Abstract
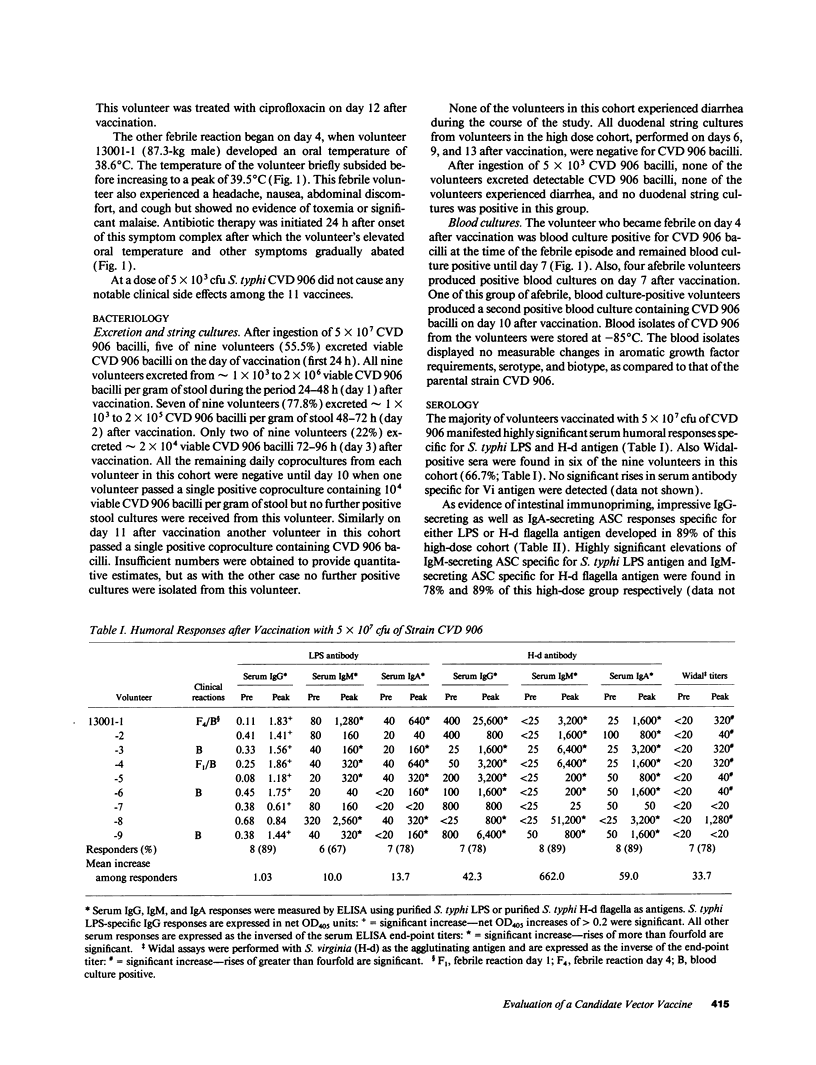
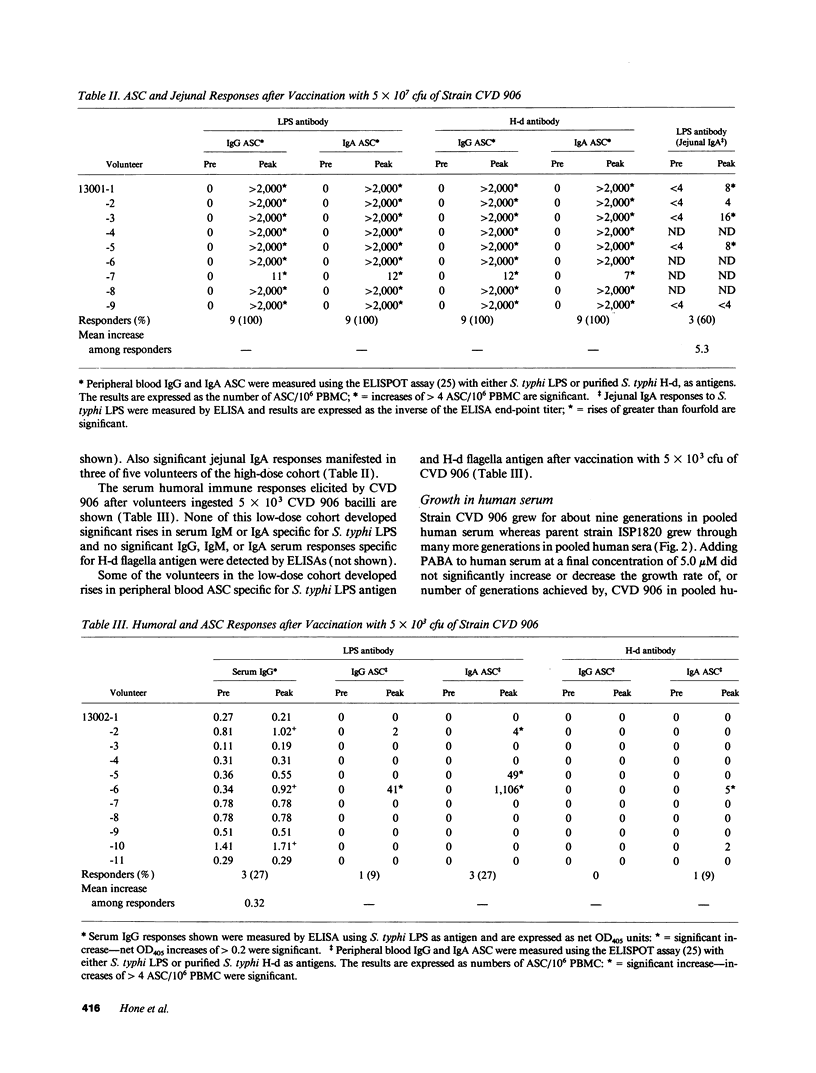
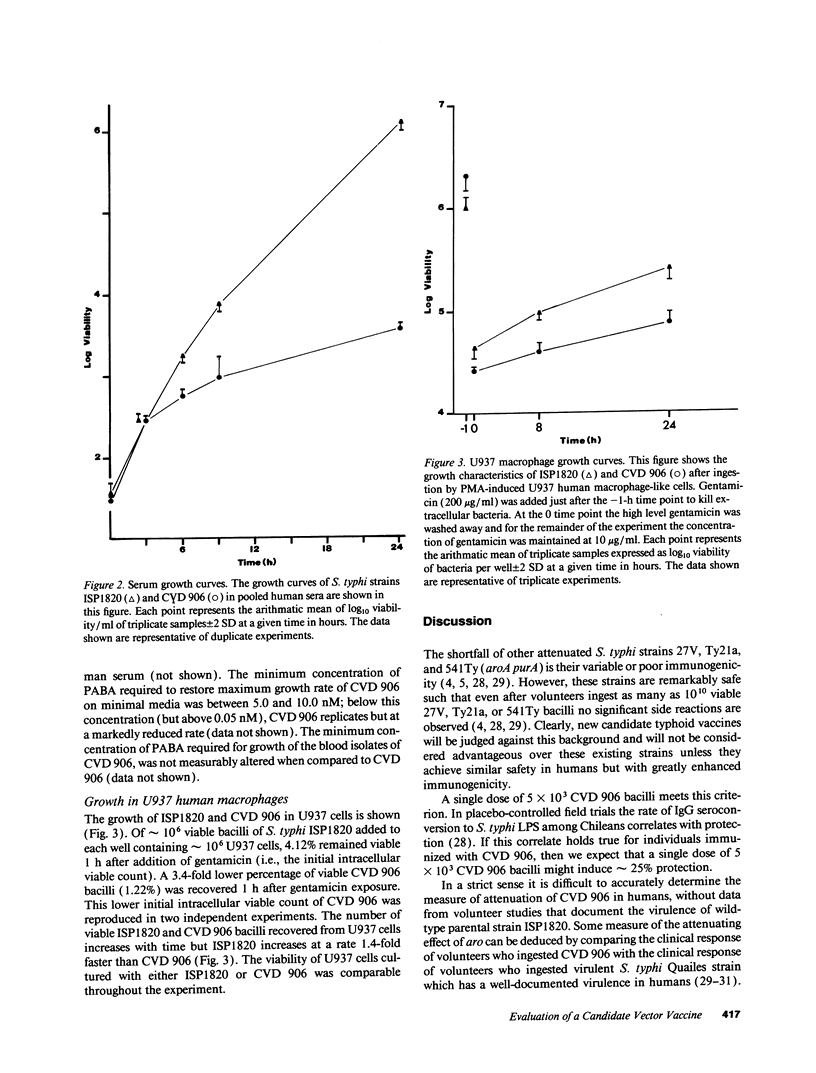
Candidate vector vaccine strain CVD 906 (aroC- and aroD- derivative of virulent Salmonella typhi strain ISP1820) was evaluated in phase 1 clinical trials. The first nine volunteers ingested a single dose of 5 x 10(7) CVD 906 bacilli. At this dose CVD 906 stimulates remarkable systemic and mucosal immune responses, inasmuch as 89% of volunteers developed marked serum antibody levels to S. typhi antigens and high numbers of antigen-specific gut-derived antibody-secreting cells. Four (44%) volunteers developed asymptomatic vaccinemia 4-10 d after immunization and all volunteers excreted CVD 906 on at least one occasion. However, two volunteers developed febrile adverse reactions, one on the day of vaccination and the other on day 4. Of 11 volunteers who ingested a single dose of 5 x 10(3) CVD 906 bacilli, none displayed side effects but 27% developed significant serum responses to S. typhi LPS. In vitro, CVD 906 replicates for only nine generations in pooled human serum, indicating that CVD 906 growth is limited in this physiologically relevant medium. In phorbol myristate acetate-induced U937 human macrophage-like cells, CVD 906 replicates intracellularly to a lesser extent than parent strain ISP1820. Although, strain CVD 906 is attenuated and highly immunogenic, the occasional febrile reactions at high doses indicate that further attenuation of this strain is necessary.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acharya I. L., Lowe C. U., Thapa R., Gurubacharya V. L., Shrestha M. B., Cadoz M., Schulz D., Armand J., Bryla D. A., Trollfors B. Prevention of typhoid fever in Nepal with the Vi capsular polysaccharide of Salmonella typhi. A preliminary report. N Engl J Med. 1987 Oct 29;317(18):1101–1104. doi: 10.1056/NEJM198710293171801. [DOI] [PubMed] [Google Scholar]
- Aggarwal A., Kumar S., Jaffe R., Hone D., Gross M., Sadoff J. Oral Salmonella: malaria circumsporozoite recombinants induce specific CD8+ cytotoxic T cells. J Exp Med. 1990 Oct 1;172(4):1083–1090. doi: 10.1084/jem.172.4.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BACON G. A., BURROWS T. W., YATES M. The effects of biochemical mutation on the virulence of Bacterium typhosum: the virulence of mutants. Br J Exp Pathol. 1950 Dec;31(6):714–724. [PMC free article] [PubMed] [Google Scholar]
