Summary
The Ccr4-Not complex regulates eukaryotic gene expression at multiple levels, including mRNA turnover, translational repression, and transcription. We have studied the ubiquitylation module of the yeast Ccr4-Not complex and addressed how E3 ligase binds cognate E2 and how it is tethered to the complex. The 2.8-Å resolution crystal structure of the N-terminal RING domain of Not4 in complex with Ubc4 shows the detailed interactions of this E3-E2 complex. The 3.6-Å resolution crystal structure of the C-terminal domain of the yeast Not4 in complex with the C-terminal domain of Not1 reveals how a largely extended region at the C-terminus of Not4 wraps around a HEAT-repeat region of Not1. This C-terminal region of Not4 is only partly conserved in metazoans, rationalizing its weaker Not1-binding properties. The structural and biochemical data show how Not1 can incorporate both the ubiquitylation module and the Not2-Not3/5 module concomitantly in the Ccr4-Not complex.
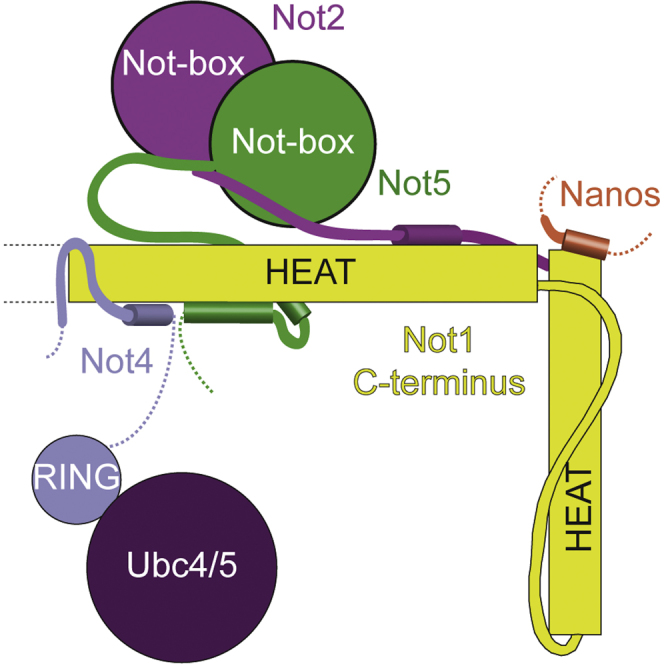
Graphical Abstract
Highlights
-
•
The Not1 C-terminal domain tethers the Not4 ubiquitylation module to yeast Ccr4-Not
-
•
A low-complexity region of Not4 wraps around the C-terminal HEAT repeats of Not1
-
•
In metazoans, Not4 lacks residues that confer high affinity binding to Not1 in yeast
-
•
Not1C can recruit Not4 and Not2-Not5 concomitantly to the Ccr4-Not complex
Bhaskar et al. have determined the crystal structures of the complexes of the Not4 C-terminal domain with Not1 and the Not4 N-terminal RING domain with Ubc4. The structural and biochemical data highlight the underlying specificity of their interaction and rationalize the concomitant binding of Not4 and Not2-Not3/5 modules to Not1.
Introduction
The Ccr4-Not complex is a crucial player in the regulation of eukaryotic gene expression (reviewed in Wahle and Winkler, 2013). Ccr4-Not was originally discovered as a transcriptional regulator in yeast (Collart and Struhl, 1994; Draper et al., 1994). Subsequent experiments revealed its fundamental function in cytoplasmic mRNA turnover, as a deadenylase that shortens the poly(A) tail at the 3′ end of mRNAs (Daugeron et al., 2001; Tucker et al., 2002). More recently, Ccr4-Not was also shown to act as a translational repressor (reviewed in Chapat and Corbo, 2014) and to be implicated in co-translational quality control (Panasenko, 2014; Matsuda et al., 2014).
Purification of the Ccr4-Not core complex from endogenous sources has revealed the presence of a large macromolecular assembly containing several evolutionary conserved proteins and a few proteins that are instead species specific (Chen et al., 2001; Lau et al., 2009; Temme et al., 2010; Erben et al., 2014). Ccr4-Not is assembled around Not1, a ∼240 kDa protein that is built by consecutive helical domains. The individual domains of Not1 recruit the other core components of the complex forming structurally and functionally distinct modules. The Not1 N-terminal domain is an elongated HEAT-repeat fold (Basquin et al., 2012) and appears to bind species-specific subunits (CNOT10-CNOT11 in metazoans, Caf130 in yeast) (Chen et al., 2001; Mauxion et al., 2013; Bawankar et al., 2013). The Not1 central MIF4G domain is next and recruits Caf1 (also known as Pop2 in yeast) and Ccr4, forming the deadenylase module of the complex (Draper et al., 1994; Bai et al., 1999). This is followed by the Not1 helical bundle domain, which binds Caf40 (Bawankar et al., 2013). Last is the Not1 C-terminal domain, an elongated HEAT-repeat fold that binds Not2 and Not5 (and in yeast also the paralog Not3), forming the Not module of the complex (Bai et al., 1999). The C-terminal domain of Not1 also binds Not4, another core component of the yeast Ccr4-Not complex (Bai et al., 1999). Finally, several peripheral proteins are recruited to the core complex, such as DDX6, Nanos, tristetraprolin, and GW182 in metazoans (Maillet and Collart, 2002; Suzuki et al., 2010; Sandler et al., 2011; Braun et al., 2011; Chekulaeva et al., 2011; Fabian et al., 2011).
