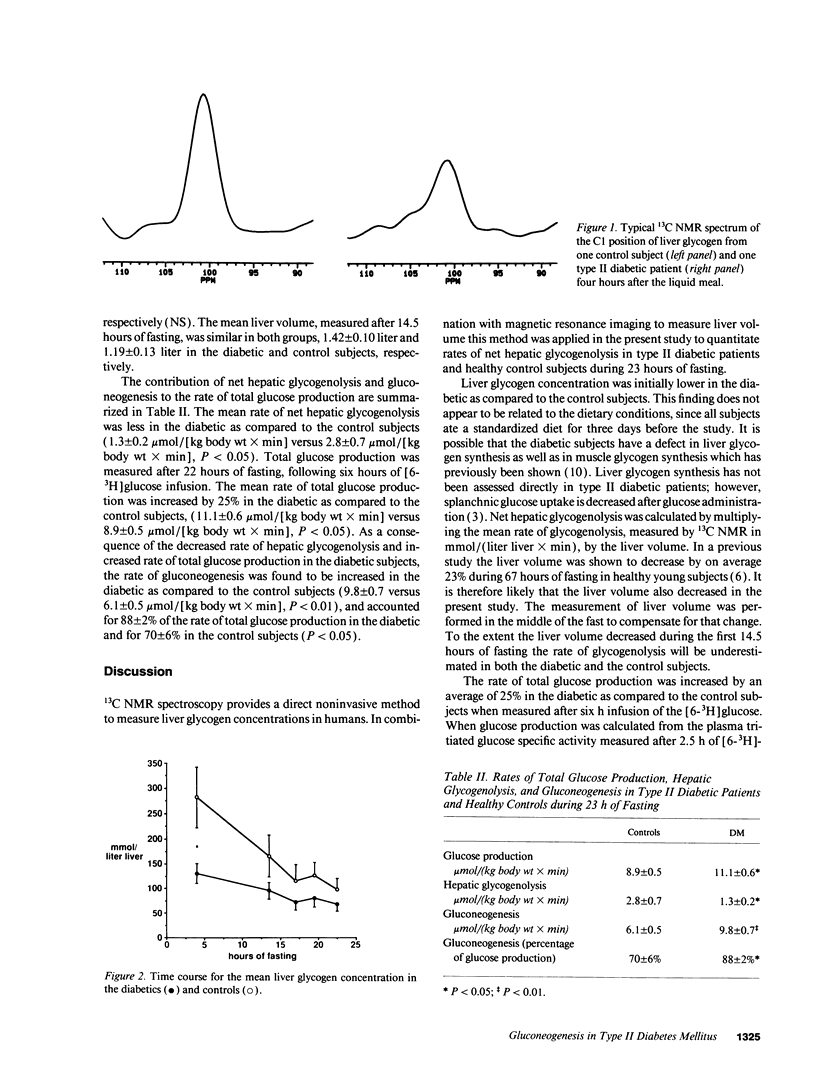
Abstract
To quantitate hepatic glycogenolysis, liver glycogen concentration was measured with 13C nuclear magnetic resonance spectroscopy in seven type II diabetic and five control subjects during 23 h of fasting. Net hepatic glycogenolysis was calculated by multiplying the rate of glycogen breakdown by the liver volume, determined from magnetic resonance images. Gluconeogenesis was calculated by subtracting the rate of hepatic glycogenolysis from the whole body glucose production rate, measured using [6-3H]glucose. Liver glycogen concentration 4 h after a meal was lower in the diabetics than in the controls; 131 +/- 20 versus 282 +/- 60 mmol/liter liver (P < 0.05). Net hepatic glycogenolysis was decreased in the diabetics, 1.3 +/- 0.2 as compared to 2.8 +/- 0.7 mumol/(kg body wt x min) in the controls (P < 0.05). Whole body glucose production was increased in the diabetics as compared to the controls, 11.1 +/- 0.6 versus 8.9 +/- 0.5 mumol/(kg body wt x min) (P < 0.05). Gluconeogenesis was consequently increased in the diabetics, 9.8 +/- 0.7 as compared to 6.1 +/- 0.5 mumol/(kg body wt x min) in the controls (P < 0.01), and accounted for 88 +/- 2% of total glucose production as compared with 70 +/- 6% in the controls (P < 0.05). In conclusion: increased gluconeogenesis is responsible for the increased whole body glucose production in type II diabetes mellitus after an overnight fast.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bogardus C., Lillioja S., Howard B. V., Reaven G., Mott D. Relationships between insulin secretion, insulin action, and fasting plasma glucose concentration in nondiabetic and noninsulin-dependent diabetic subjects. J Clin Invest. 1984 Oct;74(4):1238–1246. doi: 10.1172/JCI111533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Capani F., Gerich J. Predominant role of gluconeogenesis in increased hepatic glucose production in NIDDM. Diabetes. 1989 May;38(5):550–557. doi: 10.2337/diab.38.5.550. [DOI] [PubMed] [Google Scholar]
- Consoli A., Nurjhan N., Reilly J. J., Jr, Bier D. M., Gerich J. E. Mechanism of increased gluconeogenesis in noninsulin-dependent diabetes mellitus. Role of alterations in systemic, hepatic, and muscle lactate and alanine metabolism. J Clin Invest. 1990 Dec;86(6):2038–2045. doi: 10.1172/JCI114940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Simonson D. C. Fasting hyperglycemia in non-insulin-dependent diabetes mellitus: contributions of excessive hepatic glucose production and impaired tissue glucose uptake. Metabolism. 