- Benjamin W. H., Jr, Turnbough C. L., Jr, Posey B. S., Briles D. E. The ability of Salmonella typhimurium to produce the siderophore enterobactin is not a virulence factor in mouse typhoid. Infect Immun. 1985 Nov;50(2):392–397. doi: 10.1128/iai.50.2.392-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Induction of Salmonella stress proteins upon infection of macrophages. Science. 1990 May 11;248(4956):730–732. doi: 10.1126/science.1970672. [DOI] [PubMed] [Google Scholar]
- Buchmeier N. A., Heffron F. Intracellular survival of wild-type Salmonella typhimurium and macrophage-sensitive mutants in diverse populations of macrophages. Infect Immun. 1989 Jan;57(1):1–7. doi: 10.1128/iai.57.1.1-7.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements J. D., El-Morshidy S. Construction of a potential live oral bivalent vaccine for typhoid fever and cholera-Escherichia coli-related diarrheas. Infect Immun. 1984 Nov;46(2):564–569. doi: 10.1128/iai.46.2.564-569.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtiss R., 3rd, Kelly S. M. Salmonella typhimurium deletion mutants lacking adenylate cyclase and cyclic AMP receptor protein are avirulent and immunogenic. Infect Immun. 1987 Dec;55(12):3035–3043. doi: 10.1128/iai.55.12.3035-3043.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougan G., Chatfield S., Pickard D., Bester J., O'Callaghan D., Maskell D. Construction and characterization of vaccine strains of Salmonella harboring mutations in two different aro genes. J Infect Dis. 1988 Dec;158(6):1329–1335. doi: 10.1093/infdis/158.6.1329. [DOI] [PubMed] [Google Scholar]
- Dragunsky E. M., Rivera E., Hochstein H. D., Levenbook I. S. In vitro characterization of Salmonella typhi mutant strains for live oral vaccines. Vaccine. 1990 Jun;8(3):263–268. doi: 10.1016/0264-410x(90)90056-r. [DOI] [PubMed] [Google Scholar]
- Edwards M. F., Stocker B. A. Construction of delta aroA his delta pur strains of Salmonella typhi. J Bacteriol. 1988 Sep;170(9):3991–3995. doi: 10.1128/jb.170.9.3991-3995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein T. K., Killar L. M., Stocker B. A., Sultzer B. M. Cellular immunity induced by avirulent Salmonella in LPS-defective C3H/HeJ mice. J Immunol. 1984 Aug;133(2):958–961. [PubMed] [Google Scholar]