In the past few years, most of the conserved interactions of the core complex as well as the interactions with several peripheral factors have been elucidated at the structural level (Basquin et al., 2012; Petit et al., 2012; Fabian et al., 2013; Boland et al., 2013; Bhaskar et al., 2013; Bhandari et al., 2014; Chen et al., 2014; Mathys et al., 2014), with the exception of Not4. Not4 is an evolutionarily conserved protein that contains an N-terminal RING domain, a central RRM domain and a C-terminal domain predicted to be unstructured. As shown for both the yeast and human orthologs, the Not4 RING domain harbors an E3 ubiquitin ligase activity (Albert et al., 2002; Mulder et al., 2007a). Consistently, Not4 has been reported to ubiquitylate a wide range of substrates (Laribee et al., 2007; Mulder et al., 2007b; Mersman et al., 2009; Cooper et al., 2012; Gulshan et al., 2012), including ribosome-associated factors (Panasenko et al., 2006; Panasenko and Collart, 2012). Although the exact function is currently debated, the enzymatic activity of Not4 has been linked to proteasomal degradation in particular in the context of mRNA quality control pathways that respond to halted translation (Dimitrova et al., 2009; Matsuda et al., 2014). The activity of the Not4 E3 ligase depends on its interaction with a specific E2, which has been identified as Ubc4/5 in yeast and the ortholog UbcH5B in humans (Albert et al., 2002; Mulder et al., 2007a). Structural studies have shown how the RING domain of human CNOT4 folds via an unusual C4C4 motif whereby eight cysteine residues coordinate two zinc ions (Hanzawa et al., 2001). A model of the human CNOT4-UbcH5B complex has been proposed based on chemical shift nuclear magnetic resonance restraints, computational docking approaches, and mutational analysis (Dominguez et al., 2004) but no crystal structure has been reported as yet.
Binding of yeast Not4 to the Ccr4-Not complex does not require the N-terminal RING domain but rather the C-terminal domain (Panasenko and Collart, 2011). The C-terminal domain of Not4, however, is the least conserved portion of the molecule. In addition, although Not4 is a bona fide Ccr4-Not subunit in yeast, it is not stably associated with the complex in human and Drosophila cells (Lau et al., 2009; Temme et al., 2010). The molecular basis for the Not1-Not4 interaction in yeast and the reason for the weaker association in higher eukaryotes are currently unknown. Also unknown is whether Not4 can bind Not1 in the context of the Not module, as Not2, Not3, and Not5 also dock to the same domain of Not1. Here, we report a structural and biochemical study that sheds light on how the E3 ligase of Not4 binds specifically its cognate E2 and how it is recruited to the Ccr4-Not complex.
Results and Discussion
Overall Structure of Saccharomyces cerevisiae Not4N Bound to Ubc4
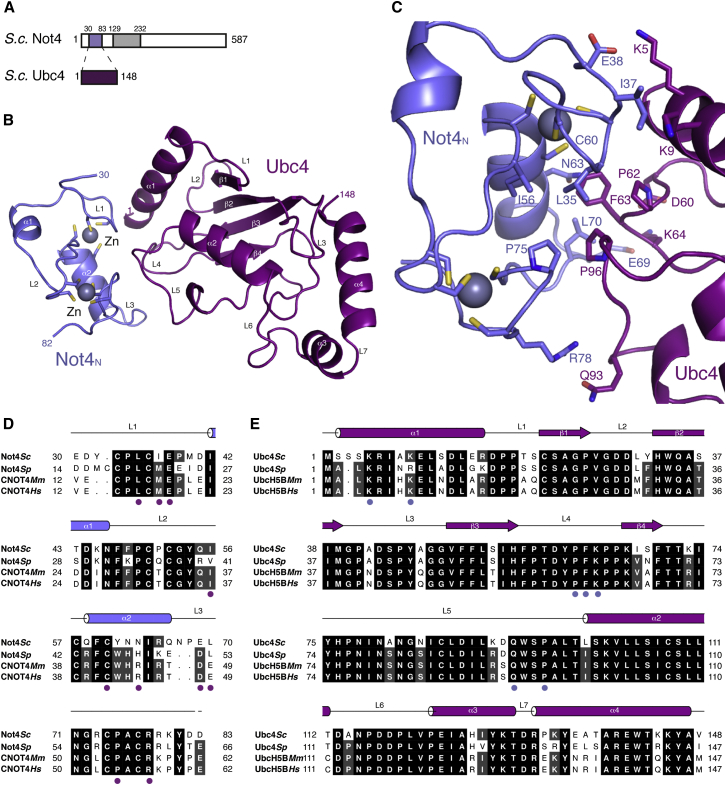
The N-terminal RING domain of Not4 (Not4N, residues 30–83 in S. cerevisiae) has been shown to interact with Ubc4 (Figure 1A) (Albert et al., 2002; Mulder et al., 2007a). To obtain crystals of the complex, we used a strategy that had been reported for another E3-E2 complex (Hodson et al., 2014) and connected the two proteins covalently via a 10-residue linker. The structure of the Not4N-Ubc4 fusion protein was determined by a combination of zinc-based single-wavelength anomalous dispersion (SAD) and molecular replacement, and was refined to 2.8-Å resolution with Rfree of 27.1%, Rfactor of 22.0%, and good stereochemistry (Table 1). The final model of Not4N-Ubc4 has well-defined electron density for most of the polypeptide, except for the connecting linker (Figure 1B).
Figure 1.
Structure of the Complex between the Not4 RING E3 and the Ubc4 E2
(A) A schematic diagram of the domain architecture of S. cerevisiae Not4 and Ubc4. The colored rectangles indicate the regions present in the crystal structure. The gray rectangle represents another folded domain, while the empty boxes represent low-complexity regions.
(B) Cartoon representation of the structure of yeast Not4N (in blue) bound to Ubc4 (in purple). The N- and C-terminal residues of the two proteins ordered in the electron density are indicated. The secondary structure elements are labeled. The two zinc ions are shown as spheres and the cysteine residues that coordinate them are shown in stick representation. This structure figure and all others in the article were generated using PyMOL (The PyMOL Molecular Graphics System, Version 1.2r3pre, Schrödinger, LLC).
(C) Close-up view of the interaction interface between Not4N and Ubc4. Interacting residues are shown and labeled.
(D and E) Structure-based sequence alignment of Not4N and Ubc4 from different species, including S. cerevisiae (Sc), Mus musculus (Mm) and Homo sapiens (Hs), highlighting the interacting residues. The secondary structure elements are shown above the sequence.
See also Figure S1.
Table 1.
