Abstract
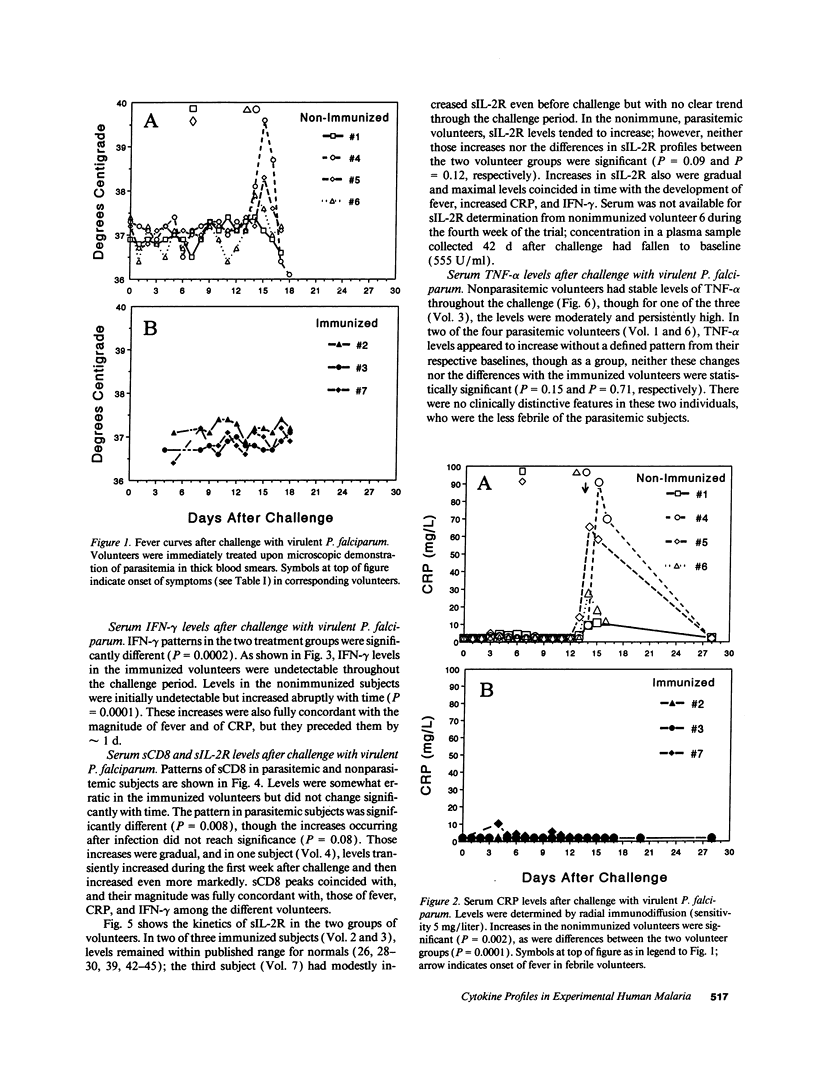
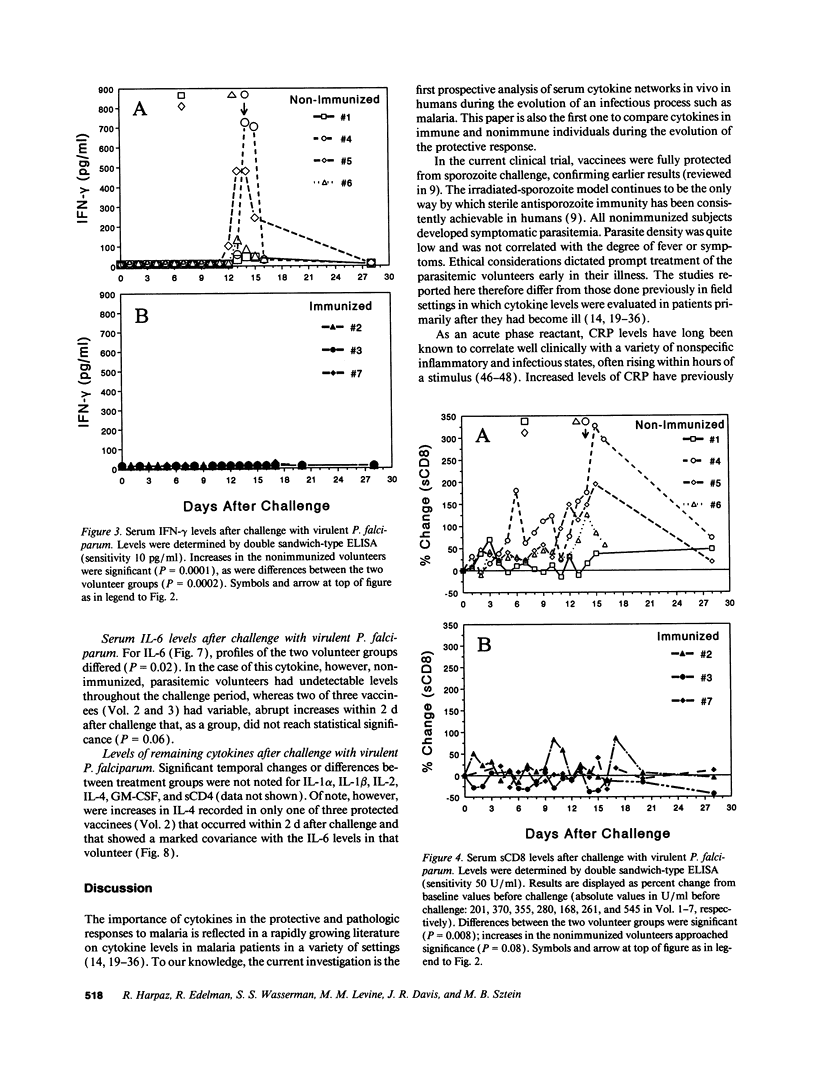
Serum cytokine profiles were evaluated in immunized and nonimmunized human volunteers after challenge with infectious Plasmodium falciparum sporozoites. Three volunteers had been immunized with x-irradiated sporozoites and were fully protected from infection. Four nonimmune volunteers all developed symptomatic infection at which time they were treated. Sera from all volunteers were collected at approximately 20 time points during the 28-d challenge period; levels of IL-1 alpha, IL-1 beta, IL-2, IFN-gamma, tumor necrosis factor-alpha, IL-4, IL-6, granulocyte macrophage-colony-stimulating factor, and soluble CD4, CD8, and IL-2 receptor (sCD4, sCD8, and sIL-2R, respectively) were determined by ELISA. C-reactive protein (CRP) was assayed by radial immunodiffusion. Parasitemic subjects developed increases in CRP and IFN-gamma, with less marked increases in sIL-2R and sCD8; the other cytokines tested did not change. CRP increases were abrupt and occurred at the onset of fever (day 14 after challenge). IFN-gamma increases were also abrupt, preceding those of fever and CRP by one day. Increases in sIL-2R and sCD8 were more gradual. Increases in fever, CRP, IFN-gamma, and sCD8 were concordant in each volunteer. Early IL-6 increases were noted in the protected vaccinees. Thus, after challenge with virulent P. falciparum, unique systemic cytokine profiles were detectable both in immunized, nonparasitemic volunteers and in unvaccinated, parasitemic subjects. The contrasting cytokine profiles in the two groups may relate to mechanisms of protection and immunopathology in experimental human malaria.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aulitzky W. E., Aulitzky W. K., Frick J., Herold M., Gastl G., Tilg H., Berger M., Huber C. Treatment of cancer patients with recombinant interferon-gamma induces release of endogenous tumor necrosis factor-alpha. Immunobiology. 1990 Jun;180(4-5):385–394. doi: 10.1016/s0171-2985(11)80300-1. [DOI] [PubMed] [Google Scholar]
- Bhattacharya S. K., Pillai C. R., Mathur M., Sen P. Effect of chloroquine and some other antimalarials on the immune mechanism in experimental animals. J Pharm Pharmacol. 1984 Apr;36(4):268–269. doi: 10.1111/j.2042-7158.1984.tb04366.x. [DOI] [PubMed] [Google Scholar]
- Bone R. C. The pathogenesis of sepsis. Ann Intern Med. 1991 Sep 15;115(6):457–469. doi: 10.7326/0003-4819-115-6-457. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Herrington D. A., Webster H. K., Clyde D. F., Sztein M. B., Davis J. R., Beier M. S., Edelman R. Urinary neopterin in volunteers experimentally infected with Plasmodium falciparum. Trans R Soc Trop Med Hyg. 1992 Mar-Apr;86(2):134–136. doi: 10.1016/0035-9203(92)90539-o. [DOI] [PubMed] [Google Scholar]