- Emmerson P. T., West S. C. Identification of protein X of Escherichia coli as the recA+/tif+ gene product. Mol Gen Genet. 1977 Sep 21;155(1):77–85. doi: 10.1007/BF00268563. [DOI] [PubMed] [Google Scholar]
- Formal S. B., Baron L. S., Kopecko D. J., Washington O., Powell C., Life C. A. Construction of a potential bivalent vaccine strain: introduction of Shigella sonnei form I antigen genes into the galE Salmonella typhi Ty21a typhoid vaccine strain. Infect Immun. 1981 Dec;34(3):746–750. doi: 10.1128/iai.34.3.746-750.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galán J. E., Curtiss R., 3rd Virulence and vaccine potential of phoP mutants of Salmonella typhimurium. Microb Pathog. 1989 Jun;6(6):433–443. doi: 10.1016/0882-4010(89)90085-5. [DOI] [PubMed] [Google Scholar]
- Gilman R. H., Hornick R. B., Woodard W. E., DuPont H. L., Snyder M. J., Levine M. M., Libonati J. P. Evaluation of a UDP-glucose-4-epimeraseless mutant of Salmonella typhi as a liver oral vaccine. J Infect Dis. 1977 Dec;136(6):717–723. doi: 10.1093/infdis/136.6.717. [DOI] [PubMed] [Google Scholar]
- Greisman S. E., Hornick R. B., Wagner H. N., Jr, Woodward W. E., Woodward T. E. The role of endotoxin during typhoid fever and tularemia in man. IV. The integrity of the endotoxin tolerance mechanisms during infection. J Clin Invest. 1969 Apr;48(4):613–629. doi: 10.1172/JCI106020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoiseth S. K., Stocker B. A. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature. 1981 May 21;291(5812):238–239. doi: 10.1038/291238a0. [DOI] [PubMed] [Google Scholar]
- Hone D. M., Attridge S. R., Forrest B., Morona R., Daniels D., LaBrooy J. T., Bartholomeusz R. C., Shearman D. J., Hackett J. A galE via (Vi antigen-negative) mutant of Salmonella typhi Ty2 retains virulence in humans. Infect Immun. 1988 May;56(5):1326–1333. doi: 10.1128/iai.56.5.1326-1333.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hone D. M., Harris A. M., Chatfield S., Dougan G., Levine M. M. Construction of genetically defined double aro mutants of Salmonella typhi. Vaccine. 1991 Nov;9(11):810–816. doi: 10.1016/0264-410x(91)90218-u. [DOI] [PubMed] [Google Scholar]