Data Collection and Refinement Statistics
| Not4N-Ubc4 |
Not1C-Not4C | ||
|---|---|---|---|
| Zinc SAD | Native | ||
| Wavelength (Å) | 1.2819 | 1 | 1 |
| Resolution range (Å)a | 37.33–2.48 | 53.56–2.80 (2.90–2.80) | 75.66–3.62 (3.75–3.62) |
| Space group | P1 | R3H | P3221 |
| Unit cell | 62.45, 62.96, 65.43 | 107.11, 107.11, 62.20 | 173.66, 173.66, 262.61 |
| α, β, γ (°) | 108.50, 107.40,108.09 | 90, 90, 120 | 90, 90, 120 |
| Total reflectionsa | 95,124 (13,582) | 67,241 (6,289) | 352,483 (34,067) |
| Unique reflectionsa | 52,704 (7,634) | 6,541 (628) | 52,676 (5,095) |
| Multiplicitya | 1.84 (1.77) | 10.30 (10.00) | 6.70 (6.70) |
| Completeness (%)a | 92.5 (83.2) | 99.4 (95.2) | 99.7 (98.6) |
| Mean I/σ(I)a | 14.66 (4.78) | 29.35 (2.10) | 11.26 (1.37) |
| SigAnoa | 1.31 (0.95) | ||
| CC1/2a | 0.997 (0.949) | 1 (0.911) | 1 (0.733) |
| Rmerge (%)a | 5.6 (97.1) | 11.2 (149.8) | |
| Rwork (%)a | 22.0 (45.1) | 26.6 (38.7) | |
| Rfree (%)a | 27.1 (50.8) | 31.9 (42.7) | |
| Number of non-hydrogen atoms | 1,575 | 24,555 | |
| Macromolecules | 1,573 | 24,555 | |
| Ligands | 2 | 0 | |
| Protein residues | 204 | 3,223 | |
| RMS (bonds) | 0.002 | 0.010 | |
| RMS (angles) | 0.52 | 0.64 | |
| Ramachandran favored (%) | 97 | 94 | |
| Ramachandran outliers (%) | 0 | 0.064 | |
| Average B-factor | 103.00 | 124.80 | |
| Macromolecules | 103.10 | 124.80 | |
| Ligands | 94.60 | ||
SigAno = mean anomalous difference in units of its estimated standard deviation. (|F(+) − F(−)|/σ). F(+), F(−) are structure factor estimates obtained from the merged intensity observations in each parity class.
Statistics for the highest-resolution shell are shown in parentheses.
The structure of yeast Not4N bound to Ubc4 is very similar to that of the human CNOT4 ortholog in isolation (Hanzawa et al., 2001). The RING domain of Not4 contains two α-helices (the short α1 helix and the long α2 helix) and two zinc ions (Figure 1B). The zinc ions are coordinated in cross bracing fashion by cysteine residues that protrude from helix α2 and from the three loops regions L1, L2, and L3. The structure of yeast Ubc4 bound to Not4N is very similar to a previously determined structure of Ubc4 in isolation (Cook et al., 1993). Briefly, Ubc4 is centered at a four-stranded antiparallel β-sheet flanked by an N-terminal α-helix (α1) and by three C-terminal α-helices (α2, α3, α4) (Figure 1B). When compared with the previously proposed model of the human CNOT4-UbcH5B complex (Dominguez et al., 2004), the experimentally determined structure of yeast Not4N-Ubc4 shows localized differences (Figure S1A).
Specific Interaction Network between the Not4N RING E3 and the Ubc4 E2
In the crystal structure, the Not4 helix α2 and the zinc-binding loops L1, L2, and L3 interact with two loops of Ubc4 that precede and follow the fourth strand of the β-sheet (L4 and L5) (Figure 1B). The central hotspot of the interaction is formed by Phe63 of Ubc4, which wedges into a hydrophobic pocket formed by Leu35, Ile56, Cys60, Asn63, Leu70, and Pro75 of Not4 and by Pro62 and Pro96 of Ubc4 (Figure 1C). In addition, Ile37 of Not4 is involved in hydrophobic interactions with the aliphatic portion of the side chain of Lys5 and Lys9 in the helix α1 of Ubc4. This hydrophobic hotspot is surrounded by polar and electrostatic contacts: a hydrogen-bond interaction involving Not4 Arg78 and Ubc4 Gln93 and two salt bridge interactions between Not4 Glu38 and Ubc4 Lys5 and between Not4 Glu69 and Ubc4 Lys64 (Figure 1C). In addition, Ubc4 Lys64 is engaged in an intra-molecular salt bridge with Ubc4 Asp60. The Glu69-Lys64-Asp60 network effectively pulls the L3 loop of Not4 toward Ubc4, closing the hydrophobic core. The interaction interface is formed by evolutionary conserved residues (Figures 1D and 1E) and is consistent with the effects of mutations previously reported (Mulder et al., 2007a).
To understand the specificity of yeast Not4 toward Ubc4/5 enzymes, we structurally aligned the known yeast E2 proteins on Ubc4 and analyzed if residues at the Not4-binding interface are conserved (Figure S1B). The Ubc2 and Ubc9 E2 proteins lack a hydrophobic residue at the corresponding position of Ubc4 Phe63. Ubc3, Ubc7, Ubc10, Ubc12, and Ubc13 lack a positively charged residue at the corresponding position of Ubc4 Lys64. Ubc1, Ubc6, Ubc8, and Ubc11 lack the equivalent of Ubc4 Gln93. These subtle differences appear to weaken the interaction network observed in the Not4N-Ubc4 structure, driving the specificity of Not4N toward Ubc4/5 (Albert et al., 2002; Mulder et al., 2007a).