- Brown A. E., Webster H. K., Teja-Isavadharm P., Keeratithakul D. Macrophage activation in falciparum malaria as measured by neopterin and interferon-gamma. Clin Exp Immunol. 1990 Oct;82(1):97–101. doi: 10.1111/j.1365-2249.1990.tb05410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher G. A., Garland T., Ajdukiewicz A. B., Clark I. A. Serum tumor necrosis factor associated with malaria in patients in the Solomon Islands. Trans R Soc Trop Med Hyg. 1990 Sep-Oct;84(5):658–661. doi: 10.1016/0035-9203(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Clark I. A., Hunt N. H., Butcher G. A., Cowden W. B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-gamma or tumor necrosis factor, and its enhancement by butylated hydroxyanisole. J Immunol. 1987 Nov 15;139(10):3493–3496. [PubMed] [Google Scholar]
- Degiannis D., Seibold J. R., Czarnecki M., Raskova J., Raska K., Jr Soluble and cellular markers of immune activation in patients with systemic sclerosis. Clin Immunol Immunopathol. 1990 Aug;56(2):259–270. doi: 10.1016/0090-1229(90)90147-i. [DOI] [PubMed] [Google Scholar]
- Deloron P., Lepers J. P., Coulanges P. Evolution of the levels of soluble interleukin-2 receptors during Plasmodium falciparum and P. vivax malaria. J Clin Microbiol. 1989 Aug;27(8):1887–1889. doi: 10.1128/jcm.27.8.1887-1889.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demetri G. D., Spriggs D. R., Sherman M. L., Arthur K. A., Imamura K., Kufe D. W. A phase I trial of recombinant human tumor necrosis factor and interferon-gamma: effects of combination cytokine administration in vivo. J Clin Oncol. 1989 Oct;7(10):1545–1553. doi: 10.1200/JCO.1989.7.10.1545. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Schofield L., Enea V., Schellekens H., van der Meide P., Collins W. E., Nussenzweig R. S., Nussenzweig V. Inhibition of development of exoerythrocytic forms of malaria parasites by gamma-interferon. Science. 1986 May 16;232(4752):881–884. doi: 10.1126/science.3085218. [DOI] [PubMed] [Google Scholar]
- Fujimoto J., Levy S., Levy R. Spontaneous release of the Leu-2 (T8) molecule from human T cells. J Exp Med. 1983 Sep 1;158(3):752–766. doi: 10.1084/jem.158.3.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Bieler G., Pointaire P., De Kossodo S., Tacchini-Cotier F., Vassalli P., Piguet P. F., Lambert P. H. Significance of cytokine production and adhesion molecules in malarial immunopathology. Immunol Lett. 1990 Aug;25(1-3):189–194. doi: 10.1016/0165-2478(90)90113-5. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Frei K., Piguet P. F., Fontana A., Heremans H., Billiau A., Vassalli P., Lambert P. H. Interleukin 6 production in experimental cerebral malaria: modulation by anticytokine antibodies and possible role in hypergammaglobulinemia. J Exp Med. 1990 Nov 1;172(5):1505–1508. doi: 10.1084/jem.172.5.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Heremans H., Piguet P. F., Pointaire P., Lambert P. H., Billiau A., Vassalli P. Monoclonal antibody against interferon gamma can prevent experimental cerebral malaria and its associated overproduction of tumor necrosis factor. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5572–5574. doi: 10.1073/pnas.86.14.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grau G. E., Piguet P. F., Vassalli P., Lambert P. H. Tumor-necrosis factor and other cytokines in cerebral malaria: experimental and clinical data. Immunol Rev. 1989 Dec;112:49–70. doi: 10.1111/j.1600-065x.1989.tb00552.x. [DOI] [PubMed] [Google Scholar]
- Grau G. E., Taylor T. E., Molyneux M. E., Wirima J. J., Vassalli P., Hommel M., Lambert P. H. Tumor necrosis factor and disease severity in children with falciparum malaria. N Engl J Med. 1989 Jun 15;320(24):1586–1591. doi: 10.1056/NEJM198906153202404. [DOI] [PubMed] [Google Scholar]
- Grifo J. A., Jeremias J., Ledger W. J., Witkin S. S. Interferon-gamma in the diagnosis and pathogenesis of pelvic inflammatory disease. Am J Obstet Gynecol. 1989 Jan;160(1):26–31. doi: 10.1016/0002-9378(89)90080-x. [DOI] [PubMed] [Google Scholar]
- Hemmer C. J., Kern P., Holst F. G., Radtke K. P., Egbring R., Bierhaus A., Nawroth P. P., Dietrich M. Activation of the host response in human Plasmodium falciparum malaria: relation of parasitemia to tumor necrosis factor/cachectin, thrombin-antithrombin III, and protein C levels. Am J Med. 1991 Jul;91(1):37–44. doi: 10.1016/0002-9343(91)90071-5. [DOI] [PubMed] [Google Scholar]
- Herrington D. A., Clyde D. F., Murphy J. R., Baqar S., Levine M. M., do Rosario V., Hollingdale M. R. A model for Plasmodium falciparum sporozoite challenge and very early therapy of parasitaemia for efficacy studies of sporozoite vaccines. Trop Geogr Med. 1988 Apr;40(2):124–127. [PubMed] [Google Scholar]
- Herrington D., Davis J., Nardin E., Beier M., Cortese J., Eddy H., Losonsky G., Hollingdale M., Sztein M., Levine M. Successful immunization of humans with irradiated malaria sporozoites: humoral and cellular responses of the protected individuals. Am J Trop Med Hyg. 1991 Nov;45(5):539–547. doi: 10.4269/ajtmh.1991.45.539. [DOI] [PubMed] [Google Scholar]
- Ho M., Webster H. K., Green B., Looareesuwan S., Kongchareon S., White N. J. Defective production of and response to IL-2 in acute human falciparum malaria. J Immunol. 1988 Oct 15;141(8):2755–2759. [PubMed] [Google Scholar]