- Hornick R. B., Greisman S. E., Woodward T. E., DuPont H. L., Dawkins A. T., Snyder M. J. Typhoid fever: pathogenesis and immunologic control. N Engl J Med. 1970 Sep 24;283(13):686–691. doi: 10.1056/NEJM197009242831306. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Kamiya R., Yamaguchi S. Excretion of flagellin by a short-flagella mutant of Salmonella typhimurium. J Bacteriol. 1983 Jan;153(1):506–510. doi: 10.1128/jb.153.1.506-510.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JOHNSON E. M., KRAUSKOPF B., BARON L. S. GENETIC MAPPING OF VI AND SOMATIC ANTIGENIC DETERMINANTS IN SALMONELLA. J Bacteriol. 1965 Aug;90:302–308. doi: 10.1128/jb.90.2.302-308.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K., Charles I., Dougan G., Pickard D., O'Gaora P., Costa G., Ali T., Miller I., Hormaeche C. The role of a stress-response protein in Salmonella typhimurium virulence. Mol Microbiol. 1991 Feb;5(2):401–407. doi: 10.1111/j.1365-2958.1991.tb02122.x. [DOI] [PubMed] [Google Scholar]
- Kantele A., Kantele J. M., Arvilommi H., Mäkelä P. H. Active immunity is seen as a reduction in the cell response to oral live vaccine. Vaccine. 1991 Jun;9(6):428–431. doi: 10.1016/0264-410x(91)90130-x. [DOI] [PubMed] [Google Scholar]
- Kantele A., Mäkelä P. H. Different profiles of the human immune response to primary and secondary immunization with an oral Salmonella typhi Ty21a vaccine. Vaccine. 1991 Jun;9(6):423–427. doi: 10.1016/0264-410x(91)90129-t. [DOI] [PubMed] [Google Scholar]
- Levine M. M., DuPont H. L., Hornick R. B., Snyder M. J., Woodward W., Gilman R. H., Libonati J. P. Attenuated, streptomycin-dependent Salmonella typhi oral vaccine: potential deleterious effects of lyophilization. J Infect Dis. 1976 Apr;133(4):424–429. doi: 10.1093/infdis/133.4.424. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Ferreccio C., Black R. E., Tacket C. O., Germanier R. Progress in vaccines against typhoid fever. Rev Infect Dis. 1989 May-Jun;11 (Suppl 3):S552–S567. doi: 10.1093/clinids/11.supplement_3.s552. [DOI] [PubMed] [Google Scholar]
- Levine M. M., Herrington D., Murphy J. R., Morris J. G., Losonsky G., Tall B., Lindberg A. A., Svenson S., Baqar S., Edwards M. F. Safety, infectivity, immunogenicity, and in vivo stability of two attenuated auxotrophic mutant strains of Salmonella typhi, 541Ty and 543Ty, as live oral vaccines in humans. J Clin Invest. 1987 Mar;79(3):888–902. doi: 10.1172/JCI112899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonsky G. A., Ferreccio C., Kotloff K. L., Kaintuck S., Robbins J. B., Levine M. M. Development and evaluation of an enzyme-linked immunosorbent assay for serum Vi antibodies for detection of chronic Salmonella typhi carriers. J Clin Microbiol. 