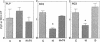Abstract
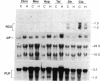
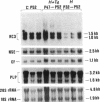
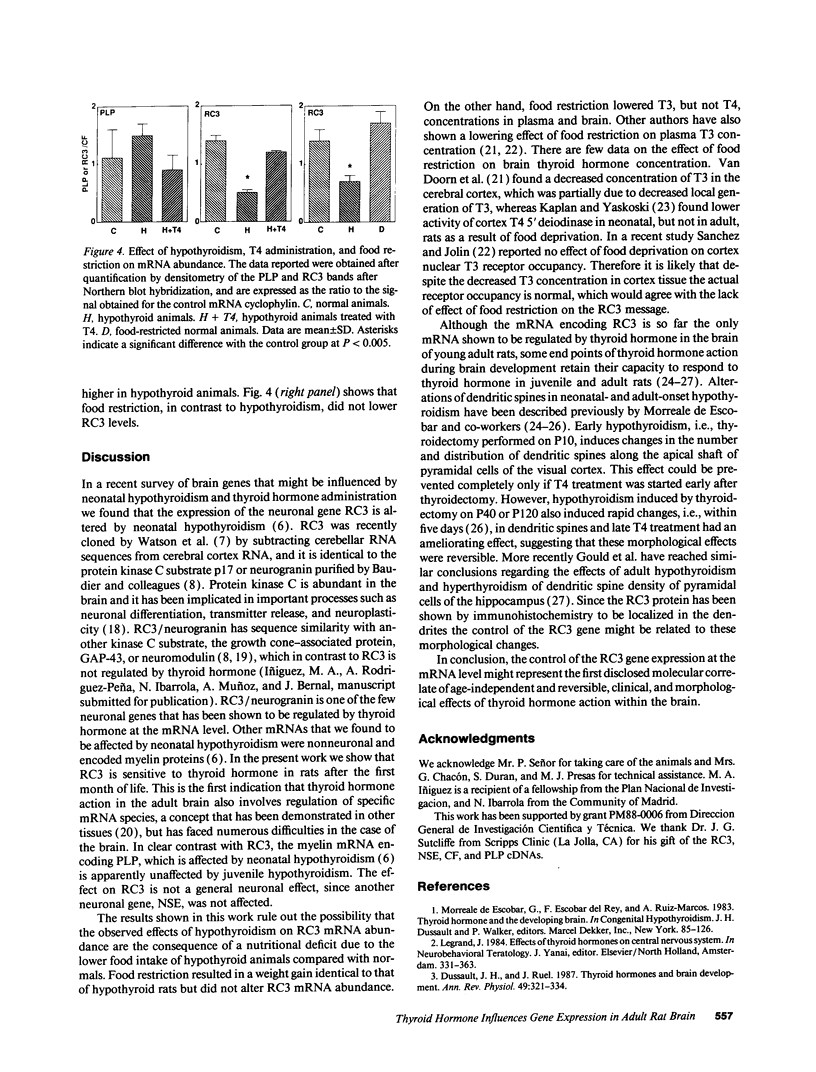
The mammalian brain is considered to be poorly responsive to thyroid hormone after the so called "critical periods" of brain development, which occur in the rat before postnatal days 15-20. In a previous work (Muñoz, A., A. Rodriguez-Peña, A. Perez-Castillo, B. Ferreiro, J.G. Sutcliffe, and J. Bernal. 1991. Mol. Endocrinol. 5:273-280) we have identified one neuronal gene, RC3, whose expression is influenced by early neonatal hypothyroidism and thyroid hormone treatment. In the present work we show that adult-onset hypothyroidism leads to a reversible decrease of RC3 mRNA. Rats thyroidectomized on postnatal day 40 and killed three months later showed a decreased RC3 mRNA concentration in the cerebral cortex and striatum. The same effect was observed in animals made hypothyroid on postnatal day 32 and killed on postnatal day 52. RC3 expression was normal when hypothyroid animals were treated with T4 five days before being killed. In contrast, the mRNA encoding myelin proteolipid protein showed no changes in either experimental situation. RC3 mRNA levels were not affected by food restriction demonstrating that the effect of hypothyroidism was not related to the lack of weight gain. The control of RC3 mRNA is so far the only molecular event known to be regulated by thyroid hormone once the critical periods of brain development are over and could represent a molecular correlate for the age-independent, reversible alterations induced by hypothyroidism in the adult brain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baudier J., Bronner C., Kligman D., Cole R. D. Protein kinase C substrates from bovine brain. Purification and characterization of neuromodulin, a neuron-specific calmodulin-binding protein. J Biol Chem. 1989 Jan 25;264(3):1824–1828. [PubMed] [Google Scholar]
- Baudier J., Deloulme J. C., Van Dorsselaer A., Black D., Matthes H. W. Purification and characterization of a brain-specific protein kinase C substrate, neurogranin (p17). Identification of a consensus amino acid sequence between neurogranin and neuromodulin (GAP43) that corresponds to the protein kinase C phosphorylation site and the calmodulin-binding domain. J Biol Chem. 1991 Jan 5;266(1):229–237. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Danielson P. E., Forss-Petter S., Brow M. A., Calavetta L., Douglass J., Milner R. J., Sutcliffe J. G. p1B15: a cDNA clone of the rat mRNA encoding cyclophilin. DNA. 1988 May;7(4):261–267. doi: 10.1089/dna.1988.7.261. [DOI] [PubMed] [Google Scholar]
- Forss-Petter S., Danielson P., Sutcliffe J. G. Neuron-specific enolase: complete structure of rat mRNA, multiple transcriptional start sites, and evidence suggesting post-transcriptional control. J Neurosci Res. 1986;16(1):141–156. doi: 10.1002/jnr.490160114. [DOI] [PubMed] [Google Scholar]
- Glass C. K., Holloway J. M. Regulation of gene expression by the thyroid hormone receptor. Biochim Biophys Acta. 1990 Dec 11;1032(2-3):157–176. doi: 10.1016/0304-419x(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Gould E., Allan M. D., McEwen B. S. Dendritic spine density of adult hippocampal pyramidal cells is sensitive to thyroid hormone. Brain Res. 1990 Aug 20;525(2):327–329. doi: 10.1016/0006-8993(90)90884-e. [DOI] [PubMed] [Google Scholar]