- Ho M., Webster H. K., Looareesuwan S., Supanaranond W., Phillips R. E., Chanthavanich P., Warrell D. A. Antigen-specific immunosuppression in human malaria due to Plasmodium falciparum. J Infect Dis. 1986 Apr;153(4):763–771. doi: 10.1093/infdis/153.4.763. [DOI] [PubMed] [Google Scholar]
- Jodal U., Hanson L. A. Sequential determination of C-reactive protein in acute childhood pyelonephritis. Acta Paediatr Scand. 1976 May;65(3):319–322. doi: 10.1111/j.1651-2227.1976.tb04892.x. [DOI] [PubMed] [Google Scholar]
- Josimovic-Alasevic O., Feldmeier H., Zwingenberger K., Harms G., Hahn H., Shrisuphanunt M., Diamantstein T. Interleukin 2 receptor in patients with localized and systemic parasitic diseases. Clin Exp Immunol. 1988 May;72(2):249–254. [PMC free article] [PubMed] [Google Scholar]
- Keller R. J., Jackson R. A. Developmental regulation of serum interleukin-2 receptor concentrations: attenuation of the childhood peak in patients at risk for developing or having recently developed type I diabetes mellitus. J Pediatr. 1989 May;114(5):816–819. doi: 10.1016/s0022-3476(89)80146-5. [DOI] [PubMed] [Google Scholar]
- Kern P., Hemmer C. J., Van Damme J., Gruss H. J., Dietrich M. Elevated tumor necrosis factor alpha and interleukin-6 serum levels as markers for complicated Plasmodium falciparum malaria. Am J Med. 1989 Aug;87(2):139–143. doi: 10.1016/s0002-9343(89)80688-6. [DOI] [PubMed] [Google Scholar]
- Koj A. The role of interleukin-6 as the hepatocyte stimulating factor in the network of inflammatory cytokines. Ann N Y Acad Sci. 1989;557:1–8. doi: 10.1111/j.1749-6632.1989.tb23994.x. [DOI] [PubMed] [Google Scholar]
- Kremsner P. G., Bienzle U. Soluble CD8 antigen in Plasmodium falciparum malaria. J Infect Dis. 1989 Aug;160(2):357–358. doi: 10.1093/infdis/160.2.357. [DOI] [PubMed] [Google Scholar]
- Kremsner P. G., Feldmeier H., Zotter G. M., Jansen-Rosseck R., Graninger W., Rocha R. M., Bienzle U. Immunological alterations in uncomplicated Plasmodium falciparum malaria. Relationship between parasitaemia and indicators of macrophage activation. Acta Trop. 1989 Oct;46(5-6):351–359. doi: 10.1016/0001-706x(89)90047-8. [DOI] [PubMed] [Google Scholar]
- Kremsner P. G., Zotter G. M., Feldmeier H., Graninger W., Rocha R. M., Jansen-Rosseck R., Bienzle U. Immune response in patients during and after Plasmodium falciparum infection. J Infect Dis. 1990 May;161(5):1025–1028. doi: 10.1093/infdis/161.5.1025. [DOI] [PubMed] [Google Scholar]
- Kumaratilake L. M., Ferrante A., Rzepczyk C. The role of T lymphocytes in immunity to Plasmodium falciparum. Enhancement of neutrophil-mediated parasite killing by lymphotoxin and IFN-gamma: comparisons with tumor necrosis factor effects. J Immunol. 1991 Jan 15;146(2):762–767. [PubMed] [Google Scholar]