1987 Dec;25(12):2266–2269. doi: 10.1128/jcm.25.12.2266-2269.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhous W. K., Weatherly N. F., Bowdre J. H., Desjardins R. E. In vitro activities of and mechanisms of resistance to antifol antimalarial drugs. Antimicrob Agents Chemother. 1985 Apr;27(4):525–530. doi: 10.1128/aac.27.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I. A., Chatfield S., Dougan G., Desilva L., Joysey H. S., Hormaeche C. Bacteriophage P22 as a vehicle for transducing cosmid gene banks between smooth strains of Salmonella typhimurium: use in identifying a role for aroD in attenuating virulent Salmonella strains. Mol Gen Genet. 1989 Jan;215(2):312–316. doi: 10.1007/BF00339734. [DOI] [PubMed] [Google Scholar]
- Miller S. I., Kukral A. M., Mekalanos J. J. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5054–5058. doi: 10.1073/pnas.86.13.5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. I., Mekalanos J. J. Constitutive expression of the phoP regulon attenuates Salmonella virulence and survival within macrophages. J Bacteriol. 1990 May;172(5):2485–2490. doi: 10.1128/jb.172.5.2485-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Callaghan D., Maskell D., Liew F. Y., Easmon C. S., Dougan G. Characterization of aromatic- and purine-dependent Salmonella typhimurium: attention, persistence, and ability to induce protective immunity in BALB/c mice. Infect Immun. 1988 Feb;56(2):419–423. doi: 10.1128/iai.56.2.419-423.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigwart D. F., Stocker B. A., Clements J. D. Effect of a purA mutation on efficacy of Salmonella live-vaccine vectors. Infect Immun. 1989 Jun;57(6):1858–1861. doi: 10.1128/iai.57.6.1858-1861.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Hoiseth S. K., Stocker B. A., Habasha F., Johnson E., Merritt F. Aromatic-dependent Salmonella typhimurium as modified live vaccines for calves. Am J Vet Res. 1984 Jan;45(1):59–66. [PubMed] [Google Scholar]
- Smith B. P., Reina-Guerra M., Stocker B. A., Hoiseth S. K., Johnson E. H. Vaccination of calves against Salmonella dublin with aromatic-dependent Salmonella typhimurium. Am J Vet Res. 1984 Sep;45(9):1858–1861. [PubMed] [Google Scholar]
- Stocker B. A. Auxotrophic Salmonella typhi as live vaccine. Vaccine. 1988 Apr;6(2):141–145. doi: 10.1016/s0264-410x(88)80017-3. [DOI] [PubMed] [Google Scholar]
