Abstract
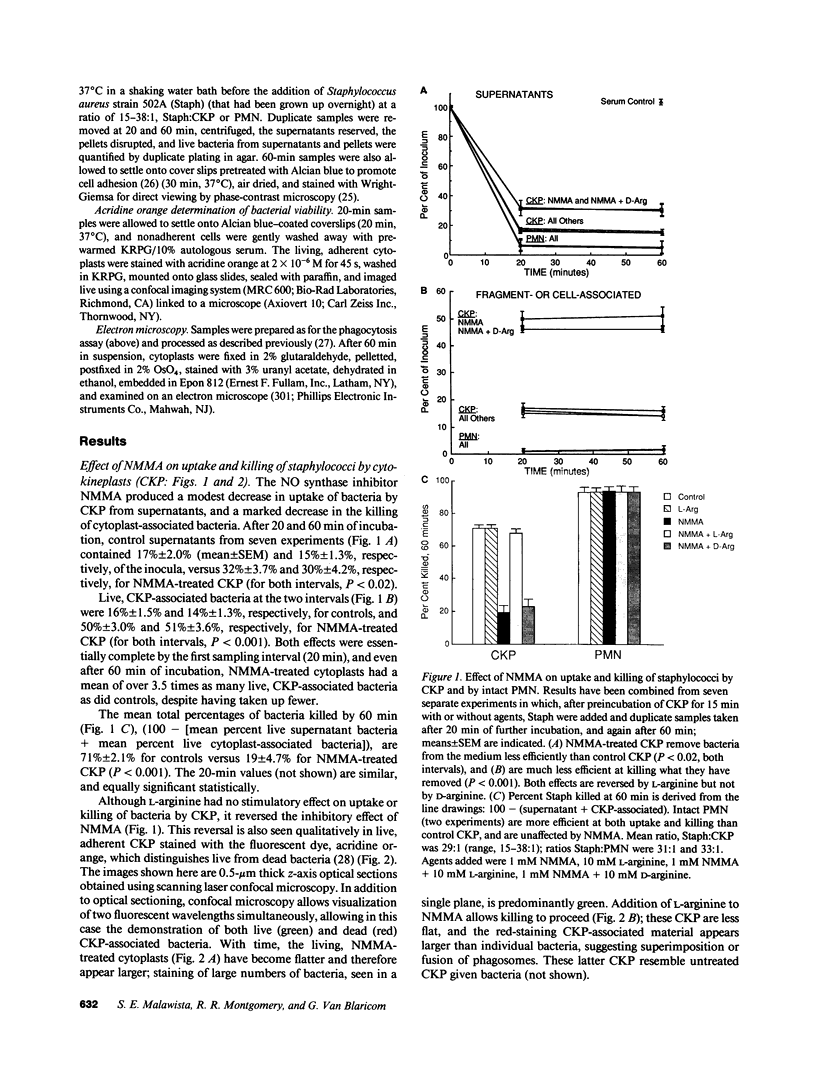
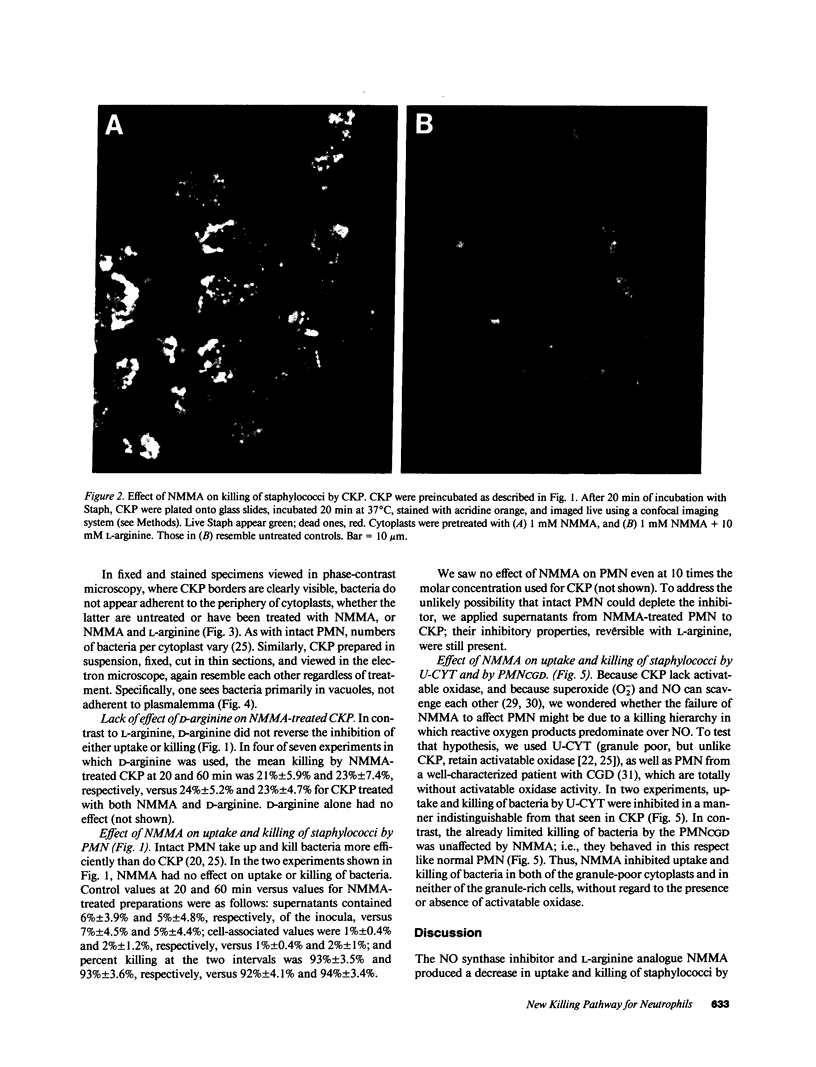
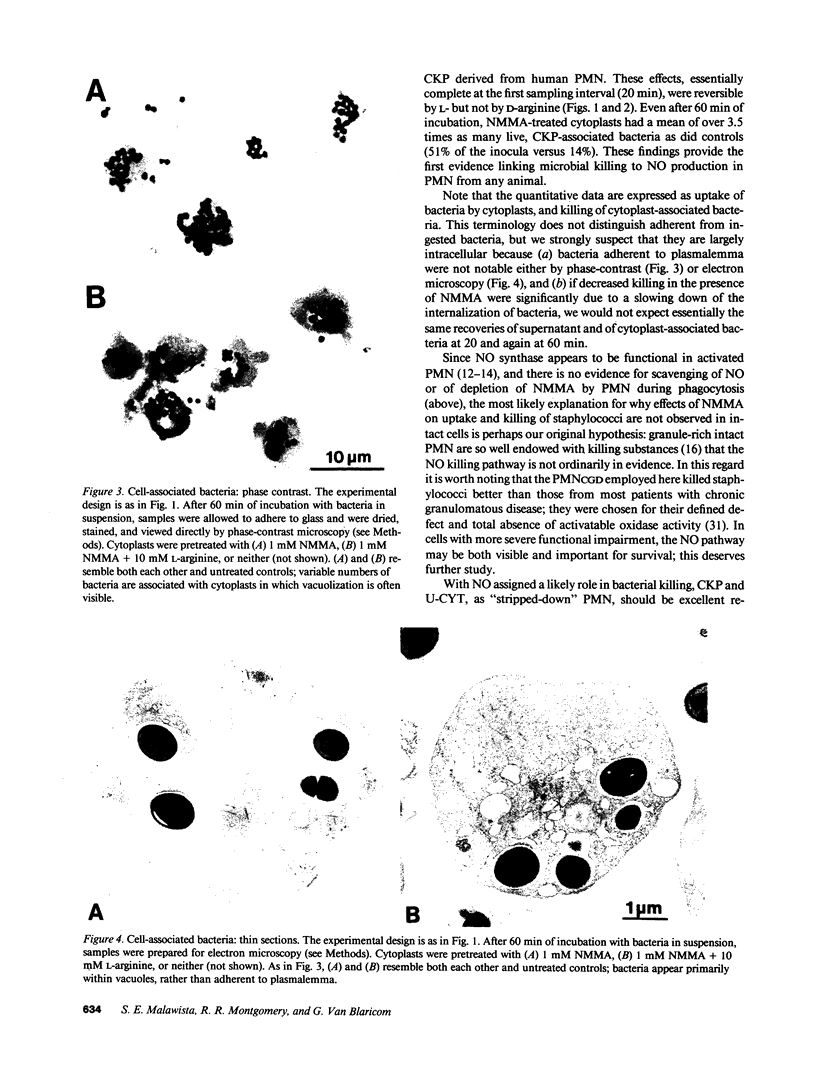
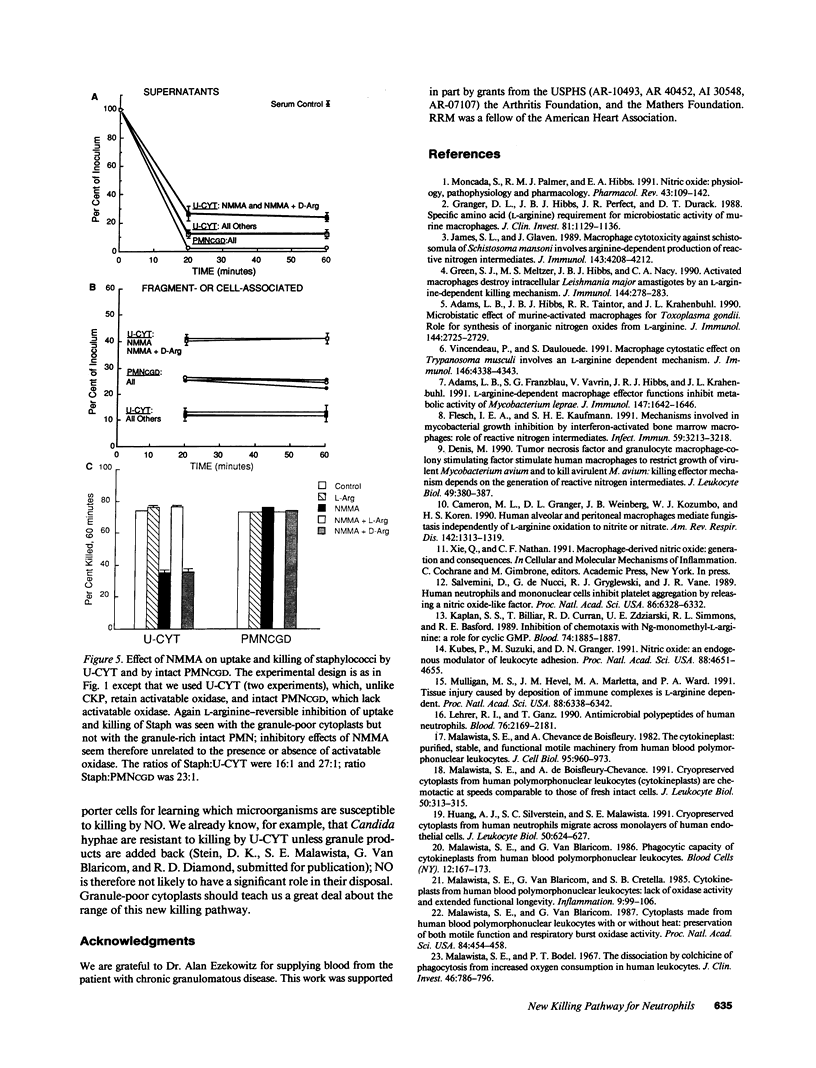
In anucleate, granule-poor, motile fragments from human blood neutrophils (cytokineplasts; CKP), the nitric oxide synthase inhibitor N omega-monomethyl-L-arginine (NMMA) produced a modest decrease in uptake of staphylococci from supernatants (P less than 0.02, n = 7), and a marked decrease in the killing of cytoplast-associated bacteria (P less than 0.001, n = 7). After 60 min of incubation with bacteria, NMMA-treated cytoplasts had a mean of over 3.5 times as many live, CKP-associated staphylococci as did controls (51% of the inocula versus 14%), despite having taken up fewer. Effects on both uptake and killing were reversible by L-arginine but not by D-arginine. Results were the same with other granule-poor cytoplasts (U-cytoplasts, U-CYT), which, unlike CKP, retain activatable oxidase activity. Killing by intact PMN, including those from a patient with chronic granulomatous disease, was not inhibited by NMMA. Thus, the ability to discern effects of NMMA correlated with the paucity of granules, without regard to the presence or absence of activatable oxidase. We propose that the generation of reactive nitrogen intermediates serves as an additional microbial killing pathway in PMN, and that cytoplasts can be used to help delineate the spectrum of susceptible targets.