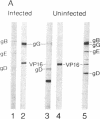
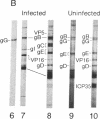
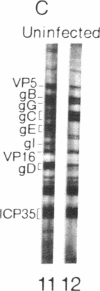
Abstract
Western blot analysis was used to compare the herpes simplex virus (HSV)-2 antibody profiles of 40 infants less than 2 wk of age who had been exposed to maternal genital HSV-2 at birth. 4 mothers were HSV seronegative at delivery and seroconverted to HSV-2 ("primary infection"), 9 had HSV-1 antibodies and seroconverted to HSV-2 ("nonprimary first episode infection"), and 27 were HSV-2 seropositive ("recurrent infection"). Neonatal herpes infections developed in 1 of 4 infants of women with primary infection, in 3 of 9 infants of women with nonprimary first episode infection, and in none of the 27 infants of women with recurrent HSV-2. Antibodies to HSV-2 proteins gG-2, VP5, and ICP35 were detected in 83, 89, and 72% of the 36 uninfected infants, respectively. None of the four infected infants had detectable antibodies to gG-2 and only one (25%) had antibodies to VP5 or ICP35. The more limited profiles of the 13 infants born to mothers with first episodes of HSV-2 were then analyzed separately; these profiles were similar among infected and uninfected infants except for gG-2, which elicits antibodies that are type specific for HSV-2. None of the infected infants versus seven of nine (78%) uninfected infants were gG-2 seropositive. These comparisons suggest that maternal type-specific antibodies may play a role in preventing neonatal infection after exposure to HSV-2.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. L., Militoni J., Lee F., Nahmias A., Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988 Apr;26(4):662–667. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron S., Worthington M. G., Williams J., Gaines J. W. Postexposure serum prophylaxis of neonatal herpes simplex virus infection of mice. Nature. 1976 Jun 10;261(5560):505–506. doi: 10.1038/261505a0. [DOI] [PubMed] [Google Scholar]
- Braun D. K., Roizman B., Pereira L. Characterization of post-translational products of herpes simplex virus gene 35 proteins binding to the surfaces of full capsids but not empty capsids. J Virol. 1984 Jan;49(1):142–153. doi: 10.1128/jvi.49.1.142-153.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown Z. A., Benedetti J., Ashley R., Burchett S., Selke S., Berry S., Vontver L. A., Corey L. Neonatal herpes simplex virus infection in relation to asymptomatic maternal infection at the time of labor. N Engl J Med. 1991 May 2;324(18):1247–1252. doi: 10.1056/NEJM199105023241804. [DOI] [PubMed] [Google Scholar]
- Gold D., Ashley R., Handsfield H. H., Verdon M., Leach L., Mills J., Drew L., Corey L. Immunoblot analysis of the humoral immune response in primary cytomegalovirus infection. J Infect Dis. 1988 Feb;157(2):319–326. doi: 10.1093/infdis/157.2.319. [DOI] [PubMed] [Google Scholar]
- Kohl S., Loo L. S., Greenberg S. B. Protection of newborn mice from a lethal herpes simplex virus infection by human interferon, antibody, and leukocytes. J Immunol. 1982 Mar;128(3):1107–1111. [PubMed] [Google Scholar]
- Kohl S., Loo L. S. Protection of neonatal mice against herpes simplex virus infection: probable in vivo antibody-dependent cellular cytotoxicity. J Immunol. 1982 Jul;129(1):370–376. [PubMed] [Google Scholar]
- Kohl S., Strynadka N. C., Hodges R. S., Pereira L. Analysis of the role of antibody-dependent cellular cytotoxic antibody activity in murine neonatal herpes simplex virus infection with antibodies to synthetic peptides of glycoprotein D and monoclonal antibodies to glycoprotein B. J Clin Invest. 1990 Jul;86(1):273–278. doi: 10.1172/JCI114695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl S. The neonatal human's immune response to herpes simplex virus infection: a critical review. Pediatr Infect Dis J. 1989 Feb;8(2):67–74. [PubMed] [Google Scholar]
