Abstract
The impact of genetics has dramatically affected our understanding of the functions of non-collagenous proteins. Specifically, mutations and knockouts have defined their cellular spectrum of actions. However, the biochemical mechanisms mediated by non-collagenous proteins in biomineralization remain elusive. It is likely that this understanding will require more focused functional testing at the protein, cell, and tissue level. Although initially viewed as rather redundant and static acidic calcium binding proteins, it is now clear that non-collagenous proteins in mineralizing tissues represent diverse entities capable of forming multiple protein-protein nteractions which act in positive and negative ways to regulate the process of bone mineralization. Several new examples from the author’s laboratory are provided which illustrate this theme including an apparent activating effect of hydroxyapatite crystals on metalloproteinases. This review emphasizes the view that secreted non-collagenous proteins in mineralizing bone actively participate in the mineralization process and ultimately control where and how much mineral crystal is deposited, as well as determining the quality and biomechanical properties of the mineralized matrix produced.
Keywords: Biomineralization, PHEX, Phospho 1, Bone sialoprotein, DMP1, Type XI collagen, Fetuin
2. INTRODUCTION
Mineralization of bone represents an extracellular process in which osteoblastic and osteocytic cells remain viable embedded within a matrix of their making. While mature bone matrix is predominantly composed of type I collagen, the ubiquitous expression of collagen indicates that additional factors are needed to initiate and control the formation of hydroxyapatite crystals which are deposited predominantly between the collagen fibrils. In general, investigation of the process of bone formation and mineralization has been difficult because of the restricted location of the forming sites, the temporal movement of the mineralization front as mineralization proceeds, and the mineralized nature of the bone tissue. This has necessitated a reliance upon cell culture models to generate concepts and paradigms which are then tested in explant or whole animal models. This review represents an overview of both in vitro and in vivo model systems and attempts to integrate data from both sources to reach functional concepts regarding the roles of each of the major non-collagenous proteins in biomineralization of bone.
3. PROPOSED MECHANISMS OF MINERAL NUCLEATION IN BONE
New bone formation in the vertebrate embryo is produced by two distinct condensation processes: intramembranous, which forms and persists in the calvaria and jaws, and endochondral, which forms the long bones. In intramembranous bone formation, woven or primary bone is rapidly formed without a cartilaginous precursor, whereas endochondral bone uses a previously formed vascularized cartilage template (1). Primary bone is formed in situations where a rapid deposition of bone is required—embryogenesis, fracture healing, and adaptive bone gain after mechanical loading (~3000 microstrain) (2). In adult skeletons, the two distinct types of bone formation may have common but also unique molecular and cellular control mechanisms. Primary bone is defined as bone that forms where no bone existed previously. In contrast, lamellar or mature bone can be viewed as “replacement bone” formed when bone (either woven or lamellar bone) is resorbed by osteoclasts and then replaced by new lamellar bone. “Modeling” and “remodeling” are applied to the formation of primary bone and lamellar bone, respectively. In both primary and lamellar bone, initial hydroxyapatite crystal nucleation is mediated by organic molecules. There are two essential events in mineralization: the first is nucleation of mineral to produce crystals with a C-axis length of between 15 and 20 nm (3) and the second is mineral crystal propagation, also known as the crystal growth phase (4). Several cell-derived structures have been proposed to be responsible for the initiation and control of the mineral nucleation event. These include: matrix vesicles (5–8), biomineralization foci (9–11), ‘crystal ghosts’ (12), calcospherulites (13), phosphoproteins-collagen fibrils (14, 15) and fetuin (16, 17). The contribution of each of these to the initial nucleation event continues to be debated but it is likely that their relative contributions will vary in different tissues or developmental stages.
3.1. Biomineralization Foci
We have shown that 10–25 micron in diameter BMF are the sites of initial mineral nucleation in three osteoblast cell lines, in developing periosteum, and in new primary bone produced by marrow ablation model (9, 10). Interestingly, pre-BMF can be visualized prior to addition of extra phosphate to the cultures and, following addition, nucleation of hydroxyapatite crystals occurs exclusively within BMF starting as soon as 12 h later. Proteomic analyses on laser capture micro dissected mineralized BMF indicate they are enriched in acidic phosphoproteins BSP and BAG-75, along with a range of different sized vesicles (50 nm to 2 μm) including matrix vesicles (11). Banded collagen fibrils do not appear to be present within these pre-BMF. Vesicles within BMF are the sites of initial mineral nucleation (9–11. 18, 19). Although BSP is associated with the first needle-like crystals produced in developing bone or culture models, it has not been reported to be a component of matrix vesicles. In this way, BSP appears to define a biochemically distinct population of particles which are larger than matrix vesicles and which can also become mineralized (10, 18). Importantly, based on a shared enrichment in BSP and their structure and tissue distribution, Bonucci recently concluded that crystal ghosts are a mineralized tissue equivalent of BMF in osteoblastic cultures (20).