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams L. B., Franzblau S. G., Vavrin Z., Hibbs J. B., Jr, Krahenbuhl J. L. L-arginine-dependent macrophage effector functions inhibit metabolic activity of Mycobacterium leprae. J Immunol. 1991 Sep 1;147(5):1642–1646. [PubMed] [Google Scholar]
- Adams L. B., Hibbs J. B., Jr, Taintor R. R., Krahenbuhl J. L. Microbiostatic effect of murine-activated macrophages for Toxoplasma gondii. Role for synthesis of inorganic nitrogen oxides from L-arginine. J Immunol. 1990 Apr 1;144(7):2725–2729. [PubMed] [Google Scholar]
- Cameron M. L., Granger D. L., Weinberg J. B., Kozumbo W. J., Koren H. S. Human alveolar and peritoneal macrophages mediate fungistasis independently of L-arginine oxidation to nitrite or nitrate. Am Rev Respir Dis. 1990 Dec;142(6 Pt 1):1313–1319. doi: 10.1164/ajrccm/142.6_Pt_1.1313. [DOI] [PubMed] [Google Scholar]
- Denis M. Tumor necrosis factor and granulocyte macrophage-colony stimulating factor stimulate human macrophages to restrict growth of virulent Mycobacterium avium and to kill avirulent M. avium: killing effector mechanism depends on the generation of reactive nitrogen intermediates. J Leukoc Biol. 1991 Apr;49(4):380–387. doi: 10.1002/jlb.49.4.380. [DOI] [PubMed] [Google Scholar]
- Dinauer M. C., Pierce E. A., Bruns G. A., Curnutte J. T., Orkin S. H. Human neutrophil cytochrome b light chain (p22-phox). Gene structure, chromosomal location, and mutations in cytochrome-negative autosomal recessive chronic granulomatous disease. J Clin Invest. 