 Pt 2):H558–H569. doi: 10.1152/ajpheart.1988.254.3.H558. [DOI] [PubMed] [Google Scholar]
- Natanson C., Eichenholz P. W., Danner R. L., Eichacker P. Q., Hoffman W. D., Kuo G. C., Banks S. M., MacVittie T. J., Parrillo J. E. Endotoxin and tumor necrosis factor challenges in dogs simulate the cardiovascular profile of human septic shock. J Exp Med. 1989 Mar 1;169(3):823–832. doi: 10.1084/jem.169.3.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natanson C., Fink M. P., Ballantyne H. K., MacVittie T. J., Conklin J. J., Parrillo J. E. Gram-negative bacteremia produces both severe systolic and diastolic cardiac dysfunction in a canine model that simulates human septic shock. J Clin Invest. 1986 Jul;78(1):259–270. doi: 10.1172/JCI112559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okusawa S., Gelfand J. A., Ikejima T., Connolly R. J., Dinarello C. A. Interleukin 1 induces a shock-like state in rabbits. Synergism with tumor necrosis factor and the effect of cyclooxygenase inhibition. J Clin Invest. 1988 Apr;81(4):1162–1172. doi: 10.1172/JCI113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. M., Shelhamer J. H., Bacharach S. L., Green M. V., Natanson C., Frederick T. M., Damske B. A., Parrillo J. E. Profound but reversible myocardial depression in patients with septic shock. Ann Intern Med. 1984 Apr;100(4):483–490. doi: 10.7326/0003-4819-100-4-483. [DOI] [PubMed] [Google Scholar]
- Pasque M. K., Van Trigt P., Pellom G. L., Freedman B. M., Wechsler A. S. Assessment of the intrinsic contractile status of the heart during sepsis by myocardial pressure-dimension analysis. Ann Surg. 1988 Jul;208(1):110–117. doi: 10.1097/00000658-198807000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohlman T. H., Stanness K. A., Beatty P. G., Ochs H. D., Harlan J. M. An endothelial cell surface factor(s) induced in vitro by lipopolysaccharide, interleukin 1, and tumor necrosis factor-alpha increases neutrophil adherence by a CDw18-dependent mechanism. J Immunol. 1986 Jun 15;136(12):4548–4553. [PubMed] [Google Scholar]
- Przyklenk K., Kloner R. A. Superoxide dismutase plus catalase improve contractile function in the canine model of the "stunned myocardium". Circ Res. 1986 Jan;58(1):148–156. doi: 10.1161/01.res.58.1.148. [DOI] [PubMed] [Google Scholar]
- Rankin J. S., Arentzen C. E., McHale P. A., Ling D., Anderson R. W. Viscoelastic properties of the diastolic left ventricle in the conscious dog. Circ Res. 1977 Jul;41(1):37–45. doi: 10.1161/01.res.41.1.37. [DOI] [PubMed] [Google Scholar]
- Rankin J. S., McHale P. A., Arentzen C. E., Ling D., Greenfield J. C., Jr, Anderson R. W. The three-dimensional dynamic geometry of the left ventricle in the conscious dog. Circ Res. 1976 Sep;39(3):304–313. doi: 10.1161/01.res.39.3.304. [DOI] [PubMed] [Google Scholar]
- Remick D. G., Kunkel R. G., Larrick J. W., Kunkel S. L. Acute in vivo effects of human recombinant tumor necrosis factor. Lab Invest. 1987 Jun;56(6):583–590. [PubMed] [Google Scholar]
- Reynolds J. M., McDonagh P. F. Early in reperfusion, leukocytes alter perfused coronary capillarity and vascular resistance. Am J Physiol. 1989 Apr;256(4 Pt 2):H982–H989. doi: 10.1152/ajpheart.1989.256.4.H982. [DOI] [PubMed] [Google Scholar]
- Romson J. L., Hook B. G., Kunkel S. L., Abrams G. D., Schork M. A., Lucchesi B. R. Reduction of the extent of ischemic myocardial injury by neutrophil depletion in the dog. Circulation. 1983 May;67(5):1016–1023. doi: 10.1161/01.cir.67.5.1016. [DOI] [PubMed] [Google Scholar]
- Rothstein J. L., Schreiber H. Synergy between tumor necrosis factor and bacterial products causes hemorrhagic necrosis and lethal shock in normal mice. Proc Natl Acad Sci U S A. 1988 Jan;85(2):607–611. doi: 10.1073/pnas.85.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer W. J., Schirmer J. M., Fry D. E. Recombinant human tumor necrosis factor produces hemodynamic changes characteristic of sepsis and endotoxemia. Arch Surg. 1989 Apr;124(4):445–448. doi: 10.1001/archsurg.1989.01410040055012. [DOI] [PubMed] [Google Scholar]