Overall Structure of Not4C Bound to Not1C
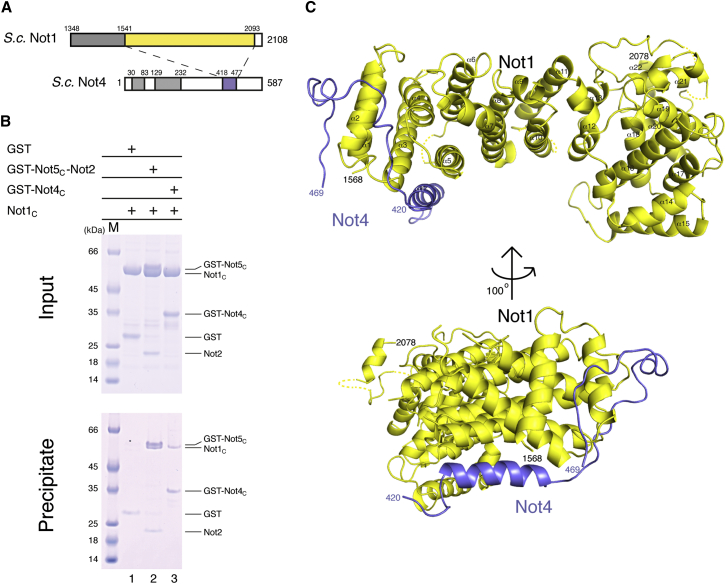
The C-terminal domain of S. cerevisiae Not4 (specifically residues 430–480) has been shown to interact with the C-terminal domain of Not1 by yeast two-hybrid and co-immunoprecipitation (co-IP) studies (Albert et al., 2002; Panasenko and Collart, 2011). To ensure the identification of the correct domain boundaries that would include all the determinants of the interaction, we used secondary structure predictions to engineer larger regions of the interacting proteins than those mapped from the co-IP experiment. We purified a complex encompassing Not1 residues 1348–2093 and Not4 residues 418–587 (Not4 Δ417). Limited proteolysis of this complex and subsequent gel filtration resulted in stable fragments that were characterized by N-terminal sequencing and mass spectrometry analysis as encompassing residues 418–477 of Not4 (Not4C) and residues 1541–2093 of Not1 (the C-terminal domain of Not1, or Not1C) (Figures 2A and S2). Consistent with the proteolysis results, GST-tagged Not4C was able to precipitate Not1C in pull-down assays (Figure 2B, lane 3). We purified the Not1C-Not4C minimal complex and obtained crystals diffracting to 3.6-Å resolution containing six copies of the complex in the asymmetric unit. We determined the structure by molecular replacement, using the previously determined structure of Not1C as the search model (Bhaskar et al., 2013). The model was built and refined to Rfree of 31.9%, Rfactor of 26.6%, and good stereochemistry (Table 1). The six independent copies of the complex in the crystals are essentially identical and include residues 1568–2078 of Not1 (with the major exception of two loops between 1791–1800 and 2065–2071) and residues 420–469 of Not4 (Figure 2C).
Figure 2.
Structure of the Complex between Not4C and Not1C
(A) A schematic diagram of the domain architecture of S. cerevisiae Not1 C-terminal region and Not4. The colored rectangles indicate the regions present in the structure. The gray rectangles represent other folded domains, while the empty boxes represent low-complexity regions.
(B) Protein co-precipitation by GST pull-down experiments. GST-Not4C, GST-Not5C-Not2 (positive control), or GST alone (negative control) were incubated with untagged Not1C in a buffer containing 150 mM NaCl before co-precipitation with GSH-Sepharose beads, as indicated. Input (upper panel) and precipitates (lower panel) were analyzed on Coomassie stained 4%–12% Bis-Tris gradient gel (NuPage, Invitrogen). The proteins are labeled on the right.
(C) Structure of the Not1C-Not4C complex shown in cartoon representation in two orientations. Not1C is colored in yellow and Not4C in blue. The N- and C-terminal residues of both proteins are marked. Disordered loops are indicated as dotted lines.
See also Figure S2.
The HEAT-repeat structure of Not1C in the Not4C-bound complex is similar to that in the Not2-Not5C-bound complex (Bhaskar et al., 2013). HEAT repeats consist of two antiparallel α-helices (termed A and B) and pack side by side in a regular fashion. The ten HEAT repeats of Not1C are organized in two units. The first unit is made of six HEAT repeats and is arranged in a perpendicular fashion with respect to the second unit, which is composed of the four C-terminal repeats (Figure 2C). The loop connecting HEATs 7 and 8 is in an extended conformation, likely due to crystal contacts. Not4C folds into an α-helix (residues 426–439) that is flanked by extended regions lacking defined secondary structure elements (residues 420–425 and 440–469) (Figure 2C).
Extensive Interaction Network between Yeast Not1C and Not4C
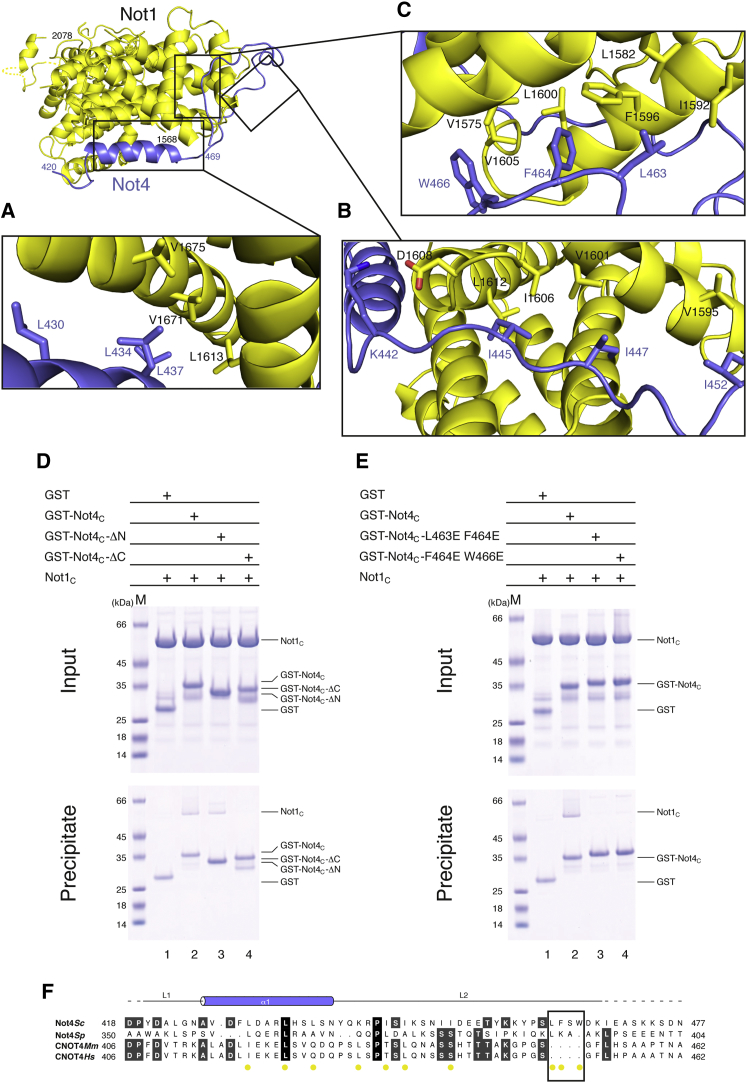
Not4C binds on the surface of the first three HEAT repeats of Not1C, extending about 100 Å in length and burying a surface area of approximately 1500 Å2 (Figure 2C). The contacts between Not4C and Not1C can be described as divided into three segments. In the first segment, the α-helix of Not4C packs against the A helices of HEAT 2 and 3 of Not1C. This interface is mainly dominated by hydrophobic interactions between Leu430, Leu434, and Leu437 of Not4C and Leu1613, Val1671, and Val1675 of Not1C (Figures 3A and S3A). In the second segment, residues 442–452 of Not4 interact extensively with two loops of Not1C connecting HEAT 1 to 2 and HEAT 2 to 3. The interactions are mediated by a salt bridge and few hydrophobic contacts (Figure 3B). In the third segment, residues 462–469 of Not4 are in extended conformation and pack between the A and B helices of the first HEAT repeat of Not1C. This interface involves hydrophobic contacts between Leu463, Phe464, and Trp466 of Not4 and Val1575, Leu1582, Ile1592, Phe1596, Leu1600, and Val1605 of Not1 (Figures 3C and S3B).