- Kushner I., Broder M. L., Karp D. Control of the acute phase response. Serum C-reactive protein kinetics after acute myocardial infarction. J Clin Invest. 1978 Feb;61(2):235–242. doi: 10.1172/JCI108932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Cannon J. G., Manogue K. R., Cerami A., Dinarello C. A., Greenwood B. M. Tumour necrosis factor production in Falciparum malaria and its association with schizont rupture. Clin Exp Immunol. 1989 Sep;77(3):361–366. [PMC free article] [PubMed] [Google Scholar]
- Kwiatkowski D., Hill A. V., Sambou I., Twumasi P., Castracane J., Manogue K. R., Cerami A., Brewster D. R., Greenwood B. M. TNF concentration in fatal cerebral, non-fatal cerebral, and uncomplicated Plasmodium falciparum malaria. Lancet. 1990 Nov 17;336(8725):1201–1204. doi: 10.1016/0140-6736(90)92827-5. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. Tumour necrosis factor, fever and fatality in falciparum malaria. Immunol Lett. 1990 Aug;25(1-3):213–216. doi: 10.1016/0165-2478(90)90117-9. [DOI] [PubMed] [Google Scholar]
- Meding S. J., Cheng S. C., Simon-Haarhaus B., Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990 Nov;58(11):3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellouk S., Maheshwari R. K., Rhodes-Feuillette A., Beaudoin R. L., Berbiguier N., Matile H., Miltgen F., Landau I., Pied S., Chigot J. P. Inhibitory activity of interferons and interleukin 1 on the development of Plasmodium falciparum in human hepatocyte cultures. J Immunol. 1987 Dec 15;139(12):4192–4195. [PubMed] [Google Scholar]
- Molyneux M. E., Taylor T. E., Wirima J. J., Grau G. E. Tumour necrosis factor, interleukin-6, and malaria. Lancet. 1991 May 4;337(8749):1098–1098. doi: 10.1016/0140-6736(91)91745-g. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Naik P., Voller A. Serum C-reactive protein levels and falciparum malaria. Trans R Soc Trop Med Hyg. 1984;78(6):812–813. doi: 10.1016/0035-9203(84)90027-0. [DOI] [PubMed] [Google Scholar]
- Naotunne T. S., Karunaweera N. D., Del Giudice G., Kularatne M. U., Grau G. E., Carter R., Mendis K. N. Cytokines kill malaria parasites during infection crisis: extracellular complementary factors are essential. J Exp Med. 1991 Mar 1;173(3):523–529. doi: 10.1084/jem.173.3.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen-Dinh P., Greenberg A. E. Increased levels of released interleukin-2 receptors in Plasmodium falciparum malaria. J Infect Dis. 1988 Dec;158(6):1403–1404. doi: 10.1093/infdis/158.6.1403. [DOI] [PubMed] [Google Scholar]