1990 Nov;86(5):1729–1737. doi: 10.1172/JCI114898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyett D. E., Malawista S. E., Naccache P. H., Sha'afi R. I. Stimulated cytokineplasts from human polymorphonuclear leukocytes mobilize calcium and polymerize actin. Cytoplasts made in cytochalasin B retain a defect in actin polymerization. J Clin Invest. 1986 Jan;77(1):34–37. doi: 10.1172/JCI112297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flesch I. E., Kaufmann S. H. Mechanisms involved in mycobacterial growth inhibition by gamma interferon-activated bone marrow macrophages: role of reactive nitrogen intermediates. Infect Immun. 1991 Sep;59(9):3213–3218. doi: 10.1128/iai.59.9.3213-3218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Gryglewski R. J., Palmer R. M., Moncada S. Superoxide anion is involved in the breakdown of endothelium-derived vascular relaxing factor. Nature. 1986 Apr 3;320(6061):454–456. doi: 10.1038/320454a0. [DOI] [PubMed] [Google Scholar]
- Huang A. J., Silverstein S. C., Malawista S. E. Cryopreserved cytoplasts from human neutrophils migrate across monolayers of human endothelial cells in response to a chemoattractant gradient. J Leukoc Biol. 1991 Dec;50(6):624–627. doi: 10.1002/jlb.50.6.624. [DOI] [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Kaplan S. S., Billiar T., Curran R. D., Zdziarski U. E., Simmons R. L., Basford R. E. Inhibition of chemotaxis Ng-monomethyl-L-arginine: a role for cyclic GMP. Blood. 1989 Nov 1;74(6):1885–1887. [PubMed] [Google Scholar]