Figure 3.
Not4C Wraps around the N-terminal HEAT Repeats of Not1C
(A–C) Close-up view of different segments of Not4C that form the Not1C interacting region. The position of each individual segment in the context of the complex is shown on the top left. The residues involved in interactions are shown as sticks and labeled.
(D and E) Pull-down experiments with GST-tagged versions of Not4 and untagged Not1C, carried out as described in Figure 2B.
(F) Structure-based sequence alignment of Not4C from different species, as mentioned in Figure 1D. The secondary structure elements are shown above the sequence.
See also Figure S3.
To test the relevance of the interacting regions, we engineered deletion mutants of Not4C and carried out pull-down assays. As the second segment of the Not1C-Not4C interface appeared the weakest from an analysis using the PISA server (Krissinel and Henrick, 2007), we constructed versions of Not4C lacking either the first hydrophobic segment (Not4C-ΔN) or the C-terminal hydrophobic segment (Not4C-ΔC). GST-tagged Not4C-ΔN precipitated Not1C to a similar extent as GST-Not4C (Figure 3D, lanes 2 and 3). In contrast, GST-tagged Not4C-ΔC failed to interact with Not1C in the pull-down assay (Figure 3D, lane 4). Next, we introduced specific mutations in the C-terminal segment of Not4C and tested them for their ability to interact with Not1C in GST pull-down assays. Mutations of Not4C either at Leu463 and Phe464 (L463E F464E) or at Phe464 and Trp466 (F464E W466E) failed to precipitate Not1C (Figure 3E, lanes 3 and 4). Altogether, these results suggest that the C-terminal segment of Not4C makes the most significant contribution to the Not1-Not4 interaction while the first and second segments of Not4C have a minor role.
Not4 Binding to Not1 Is Partially Conserved in Metazoa
To date, S. cerevisiae is the only species in which a stable association of Not4 within the Ccr4-Not core complex has been detected. This raises the question as to whether the interactions observed in the Not1C-Not4C crystal structure are likely to occur in other species, particularly as in metazoa the incorporation of Not4 in the endogenous Ccr4-Not core complex has been barely detectable (Lau et al., 2009; Temme et al., 2010). In the case of Not1, many Not4-binding residues are evolutionarily conserved in higher eukaryotes (Figure S3C). In Not4, the first hydrophobic segment of the Not1-binding region is conserved. Human CNOT4, for example, features Ile419, Leu423, and Gln426 at the equivalent positions of S. cerevisiae Leu430, Leu434, and Leu437, respectively (Figure 3F). However, the third Not1-binding segment of Not4 is not present in human CNOT4. Since the third segment is essential for stable binding of Not4 to Not1 in yeast (Figures 3D and 3E), such differences rationalize the weaker in vivo association in higher eukaryotes (Lau et al., 2009; Temme et al., 2010).
Not4 Binding to Not1 Is Independent of the Not Module
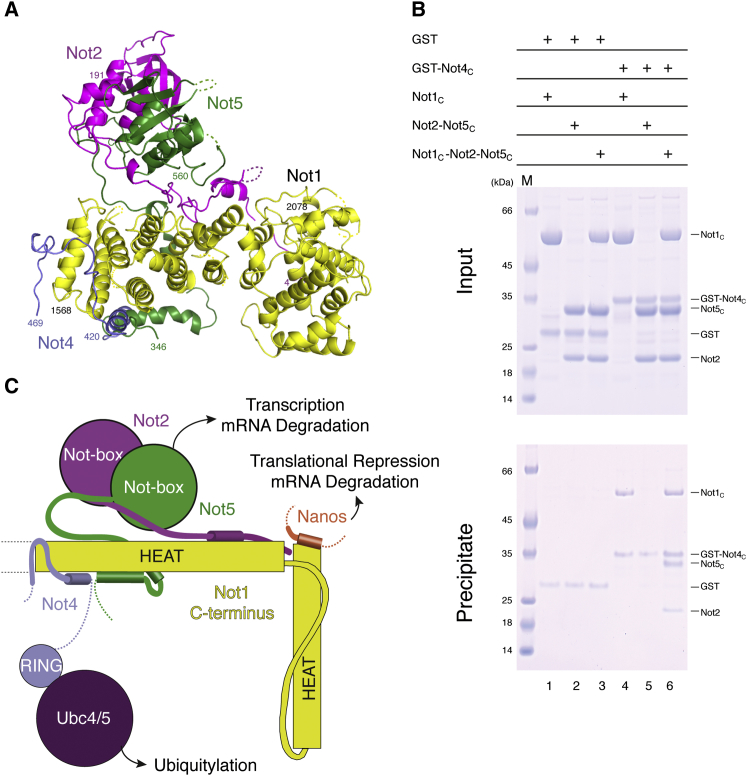
Next, we compared the structure of the ubiquitylation module with that of the Not module. We superposed the structure of yeast Not1C-Not4C with those of yeast Not1C-Not2-Not5C (Bhaskar et al., 2013) and human CNOT1C-CNOT2C-CNOT3C (Boland et al., 2013). While Not4C binds the side surface of the first HEAT-repeat unit of Not1C, yeast Not2-Not5C and human CNOT2C-CNOT3C bind the top and the bottom surfaces (Figure 4A). Although there is a small overlap between the N-terminal helix of Not4C and the N-terminal region of Not5C as observed in the yeast Not1C-Not2-Not5C complex, the structural analysis indicates that the interactions of Not4C and Not2-Not5C occur at largely separate surfaces of Not1C. Indeed, pull-down assays showed that GST-tagged Not4C could precipitate Not2-Not5C in the presence of Not1C (Figure 4B). Thus, the ubiquitylation module and the Not module can form simultaneously on the C-terminal domain of Not1. Finally, Not4C binds at a completely different surface compared with the protein Nanos, which in metazoa is recognized by the C-terminal HEAT-repeat unit of CNOT1C. Thus, the interactions of metazoan CNOT1 with CNOT2-CNOT3, CNOT4, and Nanos can in principle also occur simultaneously (Figure 4C). Whether and how bringing these proteins into close proximity by their concomitant interaction on the Not1C platform affects the regulation or coordination of their functions are open questions for future studies.