- Norment A. M., Lonberg N., Lacy E., Littman D. R. Alternatively spliced mRNA encodes a secreted form of human CD8 alpha. Characterization of the human CD8 alpha gene. J Immunol. 1989 May 1;142(9):3312–3319. [PubMed] [Google Scholar]
- Nussenzweig V., Nussenzweig R. S. Rationale for the development of an engineered sporozoite malaria vaccine. Adv Immunol. 1989;45:283–334. doi: 10.1016/s0065-2776(08)60695-1. [DOI] [PubMed] [Google Scholar]
- Nussler A., Pied S., Pontet M., Miltgen F., Renia L., Gentilini M., Mazier D. Inflammatory status and preerythrocytic stages of malaria: role of the C-reactive protein. Exp Parasitol. 1991 Jan;72(1):1–7. doi: 10.1016/0014-4894(91)90114-c. [DOI] [PubMed] [Google Scholar]
- Nüssler A., Drapier J. C., Rénia L., Pied S., Miltgen F., Gentilini M., Mazier D. L-arginine-dependent destruction of intrahepatic malaria parasites in response to tumor necrosis factor and/or interleukin 6 stimulation. Eur J Immunol. 1991 Jan;21(1):227–230. doi: 10.1002/eji.1830210134. [DOI] [PubMed] [Google Scholar]
- Orson F. M., Saadeh C. K., Lewis D. E., Nelson D. L. Interleukin 2 receptor expression by T cells in human aging. Cell Immunol. 1989 Dec;124(2):278–291. doi: 10.1016/0008-8749(89)90131-7. [DOI] [PubMed] [Google Scholar]
- Pepys M. B., Baltz M. L. Acute phase proteins with special reference to C-reactive protein and related proteins (pentaxins) and serum amyloid A protein. Adv Immunol. 1983;34:141–212. doi: 10.1016/s0065-2776(08)60379-x. [DOI] [PubMed] [Google Scholar]
- Peyron F., Vuillez J. P., Barbe G., Boudin C., Picot S., Ambroise-Thomas P. Plasma levels of tumor necrosis factor during a longitudinal survey in an endemic area of malaria. Acta Trop. 1990 Jan;47(1):47–51. doi: 10.1016/0001-706x(90)90006-l. [DOI] [PubMed] [Google Scholar]
- Picot S., Peyron F., Vuillez J. P., Polack B., Ambroise-Thomas P. Chloroquine inhibits tumor necrosis factor production by human macrophages in vitro. J Infect Dis. 1991 Oct;164(4):830–830. doi: 10.1093/infdis/164.4.830. [DOI] [PubMed] [Google Scholar]
- Pied S., Nussler A., Pontent M., Miltgen F., Matile H., Lambert P. H., Mazier D. C-reactive protein protects against preerythrocytic stages of malaria. Infect Immun. 1989 Jan;57(1):278–282. doi: 10.1128/iai.57.1.278-282.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes-Feuillette A., Bellosguardo M., Druilhe P., Ballet J. J., Chousterman S., Canivet M., Périès J. The interferon compartment of the immune response in human malaria: II. Presence of serum-interferon gamma following the acute attack. J Interferon Res. 1985 Winter;5(1):169–178. doi: 10.1089/jir.1985.5.169. [DOI] [PubMed] [Google Scholar]