- Kubes P., Suzuki M., Granger D. N. Nitric oxide: an endogenous modulator of leukocyte adhesion. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4651–4655. doi: 10.1073/pnas.88.11.4651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrer R. I., Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990 Dec 1;76(11):2169–2181. [PubMed] [Google Scholar]
- Malawista S. E., Bodel P. T. The dissociation by colchicine of phagocytosis from increased oxygen consumption in human leukocytes. J Clin Invest. 1967 May;46(5):786–796. doi: 10.1172/JCI105579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., De Boisfleury Chevance A. The cytokineplast: purified, stable, and functional motile machinery from human blood polymorphonuclear leukocytes. J Cell Biol. 1982 Dec;95(3):960–973. doi: 10.1083/jcb.95.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G., Breitenstein M. G. Cryopreservable neutrophil surrogates. Stored cytoplasts from human polymorphonuclear leukocytes retain chemotactic, phagocytic, and microbicidal function. J Clin Invest. 1989 Feb;83(2):728–732. doi: 10.1172/JCI113939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G., Cretella S. B. Cytokineplasts from human blood polymorphonuclear leukocytes. Lack of oxidase activity and extended functional longevity. Inflammation. 1985 Mar;9(1):99–106. doi: 10.1007/BF00915416. [DOI] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G. Cytoplasts made from human blood polymorphonuclear leukocytes with or without heat: preservation of both motile function and respiratory burst oxidase activity. Proc Natl Acad Sci U S A. 1987 Jan;84(2):454–458. doi: 10.1073/pnas.84.2.