Figure 4.
Not4C Binds Not1C Independently of Not2 and Not5
(A) Superposition of the yeast Not1C-Not4C and Not1C-Not2-Not5C structures. Not1C is in yellow, Not2 in magenta, Not5C in green, and Not4C in blue.
(B) Pull-down experiments with GST-tagged Not4C with untagged Not1C and/or Not2-Not5C, carried out as described in Figure 2B.
(C) Schematic diagram of the C-terminal domain of Not1 with the positions of the interacting proteins Not4, Not2-Not5 (or CNOT2-CNOT3 in humans), and Nanos.
Experimental Procedures
Protein Purification
All proteins were cloned, expressed, and purified as previously described (Bhaskar et al., 2013) (see Supplemental Experimental Procedures).
Crystallization and Structure Determination
All crystals were obtained by vapor diffusion at room temperature. All data were collected at the PXII and PXIII beamlines of the Swiss Light Source, processed using XDS (Kabsch, 2010), and scaled and merged using Aimless (Evans and Murshudov, 2013). The structures were obtained after iterative rounds of model building using the program Coot (Emsley et al., 2010) and/or BUCCANEER (Cowtan, 2006) and refined using PHENIX.REFINE (Adams et al., 2010). The Not4N-Ubc4 complex was crystallized at 48 mg ml−1 (see Supplemental Experimental Procedures). Synchrotron data collected at the zinc edge (wavelength 1.28 Å) were used to solve the structure by molecular replacement-SAD in Phaser using the Ubc4 structure as a search model for molecular replacement and anomalous signal from the zinc atom (Cook et al., 1993; McCoy et al., 2007). The final model was refined against a 2.8-Å resolution native dataset (collected at 1 Å wavelength).
Not1C-Not4C was crystallized at 12 mg ml−1 (see Supplemental Experimental Procedures). The crystals belong to space group P3221 with six copies in an asymmetric unit related by translational noncrystallographic symmetry (NCS). The structure was determined by molecular replacement using Not1C from the Not1C-Not2-Not5C structure as the search model (Bhaskar et al., 2013). The model was refined for individual sites and individual B-factors along with torsion angle NCS restraints (in the initial rounds of refinement) that allow local conformational changes between the NCS-related copies.
Pull-down Assays
Pull-down assays of GST-tagged Not4 constructs with untagged Not1C and/or Not2-Not5C complex were performed as described in Bhaskar et al. (2013) (see Supplemental Experimental Procedures).
Author Contributions
V.B. and J.B. performed the experiments; E.C. supervised the project; E.C. and V.B. wrote the manuscript.
Acknowledgments
We would like to thank the Max-Planck-Institute of Biochemistry Core Facility and Crystallization Facility; the staff members at the beamlines PXII and PXIII of the Swiss Light Source, Airlie McCoy and Pavel Afonine for suggestions on NCS treatment; members of our lab for useful discussions and critical reading of the manuscript. This study was supported by the Max-Planck Gesellschaft, the European Commission (ERC Advanced Investigator Grant 294371, Marie Curie ITN RNPnet), and the Deutsche Forschungsgemeinschaft (DFG SFB646, SFB1035, GRK1721, FOR1680, CIPSM) to E.C.
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Accession Numbers
The PDB accession numbers for the structure of Not4N-Ubc4 and Not1C-Not4C reported in this paper are 5AIE and 5AJD, respectively.
Supplemental Information
References
- Adams P.D., Afonine P.V., Bunkóczi G., Chen V.B., Davis I.W., Echols N., Headd J.J., Hung L.-W., Kapral G.J., Grosse-Kunstleve R.W. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert T.K., Hanzawa H., Legtenberg Y.I.A., de Ruwe M.J., van den Heuvel F.A.J., Collart M.A., Boelens R., Timmers H.T.M. Identification of a ubiquitin-protein ligase subunit within the CCR4-NOT transcription repressor complex. EMBO J. 2002;21:355–364. doi: 10.1093/emboj/21.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai Y., Salvadore C., Chiang Y.C., Collart M.A., Liu H.Y., Denis C.L. The CCR4 and CAF1 proteins of the CCR4-NOT complex are physically and functionally separated from NOT2, NOT4, and NOT5. Mol. Cell. Biol. 1999;19:6642–6651. doi: 10.1128/mcb.19.10.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basquin J., Roudko V.V., Rode M., Basquin C., Séraphin B., Conti E. Architecture of the nuclease module of the yeast Ccr4-not complex: the Not1-Caf1-Ccr4 interaction. Mol. Cell. 2012;48:207–218. doi: 10.1016/j.molcel.2012.08.014. [DOI] [PubMed] [Google Scholar]
- Bawankar P., Loh B., Wohlbold L., Schmidt S., Izaurralde E. NOT10 and C2orf29/NOT11 form a conserved module of the CCR4-NOT complex that docks onto the NOT1 N-terminal domain. RNA Biol. 2013;10:228–244. doi: 10.4161/rna.23018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari D., Raisch T., Weichenrieder O., Jonas S., Izaurralde E. Structural basis for the Nanos-mediated recruitment of the CCR4-NOT complex and translational repression. Genes Dev. 2014;28:888–901. doi: 10.1101/gad.237289.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhaskar V., Roudko V., Basquin J., Sharma K., Urlaub H., Séraphin B., Conti E. Structure and RNA-binding properties of the Not1-Not2-Not5 module of the yeast Ccr4-Not complex. Nat. Struct. Mol. Biol. 2013;20:1281–1288. doi: 10.1038/nsmb.2686. [DOI] [PubMed] [Google Scholar]
- Boland A., Chen Y., Raisch T., Jonas S., Kuzuoğlu-Öztürk D., Wohlbold L., Weichenrieder O., Izaurralde E. Structure and assembly of the NOT module of the human CCR4-NOT complex. Nat. Struct. Mol. Biol. 2013;20:1289–1297. doi: 10.1038/nsmb.2681. [DOI] [PubMed] [Google Scholar]