- Herrin D. L., Schmidt G. W. Rapid, reversible staining of northern blots prior to hybridization. Biotechniques. 1988 Mar;6(3):196-7, 199-200. [PubMed] [Google Scholar]
- Kaplan M. M., Yaskoski K. A. Effects of congenital hypothyroidism and partial and complete food deprivation on phenolic and tyrosyl ring iodothyronine deiodination in rat brain. Endocrinology. 1982 Mar;110(3):761–767. doi: 10.1210/endo-110-3-761. [DOI] [PubMed] [Google Scholar]
- Milner R. J., Lai C., Nave K. A., Lenoir D., Ogata J., Sutcliffe J. G. Nucleotide sequences of two mRNAs for rat brain myelin proteolipid protein. Cell. 1985 Oct;42(3):931–939. doi: 10.1016/0092-8674(85)90289-2. [DOI] [PubMed] [Google Scholar]
- Muñoz A., Rodriguez-Peña A., Perez-Castillo A., Ferreiro B., Sutcliffe J. G., Bernal J. Effects of neonatal hypothyroidism on rat brain gene expression. Mol Endocrinol. 1991 Feb;5(2):273–280. doi: 10.1210/mend-5-2-273. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Obregon M. J., Pascual A., de Escobar G. M., Escobar del Rey F. Pituitary and plasma thyrotropin, thyroxine, and triiodothyronine after hyperthyroidism. Endocrinology. 1979 May;104(5):1467–1473. doi: 10.1210/endo-104-5-1467. [DOI] [PubMed] [Google Scholar]
- Ruiz de Oña C., Morreale de Escobar G., Calvo R., Escobar del Rey F., Obregón M. J. Thyroid hormones and 5'-deiodinase in the rat fetus late in gestation: effects of maternal hypothyroidism. Endocrinology. 1991 Jan;128(1):422–432. doi: 10.1210/endo-128-1-422. [DOI] [PubMed] [Google Scholar]
- Ruiz-Marcos A., Cartagena Abella P., García García A., Escobar del Rey F., Morreale de Escobar G. Rapid effects of adult-onset hypothyroidism on dendritic spines of pyramidal cells of the rat cerebral cortex. Exp Brain Res. 1988;73(3):583–588. doi: 10.1007/BF00406617. [DOI] [PubMed] [Google Scholar]
- Ruiz-Marcos A., Sanchez-Toscano F., Obregon M. J., Escobar del Rey F., Morreale de Escobar G. Thyroxine treatment and recovery of hypothyroidism-induced pyramidal cell damage. Brain Res. 1982 May 13;239(2):559–574. doi: 10.1016/0006-8993(82)90530-3. [DOI] [PubMed] [Google Scholar]
- Ruiz-Marcos A., Sánchez-Toscano F., Escobar del Rey F., Morreale de Escobar G. Reversible morphological alterations of cortical neurons in juvenile and adult hypothyroidism in the rat. Brain Res. 1980 Mar 3;185(1):91–102. doi: 10.1016/0006-8993(80)90674-5. [DOI] [PubMed] [Google Scholar]
- Sanchez B., Jolin T. Triiodothyronine-receptor complex in rat brain: effects of thyroidectomy, fasting, food restriction, and diabetes. Endocrinology. 1991 Jul;129(1):361–367. doi: 10.1210/endo-129-1-361. [DOI] [PubMed] [Google Scholar]
- Watson J. B., Battenberg E. F., Wong K. K., Bloom F. E., Sutcliffe J. G. Subtractive cDNA cloning of RC3, a rodent cortex-enriched mRNA encoding a novel 78 residue protein. J Neurosci Res. 1990 Aug;26(4):397–408. doi: 10.1002/jnr.490260402. [DOI] [PubMed] [Google Scholar]
- van Doorn J., van der Heide D., Roelfsema F. The influence of partial food deprivation on the quantity and source of triiodothyronine in several tissues of athyreotic thyroxine-maintained rats. Endocrinology. 1984 Aug;115(2):705–711. doi: 10.1210/endo-115-2-705. [DOI] [PubMed] [Google Scholar]