- Simpson P. J., Todd R. F., 3rd, Fantone J. C., Mickelson J. K., Griffin J. D., Lucchesi B. R. Reduction of experimental canine myocardial reperfusion injury by a monoclonal antibody (anti-Mo1, anti-CD11b) that inhibits leukocyte adhesion. J Clin Invest. 1988 Feb;81(2):624–629. doi: 10.1172/JCI113364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs D. R., Sherman M. L., Michie H., Arthur K. A., Imamura K., Wilmore D., Frei E., 3rd, Kufe D. W. Recombinant human tumor necrosis factor administered as a 24-hour intravenous infusion. A phase I and pharmacologic study. J Natl Cancer Inst. 1988 Sep 7;80(13):1039–1044. doi: 10.1093/jnci/80.13.1039. [DOI] [PubMed] [Google Scholar]
- Stahl T. J., Alden P. B., Ring W. S., Madoff R. C., Cerra F. B. Sepsis-induced diastolic dysfunction in chronic canine peritonitis. Am J Physiol. 1990 Mar;258(3 Pt 2):H625–H633. doi: 10.1152/ajpheart.1990.258.3.H625. [DOI] [PubMed] [Google Scholar]
- Sun X. M., Hsueh W. Bowel necrosis induced by tumor necrosis factor in rats is mediated by platelet-activating factor. J Clin Invest. 1988 May;81(5):1328–1331. doi: 10.1172/JCI113459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracey K. J., Beutler B., Lowry S. F., Merryweather J., Wolpe S., Milsark I. W., Hariri R. J., Fahey T. J., 3rd, Zentella A., Albert J. D. Shock and tissue injury induced by recombinant human cachectin. Science. 1986 Oct 24;234(4775):470–474. doi: 10.1126/science.3764421. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Fong Y., Hesse D. G., Manogue K. R., Lee A. T., Kuo G. C., Lowry S. F., Cerami A. Anti-cachectin/TNF monoclonal antibodies prevent septic shock during lethal bacteraemia. Nature. 1987 Dec 17;330(6149):662–664. doi: 10.1038/330662a0. [DOI] [PubMed] [Google Scholar]
- Tracey K. J., Lowry S. F., Fahey T. J., 3rd, Albert J. D., Fong Y., Hesse D., Beutler B., Manogue K. R., Calvano S., Wei H. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog. Surg Gynecol Obstet. 1987 May;164(5):415–422. [PubMed] [Google Scholar]
- Tsujimoto M., Yokota S., Vilcek J., Weissmann G. Tumor necrosis factor provokes superoxide anion generation from neutrophils. Biochem Biophys Res Commun. 1986 Jun 30;137(3):1094–1100. doi: 10.1016/0006-291x(86)90337-2. [DOI] [PubMed] [Google Scholar]
- Turner C. R., Esser K. M., Wheeldon E. B., Slivjak M., Smith E. F., 3rd Cardiovascular and pulmonary effects of human recombinant tumor necrosis factor in the conscious rat. Circ Shock. 1989 Aug;28(4):369–384. [PubMed] [Google Scholar]
- Waage A., Espevik T., Lamvik J. Detection of tumour necrosis factor-like cytotoxicity in serum from patients with septicaemia but not from untreated cancer patients. Scand J Immunol. 1986 Dec;24(6):739–743. doi: 10.1111/j.1365-3083.1986.tb02194.x. [DOI] [PubMed] [Google Scholar]
- Waage A., Halstensen A., Espevik T. Association between tumour necrosis factor in serum and fatal outcome in patients with meningococcal disease. Lancet. 1987 Feb 14;1(8529):355–357. doi: 10.1016/s0140-6736(87)91728-4. [DOI] [PubMed] [Google Scholar]
- Weisman H. F., Bush D. E., Mannisi J. A., Weisfeldt M. L., Healy B. Cellular mechanisms of myocardial infarct expansion. Circulation. 1988 Jul;78(1):186–201. doi: 10.1161/01.cir.78.1.186. [DOI] [PubMed] [Google Scholar]
- Zhao M. J., Zhang H., Robinson T. F., Factor S. M., Sonnenblick E. H., Eng C. Profound structural alterations of the extracellular collagen matrix in postischemic dysfunctional ("stunned") but viable myocardium. J Am Coll Cardiol. 1987 Dec;10(6):1322–1334. doi: 10.1016/s0735-1097(87)80137-7. [DOI] [PubMed] [Google Scholar]