- Braun J.E., Huntzinger E., Fauser M., Izaurralde E. GW182 proteins directly recruit cytoplasmic deadenylase complexes to miRNA targets. Mol. Cell. 2011;44:120–133. doi: 10.1016/j.molcel.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Chapat C., Corbo L. Novel roles of the CCR4-NOT complex. Wiley Interdiscip. Rev. RNA. 2014;5:883–901. doi: 10.1002/wrna.1254. [DOI] [PubMed] [Google Scholar]
- Chekulaeva M., Mathys H., Zipprich J.T., Attig J., Colic M., Parker R., Filipowicz W. miRNA repression involves GW182-mediated recruitment of CCR4-NOT through conserved W-containing motifs. Nat. Struct. Mol. Biol. 2011;18:1218–1226. doi: 10.1038/nsmb.2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Rappsilber J., Chiang Y.C., Russell P., Mann M., Denis C.L. Purification and characterization of the 1.0 MDa CCR4-NOT complex identifies two novel components of the complex. J. Mol. Biol. 2001;314:683–694. doi: 10.1006/jmbi.2001.5162. [DOI] [PubMed] [Google Scholar]
- Chen Y., Boland A., Kuzuoğlu-Öztürk D., Bawankar P., Loh B., Chang C.-T., Weichenrieder O., Izaurralde E. A DDX6-CNOT1 complex and W-binding pockets in CNOT9 reveal direct links between miRNA target recognition and silencing. Mol. Cell. 2014;54:737–750. doi: 10.1016/j.molcel.2014.03.034. [DOI] [PubMed] [Google Scholar]
- Collart M.A., Struhl K. NOT1(CDC39), NOT2(CDC36), NOT3, and NOT4 encode a global-negative regulator of transcription that differentially affects TATA-element utilization. Genes Dev. 1994;8:525–537. doi: 10.1101/gad.8.5.525. [DOI] [PubMed] [Google Scholar]
- Cook W.J., Jeffrey L.C., Xu Y., Chau V. Tertiary structures of class I ubiquitin-conjugating enzymes are highly conserved: crystal structure of yeast Ubc4. Biochemistry. 1993;32:13809–13817. doi: 10.1021/bi00213a009. [DOI] [PubMed] [Google Scholar]
- Cooper K.F., Scarnati M.S., Krasley E., Mallory M.J., Jin C., Law M.J., Strich R. Oxidative-stress-induced nuclear to cytoplasmic relocalization is required for Not4-dependent cyclin C destruction. J. Cell Sci. 2012;125:1015–1026. doi: 10.1242/jcs.096479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowtan K. The Buccaneer software for automated model building. 1. Tracing protein chains. Acta Crystallogr. D Biol. Crystallogr. 2006;62:1002–1011. doi: 10.1107/S0907444906022116. [DOI] [PubMed] [Google Scholar]
- Daugeron M.C., Mauxion F., Séraphin B. The yeast POP2 gene encodes a nuclease involved in mRNA deadenylation. Nucleic Acids Res. 2001;29:2448–2455. doi: 10.1093/nar/29.12.2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitrova L.N., Kuroha K., Tatematsu T., Inada T. Nascent peptide-dependent translation arrest leads to Not4p-mediated protein degradation by the proteasome. J. Biol. Chem. 2009;284:10343–10352. doi: 10.1074/jbc.M808840200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez C., Bonvin A.M.J.J., Winkler G.S., van Schaik F.M.A., Timmers H.T.M., Boelens R. Structural model of the UbcH5B/CNOT4 complex revealed by combining NMR, mutagenesis, and docking approaches. Structure. 2004;12:633–644. doi: 10.1016/j.str.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Draper M.P., Liu H.Y., Nelsbach A.H., Mosley S.P., Denis C.L. Ccr4 is a glucose-regulated transcription factor whose leucine-rich repeat binds several proteins important for placing Ccr4 in its proper promoter context. Mol. Cell. Biol. 1994;14:4522–4531. doi: 10.1128/mcb.14.7.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley P., Lohkamp B., Scott W.G., Cowtan K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010;66:486–501. doi: 10.1107/S0907444910007493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben E., Chakraborty C., Clayton C. The CAF1-NOT complex of trypanosomes. Front. Genet. 2014;4:299. doi: 10.3389/fgene.2013.00299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P.R., Murshudov G.N. How good are my data and what is the resolution? Acta Crystallogr. D Biol. Crystallogr. 2013;69:1204–1214. doi: 10.1107/S0907444913000061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M.R., Cieplak M.K., Frank F., Morita M., Green J., Srikumar T., Nagar B., Yamamoto T., Raught B., Duchaine T.F. miRNA-mediated deadenylation is orchestrated by GW182 through two conserved motifs that interact with CCR4-NOT. Nat. Struct. Mol. Biol. 2011;18:1211–1217. doi: 10.1038/nsmb.2149. [DOI] [PubMed] [Google Scholar]
- Fabian M.R., Frank F., Rouya C., Siddiqui N., Lai W.S., Karetnikov A., Blackshear P.J., Nagar B., Sonenberg N. Structural basis for the recruitment of the human CCR4-NOT deadenylase complex by tristetraprolin. Nat. Struct. Mol. Biol. 2013;20:735–739. doi: 10.1038/nsmb.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulshan K., Thommandru B., Moye-Rowley W.S. Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J. Biol. Chem. 2012;287:26796–26805. doi: 10.1074/jbc.M112.384719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanzawa H., de Ruwe M.J., Albert T.K., van Der Vliet P.C., Timmers H.T., Boelens R. The structure of the C4C4 ring finger of human NOT4 reveals features distinct from those of C3HC4 RING fingers. J. Biol. Chem. 2001;276:10185–10190. doi: 10.1074/jbc.M009298200. [DOI] [PubMed] [Google Scholar]
- Hodson C., Purkiss A., Miles J.A., Walden H. Structure of the human FANCL RING-Ube2T complex reveals determinants of cognate E3-E2 selection. Structure. 2014;22:337–344. doi: 10.1016/j.str.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kabsch W. XDS. Acta Crystallogr. D Biol. Crystallogr. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krissinel E., Henrick K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007;372:774–797. doi: 10.1016/j.jmb.2007.05.022. [DOI] [PubMed] [Google Scholar]