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malawista S. E., Van Blaricom G. Phagocytic capacity of cytokineplasts from human blood polymorphonuclear leukocytes. Blood Cells. 1986;12(1):167–177. [PubMed] [Google Scholar]
- Malawista S. E., de Boisfleury-Chevance A. Cryopreserved cytoplasts from human polymorphonuclear leukocytes (cytokineplasts) are chemotactic at speeds comparable to those of fresh intact cells. J Leukoc Biol. 1991 Sep;50(3):313–315. doi: 10.1002/jlb.50.3.313. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Montgomery R. R., Webster P., Mellman I. Accumulation of indigestible substances reduces fusion competence of macrophage lysosomes. J Immunol. 1991 Nov 1;147(9):3087–3095. [PubMed] [Google Scholar]
- Mulligan M. S., Hevel J. M., Marletta M. A., Ward P. A. Tissue injury caused by deposition of immune complexes is L-arginine dependent. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6338–6342. doi: 10.1073/pnas.88.14.6338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantazis C. G., Kniker W. T. Assessment of blood leukocyte microbial killing by using a new fluorochrome microassay. J Reticuloendothel Soc. 1979 Aug;26(2):155–170. [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincendeau P., Daulouède S. Macrophage cytostatic effect on Trypanosoma musculi involves an L-arginine-dependent mechanism. J Immunol. 1991 Jun 15;146(12):4338–4343. [PubMed] [Google Scholar]