Recently we showed that mineral nucleation within UMR106-01 and fetal mouse calvarial osteoblastic cells could be blocked by 100 μM AEBSF, a covalent serine protease inhibitor, without impacting cell viability (11). In contrast, fifteen general and specific inhibitors against acidic, metallo-, and sulfhydryl proteases were without effect. Partial fragmentation of BMF biomarkers BSP and BAG-75 was also inhibited by AEBSF, along with that for procollagen C-terminal propeptidase enhancer1 (PCOLCE1) and 1,25-vit. D3-MARRS protein (Membrane Associated, Rapid Response Steroid binding receptor). The 50 kDa fragment of BAG-75 was detected using antibodies against the N-terminal peptide (VARYQNTEEEE) (21); in separate studies we have shown that these antibodies detect a 50 kDa band in serum which is induced 3-fold in rats stimulated to undergo new bone formation (unpublished result, Gorski). Subsequent work has sought to establish the mechanism for AEBSF action.
A detailed literature search identified a candidate protease sensitive to AEBSF inhibition, proprotein convertase SKI-1 (S1P) (22). Analysis of BSP, 1,25-D3-MARRS (Membrane Associated, Rapid Response Steroid binding receptor), and procollagen C-proteinase enhancer protein 1 (PCOLCE1), three proteins whose cleavage is blocked by AEBSF (14), reveals each contains several SKI-1 candidate cleavage sequences which appear capable of generating the observed fragments (unpublished result, Huffman, Seidah and Gorski). We have also recently shown that a catalytically active 105 kDa form of SKI-1 (23, 24) is highly expressed within laser capture microscope purified BMF (25).
3.2. Calcospherulites
Midura et al. (29, 30) recently isolated calcospherulites (from the mineralization front of tibial periosteum) and used them to induce the mineralization of type I collagen in vitro. Calcospherulites were identified as 0.5 um diameter particles which had taken up calcein within the 24 h following in vivo injection. The particles exhibited a Ca/P ratio of 1.3 which rose to 1.6 after treatment with ice cold ethanol and were enriched in bone matrix phosphoproteins BSP and BAG-75. When incubated in collagen hydrogels, calcospherulites seeded a mineralization reaction on type I fibrils. When compared with BMF from osteoblastic cells, we conclude that calcospherulites represent mineralized vesicles comparable to those which are the sites of initial mineral deposition within BMF. We believe their membrane bilayer acts as a barrier to proteolysis and permits their isolation based upon their mineral and calcein content, whereas we presume other BMF components are degraded by the dispase used (28,29). In this way, calcospherulites likely represent a protease resistant, mineral containing fraction of BMF (9–11, 18).
3.3. Matrix vesicles
First identified and characterized by Bonucci (31) and by Anderson (32), matrix vesicles have a long and controversial history in bone. However, recent work with BMF and calcospherulites indicates that matrix vesicles, and other vesicles, are present in bone and in mineralizing osteoblastic cultures (9–11, 18, 28, 29). However, while recent work shows that mineral crystals in developing bone are deposited within vesicles (8, 33), it does not show that matrix vesicles are the exclusive sites of mineral deposition in bone. Rather, careful analysis suggests that different sizes and distinct compositional populations of vesicles exist in mineralizing bone, including matrix vesicles, and participate in the initial mineral deposition.
Xiao Z et al. (34) recently characterized the proteome of extracellular matrix vesicles isolated from mineralizing MC3T3-E1 osteoblasts using proteolysis. This confirmed a number of well known matrix vesicle components, including annexin V, alkaline phosphatase, aminopeptidase A, and Emilin-1. In addition, over 125 other proteins were identified in common between the two matrix vesicle preparation methods compared. The results indicate that the protein composition of matrix vesicles is the result of a molecular sorting process which yields a different more specialized composition than expressed on the plasma membranes of differentiated osteoblastic cells (34).
4. THE ROLE OF INDIVIDUAL NON-COLLAGENOUS PROTEINS
As the most prevalent protein in the body, type I collagen is distributed widely among mineralized and non-mineralized tissues. On this basis, it is apparent that type I collagen alone cannot be solely responsible for the unique capacity of bone to mineralize. With the abovementioned initiation systems in mind, we now turn to a consideration of the functional roles of individual non-collagenous proteins. The reader is referred to previous reviews for a more comprehensive treatment of the older literature (3, 35–37).