- Laribee R.N., Shibata Y., Mersman D.P., Collins S.R., Kemmeren P., Roguev A., Weissman J.S., Briggs S.D., Krogan N.J., Strahl B.D. CCR4/NOT complex associates with the proteasome and regulates histone methylation. Proc. Natl. Acad. Sci. USA. 2007;104:5836–5841. doi: 10.1073/pnas.0607996104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau N.-C., Kolkman A., van Schaik F.M.A., Mulder K.W., Pijnappel W.W.M.P., Heck A.J.R., Timmers H.T.M. Human Ccr4-not complexes contain variable deadenylase subunits. Biochem. J. 2009;422:443–453. doi: 10.1042/BJ20090500. [DOI] [PubMed] [Google Scholar]
- Maillet L., Collart M.A. Interaction between Not1p, a component of the Ccr4-not complex, a global regulator of transcription, and Dhh1p, a putative RNA helicase. J. Biol. Chem. 2002;277:2835–2842. doi: 10.1074/jbc.M107979200. [DOI] [PubMed] [Google Scholar]
- Mathys H., Basquin J., Ozgur S., Czarnocki-Cieciura M., Bonneau F., Aartse A., Dziembowski A., Nowotny M., Conti E., Filipowicz W. Structural and biochemical insights to the role of the CCR4-NOT complex and DDX6 ATPase in microRNA repression. Mol. Cell. 2014;54:751–765. doi: 10.1016/j.molcel.2014.03.036. [DOI] [PubMed] [Google Scholar]
- Matsuda R., Ikeuchi K., Nomura S., Inada T. Protein quality control systems associated with no-go and nonstop mRNA surveillance in yeast. Genes Cells. 2014;19:1–12. doi: 10.1111/gtc.12106. [DOI] [PubMed] [Google Scholar]
- Mauxion F., Prève B., Séraphin B. C2ORF29/CNOT11 and CNOT10 form a new module of the CCR4-NOT complex. RNA Biol. 2013;10:267–276. doi: 10.4161/rna.23065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy A.J., Grosse-Kunstleve R.W., Adams P.D., Winn M.D., Storoni L.C., Read R.J. Phaser crystallographic software. J. Appl. Crystallogr. 2007;40:658–674. doi: 10.1107/S0021889807021206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mersman D.P., Du H.-N., Fingerman I.M., South P.F., Briggs S.D. Polyubiquitination of the demethylase Jhd2 controls histone methylation and gene expression. Genes Dev. 2009;23:951–962. doi: 10.1101/gad.1769209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K.W., Inagaki A., Cameroni E., Mousson F., Winkler G.S., De Virgilio C., Collart M.A., Timmers H.T.M. Modulation of Ubc4p/Ubc5p-mediated stress responses by the RING-finger-dependent ubiquitin-protein ligase Not4p in Saccharomyces cerevisiae. Genetics. 2007;176:181–192. doi: 10.1534/genetics.106.060640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder K.W., Brenkman A.B., Inagaki A., van den Broek N.J.F., Timmers H.T.M. Regulation of histone H3K4 tri-methylation and PAF complex recruitment by the Ccr4-Not complex. Nucleic Acids Res. 2007;35:2428–2439. doi: 10.1093/nar/gkm175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko O.O. The role of the E3 ligase Not4 in cotranslational quality control. Front. Genet. 2014;5:141. doi: 10.3389/fgene.2014.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko O.O., Collart M.A. Not4 E3 ligase contributes to proteasome assembly and functional integrity in part through Ecm29. Mol. Cell. Biol. 2011;31:1610–1623. doi: 10.1128/MCB.01210-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panasenko O.O., Collart M.A. Presence of Not5 and ubiquitinated Rps7A in polysome fractions depends upon the Not4 E3 ligase. Mol. Microbiol. 2012;83:640–653. doi: 10.1111/j.1365-2958.2011.07957.x. [DOI] [PubMed] [Google Scholar]
- Panasenko O., Landrieux E., Feuermann M., Finka A., Paquet N., Collart M.A. The yeast Ccr4-Not complex controls ubiquitination of the nascent-associated polypeptide (NAC-EGD) complex. J. Biol. Chem. 2006;281:31389–31398. doi: 10.1074/jbc.M604986200. [DOI] [PubMed] [Google Scholar]
- Petit A.P., Wohlbold L., Bawankar P., Huntzinger E., Schmidt S., Izaurralde E., Weichenrieder O. The structural basis for the interaction between the CAF1 nuclease and the NOT1 scaffold of the human CCR4-NOT deadenylase complex. Nucleic Acids Res. 2012;40:11058–11072. doi: 10.1093/nar/gks883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandler H., Kreth J., Timmers H.T.M., Stoecklin G. Not1 mediates recruitment of the deadenylase Caf1 to mRNAs targeted for degradation by tristetraprolin. Nucleic Acids Res. 2011;39:4373–4386. doi: 10.1093/nar/gkr011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Igarashi K., Aisaki K.-I., Kanno J., Saga Y. NANOS2 interacts with the CCR4-NOT deadenylation complex and leads to suppression of specific RNAs. Proc. Natl. Acad. Sci. USA. 2010;107:3594–3599. doi: 10.1073/pnas.0908664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temme C., Zhang L., Kremmer E., Ihling C., Chartier A., Sinz A., Simonelig M., Wahle E. Subunits of the drosophila CCR4-NOT complex and their roles in mRNA deadenylation. RNA. 2010;16:1356–1370. doi: 10.1261/rna.2145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker M., Staples R.R., Valencia-Sanchez M.A., Muhlrad D., Parker R. Ccr4p is the catalytic subunit of a Ccr4p/Pop2p/Notp mRNA deadenylase complex in Saccharomyces cerevisiae. EMBO J. 2002;21:1427–1436. doi: 10.1093/emboj/21.6.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahle E., Winkler S. RNA decay machines: deadenylation by the Ccr4-Not and Pan2-Pan3 complexes. Biochim. Biophys. Acta. 2013;1829:561–570. doi: 10.1016/j.bbagrm.2013.01.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.