1989 Apr;38(4):387–395. doi: 10.1016/0026-0495(89)90129-7. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Jacot E., Jequier E., Maeder E., Wahren J., Felber J. P. The effect of insulin on the disposal of intravenous glucose. Results from indirect calorimetry and hepatic and femoral venous catheterization. Diabetes. 1981 Dec;30(12):1000–1007. doi: 10.2337/diab.30.12.1000. [DOI] [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of maturity-onset diabetes on splanchnic glucose balance after oral glucose ingestion. Diabetes. 1978 Feb;27(2):121–126. doi: 10.2337/diab.27.2.121. [DOI] [PubMed] [Google Scholar]
- Hother-Nielsen O., Beck-Nielsen H. On the determination of basal glucose production rate in patients with type 2 (non-insulin-dependent) diabetes mellitus using primed-continuous 3-3H-glucose infusion. Diabetologia. 1990 Oct;33(10):603–610. doi: 10.1007/BF00400204. [DOI] [PubMed] [Google Scholar]
- Kreisberg R. A., Siegal A. M., Owen W. C. Alanine and gluconeogenesis in man: effect of ethanol. J Clin Endocrinol Metab. 1972 May;34(5):876–883. doi: 10.1210/jcem-34-5-876. [DOI] [PubMed] [Google Scholar]
- Nilsson L. H. Liver glycogen content in man in the postabsorptive state. Scand J Clin Lab Invest. 1973 Dec;32(4):317–323. doi: 10.3109/00365517309084354. [DOI] [PubMed] [Google Scholar]
- Rothman D. L., Magnusson I., Katz L. D., Shulman R. G., Shulman G. I. Quantitation of hepatic glycogenolysis and gluconeogenesis in fasting humans with 13C NMR. Science. 1991 Oct 25;254(5031):573–576. doi: 10.1126/science.1948033. [DOI] [PubMed] [Google Scholar]
- Schumann W. C., Magnusson I., Chandramouli V., Kumaran K., Wahren J., Landau B. R. Metabolism of [2-14C]acetate and its use in assessing hepatic Krebs cycle activity and gluconeogenesis. J Biol Chem. 1991 Apr 15;266(11):6985–6990. [PubMed] [Google Scholar]
- Shulman G. I., Rothman D. L., Jue T., Stein P., DeFronzo R. A., Shulman R. G. Quantitation of muscle glycogen synthesis in normal subjects and subjects with non-insulin-dependent diabetes by 13C nuclear magnetic resonance spectroscopy. N Engl J Med. 1990 Jan 25;322(4):223–228. doi: 10.1056/NEJM199001253220403. [DOI] [PubMed] [Google Scholar]
- Wahren J., Felig P. Renal substrate exchange in human diabetes mellitus. Diabetes. 1975 Aug;24(8):730–734. doi: 10.2337/diab.24.8.730. [DOI] [PubMed] [Google Scholar]
- Zawadzki J. K., Wolfe R. R., Mott D. M., Lillioja S., Howard B. V., Bogardus C. Increased rate of Cori cycle in obese subjects with NIDDM and effect of weight reduction. Diabetes. 1988 Feb;37(2):154–159. doi: 10.2337/diab.37.2.154. [DOI] [PubMed] [Google Scholar]


