Abstract
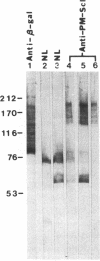
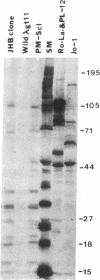
Anti-PM-Scl antibodies are associated with polymyositis-scleroderma overlap or either disease alone. Among sera from 39 patients with anti-PM-Scl, 23 recognized the 100-kD band in immunoblot against HeLa cell extract, 16 of which also stained the 70-kD band. A human thymocyte lambda gt11 cDNA expression library was screened with anti-PM-Scl serum, and two clones were identified whose products reacted with 33 and 37 of 39 anti-PM-Scl sera, respectively, but none of 26 negative control sera. Affinity-purified antibody reacting specifically with plaques of the clone stained the 100-kD band on immunoblot, reacted with nucleoli of HEp-2 cells, and immunoprecipitated the PM-Scl protein complex. Partial sequences of both inserts were identical. One insert was fully sequenced, and additional 5' and 3' sequence was obtained using a gene-specific primer to form a cDNA with HeLa cell RNA as template followed by PCR. The complete nucleotide sequence included 2,739-bp coding for a predicted full-length protein of 98,088 D. There was no homology with the PM-Scl 75-kD protein and no significant homology with other proteins. A mixed-charge cluster was identified, with 22 charged amino acids of 37. In conclusion, the full-length cDNA sequence was determined coding for the PM-Scl 100-kD protein, the most commonly antigenic protein of the PM-Scl complex.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alderuccio F., Chan E. K., Tan E. M. Molecular characterization of an autoantigen of PM-Scl in the polymyositis/scleroderma overlap syndrome: a unique and complete human cDNA encoding an apparent 75-kD acidic protein of the nucleolar complex. J Exp Med. 1991 Apr 1;173(4):941–952. doi: 10.1084/jem.173.4.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein R. M., Bunn C. C., Hughes G. R., Francoeur A. M., Mathews M. B. Cellular protein and RNA antigens in autoimmune disease. Mol Biol Med. 1984 Apr;2(2):105–120. [PubMed] [Google Scholar]
- Brendel V., Dohlman J., Blaisdell B. E., Karlin S. Very long charge runs in systemic lupus erythematosus-associated autoantigens. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1536–1540. doi: 10.1073/pnas.88.4.1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Frohman M. A., Dush M. K., Martin G. R. Rapid production of full-length cDNAs from rare transcripts: amplification using a single gene-specific oligonucleotide primer. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8998–9002. doi: 10.1073/pnas.85.23.8998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelpi C., Algueró A., Angeles Martinez M., Vidal S., Juarez C., Rodriguez-Sanchez J. L. Identification of protein components reactive with anti-PM/Scl autoantibodies. Clin Exp Immunol. 1990 Jul;81(1):59–64. doi: 10.1111/j.1365-2249.1990.tb05291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genth E., Mierau R., Genetzky P., von Mühlen C. A., Kaufmann S., von Wilmowsky H., Meurer M., Krieg T., Pollmann H. J., Hartl P. W. Immunogenetic associations of scleroderma-related antinuclear antibodies. Arthritis Rheum. 1990 May;33(5):657–665. doi: 10.1002/art.1780330508. [DOI] [PubMed] [Google Scholar]
- Itoh K., Itoh Y., Frank M. B. Protein heterogeneity in the human Ro/SSA ribonucleoproteins. The 52- and 60-kD Ro/SSA autoantigens are encoded by separate genes. J Clin Invest. 1991 Jan;87(1):177–186. doi: 10.1172/JCI114968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh E. Y., Elliott J. F., Cwirla S., Lanier L. L., Davis M. M. Polymerase chain reaction with single-sided specificity: analysis of T cell receptor delta chain. Science. 1989 Jan 13;243(4888):217–220. doi: 10.1126/science.2463672. [DOI] [PubMed] [Google Scholar]
- Reichlin M., Maddison P. J., Targoff I., Bunch T., Arnett F., Sharp G., Treadwell E., Tan E. M. Antibodies to a nuclear/nucleolar antigen in patients with polymyositis overlap syndromes. J Clin Immunol. 1984 Jan;4(1):40–44. doi: 10.1007/BF00915286. [DOI] [PubMed] [Google Scholar]
- Reimer G., Scheer U., Peters J. M., Tan E. M. Immunolocalization and partial characterization of a nucleolar autoantigen (PM-Scl) associated with polymyositis/scleroderma overlap syndromes. J Immunol. 1986 Dec 15;137(12):3802–3808. [PubMed] [Google Scholar]
- Reimer G., Steen V. D., Penning C. A., Medsger T. A., Jr, Tan E. M. Correlates between autoantibodies to nucleolar antigens and clinical features in patients with systemic sclerosis (scleroderma). Arthritis Rheum. 1988 Apr;31(4):525–532. doi: 10.1002/art.1780310409. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tabor S., Richardson C. C. DNA sequence analysis with a modified bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1987 Jul;84(14):4767–4771. doi: 10.1073/pnas.84.14.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoff I. N., Arnett F. C., Berman L., O'Brien C., Reichlin M. Anti-KJ: a new antibody associated with the syndrome of polymyositis and interstitial lung disease. J Clin Invest. 1989 Jul;84(1):162–172. doi: 10.1172/JCI114136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targoff I. N. Autoantibodies to aminoacyl-transfer RNA synthetases for isoleucine and glycine. Two additional synthetases are antigenic in myositis. J Immunol. 1990 Mar 1;144(5):1737–1743. [PubMed] [Google Scholar]
- Targoff I. N., Reichlin M. Nucleolar localization of the PM-Scl antigen. Arthritis Rheum. 1985 Feb;28(2):226–230. doi: 10.1002/art.1780280221. [DOI] [PubMed] [Google Scholar]
- Treadwell E. L., Alspaugh M. A., Wolfe J. F., Sharp G. C. Clinical relevance of PM-1 antibody and physiochemical characterization of PM-1 antigen. J Rheumatol. 1984 Oct;11(5):658–662. [PubMed] [Google Scholar]
- Wickens M. How the messenger got its tail: addition of poly(A) in the nucleus. Trends Biochem Sci. 1990 Jul;15(7):277–281. doi: 10.1016/0968-0004(90)90054-f. [DOI] [PubMed] [Google Scholar]
- Wolfe J. F., Adelstein E., Sharp G. C. Antinuclear antibody with distinct specificity for polymyositis. J Clin Invest. 1977 Jan;59(1):176–178. doi: 10.1172/JCI108616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]