- Rhodes-Feuillette A., Jaureguiberry G., Ballet J. J., Andrieu B., Druilhe P., Le Bras J., Galibert F., Périès J. The interferon compartment of the immune response in human malaria: I. Interferon inducers in Plasmodium falciparum cultures. J Interferon Res. 1985 Winter;5(1):159–168. doi: 10.1089/jir.1985.5.159. [DOI] [PubMed] [Google Scholar]
- Riley E. M., Jobe O., Whittle H. C. CD8+ T cells inhibit Plasmodium falciparum-induced lymphoproliferation and gamma interferon production in cell preparations from some malaria-immune individuals. Infect Immun. 1989 Apr;57(4):1281–1284. doi: 10.1128/iai.57.4.1281-1284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: doubt no more. Immunol Today. 1991 Aug;12(8):256–257. doi: 10.1016/0167-5699(91)90120-I. [DOI] [PubMed] [Google Scholar]
- Rubin L. A., Kurman C. C., Fritz M. E., Biddison W. E., Boutin B., Yarchoan R., Nelson D. L. Soluble interleukin 2 receptors are released from activated human lymphoid cells in vitro. J Immunol. 1985 Nov;135(5):3172–3177. [PubMed] [Google Scholar]
- Ruggiero V., Tavernier J., Fiers W., Baglioni C. Induction of the synthesis of tumor necrosis factor receptors by interferon-gamma. J Immunol. 1986 Apr 1;136(7):2445–2450. [PubMed] [Google Scholar]
- Shaffer N., Grau G. E., Hedberg K., Davachi F., Lyamba B., Hightower A. W., Breman J. G., Phuc N. D. Tumor necrosis factor and severe malaria. J Infect Dis. 1991 Jan;163(1):96–101. doi: 10.1093/infdis/163.1.96. [DOI] [PubMed] [Google Scholar]
- Shear H. L., Srinivasan R., Nolan T., Ng C. Role of IFN-gamma in lethal and nonlethal malaria in susceptible and resistant murine hosts. J Immunol. 1989 Sep 15;143(6):2038–2044. [PubMed] [Google Scholar]
- Slade S. J., Langhorne J. Production of interferon-gamma during infection of mice with Plasmodium chabaudi chabaudi. Immunobiology. 1989 Oct;179(4-5):353–365. doi: 10.1016/S0171-2985(89)80041-5. [DOI] [PubMed] [Google Scholar]
- Stevenson M. M., Tam M. F., Nowotarski M. Role of interferon-gamma and tumor necrosis factor in host resistance to Plasmodium chabaudi AS. Immunol Lett. 1990 Aug;25(1-3):115–121. doi: 10.1016/0165-2478(90)90101-u. [DOI] [PubMed] [Google Scholar]
- Teodorczyk-Injeyan J. A., Sparkes B. G., Mills G. B., Falk R. E., Peters W. J. Increase of serum interleukin 2 receptor level in thermally injured patients. Clin Immunol Immunopathol. 1989 May;51(2):205–215. doi: 10.1016/0090-1229(89)90020-2. [DOI] [PubMed] [Google Scholar]
- Tomkinson B. E., Brown M. C., Ip S. H., Carrabis S., Sullivan J. L. Soluble CD8 during T cell activation. J Immunol. 1989 Apr 1;142(7):2230–2236. [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidanz W. P., Long C. A. The role of T cells in immunity to malaria. Prog Allergy. 1988;41:215–252. [PubMed] [Google Scholar]