- Stocker B. A., Mäkelä P. H. Genetic determination of bacterial virulence, with special reference to Salmonella. Curr Top Microbiol Immunol. 1986;124:149–172. doi: 10.1007/978-3-642-70986-9_9. [DOI] [PubMed] [Google Scholar]
- Sundström C., Nilsson K. Establishment and characterization of a human histiocytic lymphoma cell line (U-937). Int J Cancer. 1976 May 15;17(5):565–577. doi: 10.1002/ijc.2910170504. [DOI] [PubMed] [Google Scholar]
- Tacket C. O., Forrest B., Morona R., Attridge S. R., LaBrooy J., Tall B. D., Reymann M., Rowley D., Levine M. M. Safety, immunogenicity, and efficacy against cholera challenge in humans of a typhoid-cholera hybrid vaccine derived from Salmonella typhi Ty21a. Infect Immun. 1990 Jun;58(6):1620–1627. doi: 10.1128/iai.58.6.1620-1627.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacket C. O., Losonsky G., Taylor D. N., Baron L. S., Kopecko D., Cryz S., Levine M. M. Lack of immune response to the Vi component of a Vi-positive variant of the Salmonella typhi live oral vaccine strain Ty21a in human studies. J Infect Dis. 1991 Apr;163(4):901–904. doi: 10.1093/infdis/163.4.901. [DOI] [PubMed] [Google Scholar]
- Tully J. G., Gaines S., Tigertt W. D. Studies on infection and immunity in experimental typhoid fever. VI. Response of chimpanzees to endotoxin and the effect of tolerance on resistance to oral challenge. J Infect Dis. 1965 Dec;115(5):445–455. doi: 10.1093/infdis/115.5.445. [DOI] [PubMed] [Google Scholar]
- Van de Verg L., Herrington D. A., Murphy J. R., Wasserman S. S., Formal S. B., Levine M. M. Specific immunoglobulin A-secreting cells in peripheral blood of humans following oral immunization with a bivalent Salmonella typhi-Shigella sonnei vaccine or infection by pathogenic S. sonnei. Infect Immun. 1990 Jun;58(6):2002–2004. doi: 10.1128/iai.58.6.2002-2004.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong K. H., Feeley J. C. Isolation of Vi antigen and a simple method for its measurement. Appl Microbiol. 1972 Oct;24(4):628–633. doi: 10.1128/am.24.4.628-633.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J. Y., Newton S., Judd A., Stocker B., Robinson W. S. Expression of immunogenic epitopes of hepatitis B surface antigen with hybrid flagellin proteins by a vaccine strain of Salmonella. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4726–4730. doi: 10.1073/pnas.86.12.4726. [DOI] [PMC free article] [PubMed] [Google Scholar]