- Kohl S., West M. S., Prober C. G., Sullender W. M., Loo L. S., Arvin A. M. Neonatal antibody-dependent cellular cytotoxic antibody levels are associated with the clinical presentation of neonatal herpes simplex virus infection. J Infect Dis. 1989 Nov;160(5):770–776. doi: 10.1093/infdis/160.5.770. [DOI] [PubMed] [Google Scholar]
- Longnecker R., Chatterjee S., Whitley R. J., Roizman B. Identification of a herpes simplex virus 1 glycoprotein gene within a gene cluster dispensable for growth in cell culture. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4303–4307. doi: 10.1073/pnas.84.12.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norrild B., Shore S. L., Nahmias A. J. Herpes simplex virus glycoproteins: participation of individual herpes simplex virus type 1 glycoprotein antigens in immunocytolysis and their correlation with previously identified glycopolypeptides. J Virol. 1979 Dec;32(3):741–748. doi: 10.1128/jvi.32.3.741-748.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson E., Schmidt O. W., Goldstein L. C., Nowinski R. C., Corey L. Typing of clinical herpes simplex virus isolates with mouse monoclonal antibodies to herpes simplex virus types 1 and 2: comparison with type-specific rabbit antisera and restriction endonuclease analysis of viral DNA. J Clin Microbiol. 1983 Jan;17(1):92–96. doi: 10.1128/jcm.17.1.92-96.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prober C. G., Sullender W. M., Yasukawa L. L., Au D. S., Yeager A. S., Arvin A. M. Low risk of herpes simplex virus infections in neonates exposed to the virus at the time of vaginal delivery to mothers with recurrent genital herpes simplex virus infections. N Engl J Med. 1987 Jan 29;316(5):240–244. doi: 10.1056/NEJM198701293160503. [DOI] [PubMed] [Google Scholar]
- Sullender W. M., Miller J. L., Yasukawa L. L., Bradley J. S., Black S. B., Yeager A. S., Arvin A. M. Humoral and cell-mediated immunity in neonates with herpes simplex virus infection. J Infect Dis. 1987 Jan;155(1):28–37. doi: 10.1093/infdis/155.1.28. [DOI] [PubMed] [Google Scholar]
- Sullender W. M., Yasukawa L. L., Schwartz M., Pereira L., Hensleigh P. A., Prober C. G., Arvin A. M. Type-specific antibodies to herpes simplex virus type 2 (HSV-2) glycoprotein G in pregnant women, infants exposed to maternal HSV-2 infection at delivery, and infants with neonatal herpes. J Infect Dis. 1988 Jan;157(1):164–171. doi: 10.1093/infdis/157.1.164. [DOI] [PubMed] [Google Scholar]
- Whitley R. J., Nahmias A. J., Visintine A. M., Fleming C. L., Alford C. A. The natural history of herpes simplex virus infection of mother and newborn. Pediatrics. 1980 Oct;66(4):489–494. [PubMed] [Google Scholar]
- Whitley R., Arvin A., Prober C., Burchett S., Corey L., Powell D., Plotkin S., Starr S., Alford C., Connor J. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med. 1991 Feb 14;324(7):444–449. doi: 10.1056/NEJM199102143240703. [DOI] [PubMed] [Google Scholar]
- Yeager A. S., Arvin A. M. Reasons for the absence of a history of recurrent genital infections in mothers of neonates infected with herpes simplex virus. Pediatrics. 1984 Feb;73(2):188–193. [PubMed] [Google Scholar]
- Yeager A. S., Arvin A. M., Urbani L. J., Kemp J. A., 3rd Relationship of antibody to outcome in neonatal herpes simplex virus infections. Infect Immun. 1980 Aug;29(2):532–538. doi: 10.1128/iai.29.2.532-538.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]