4.1. Bone Sialoprotein (BSP)
Originally cloned by Fisher and colleagues (38), BSP is thought to be related to the sialoprotein isolated by Herring in 1964 (39). Based on the work of Goldberg and colleagues (40–44), BSP is generally believed to function in bone as a crystal nucleator, although a number of studies demonstrate it is able to signal through its RGD integrin binding site and a second, cryptic cell adhesion site (45, 46). Importantly, Aubin and colleagues have produced a BSP null mouse and characterized its bone phenotype (47–51) with and without skeletal challenge. BSP null mice breed normally but their weight and size is lower than wild type mice. Hypomineralized bone is evident in embryonic and young adult bone, but not in mice over 1 year old (51). At 4 months of age, cortical bone from BSP (−/−) mice is thinner than wild type, but with greater trabecular volume with a very low bone formation rate. BSP null bone also displays fewer osteoclasts. In addition, BSP deficient mice exhibited a delay in healing response at 2 and 3 weeks after formation of cortical defects. In contrast, OPN null mice responded no differently than controls when analyzed by MicroCT or histology. Interestingly, BSP null mice lose bone normally in unloading studies, while OPN null mice do not lose bone (48). In summary, the absence of BSP results in impaired new bone formation and osteoclast activity (47–51). However, the lack of a more dramatic phenotype has been suggested to be due to a functional redundancy with other acidic phosphoproteins in bone.
Gordon et al. (52, 53) have shown that the RGD motif is required to for BSP to induce osteoblastic differentiation. When BSP was over-expressed in primary calvarial osteoblastic cells using a adenoviral vector, Runx2 and Osx expression, alkaline phosphatase, and mineralization were all increased via a pathway involving focal adhesion kinase and MAP kinase associated ERK ½ proteins. These findings seem at odds however with the phenotype of transgenic mice over-expressing BSP using the CMV promoter. The mice display mild dwarfism, lower BMD, and lower trabecular bone volume, while expressing higher levels of bone resorption markers indicating increased osteoclastic bone resorption. An explanation for these unexpected in vivo results is not yet evident.
BSP also has effects on bone resorption of osteoclasts. Curtain et al. (54) showed that dephosphorylated bone BSP preferentially inhibited the differentiation of osteoclasts in a live bone organ culture model via an intracellular tyrosine phosphorylation signaling pathway. Native bone BSP had little effect on osteoclast formation and bone resorption, whereas dephosphorylated BSP eliminated PTH-induced bone resorption. These effects were only partially blocked by integrin receptor antagonist GRGDS. Mechanistically, the effects of dBSP appeared to be mediated by down regulation of osteoblastic RANKL production in this bone organ culture system. Physiologically, Curtain et al. (54) believe that dBSP released/produced by active osteoclastic resorption of bone matrix could feedback to cells in the local environment to block or limit the resorption phase. It should be noted that Mintz et al. (45) first identified a non-RGD, cryptic cell adhesion site on BSP which was exposed upon cleavage. In addition, Sato et al. (55) also showed that BAG-75 inhibited osteoclast differentiation as well as resorption by preformed osteoclasts.
Recent work with recombinant BSP demonstrates that phosphorylation of serine-136 is critical for BSP-mediated nucleation of hydroxyapatite crystals in a steady state assay (44). BSP binding to native type I collagen increased calcium binding by 10-fold compared with free BSP. Serine-136 is a site adjacent to one of two Glu-rich regions previously implicated in hydroxyapatite nucleation, e.g., residues 42–100 and 133–205 (56). Boskey and colleagues have also used diffusion studies within a semi-solid collagenous gel to establish the ability of BSP to nucleate hydroxyapatite crystals (57).
While these data indicate that BSP is the most potent protein nucleator of hydroxyapatite, a role for BSP in the mineralization of collagen is limited by a lack of a mechanism to bind BSP specifically to the hole or gap regions of fibrillar collagen as first proposed by Glimcher (16) and by Veis and Schleuter (15). Since initial mineral crystals were found to be associated with the hole regions in the turkey tendon model of mineralization and since type I collagen is a ubiquitous component of mineralizing as well as non-mineralizing extracellular matrices, these authors argued that the associated phosphoprotein(s), bound to the hole zone of collagen fibrils, acted as a mineralization nucleator. In this way, mineralization of bone extracellular matrix was proposed to be initiated by the formation of seed crystals in the interfibrillary space which would serve to propagate crystal growth within the broader bone matrix. While an attractive hypothesis, the proposal has yet to be validated largely due the absence of a mechanism to localize phosphoproteins to the hole region. However, recent work from our laboratory has provided an interesting potential mechanism for binding of BSP near the hole region of type I collagen fibrils.
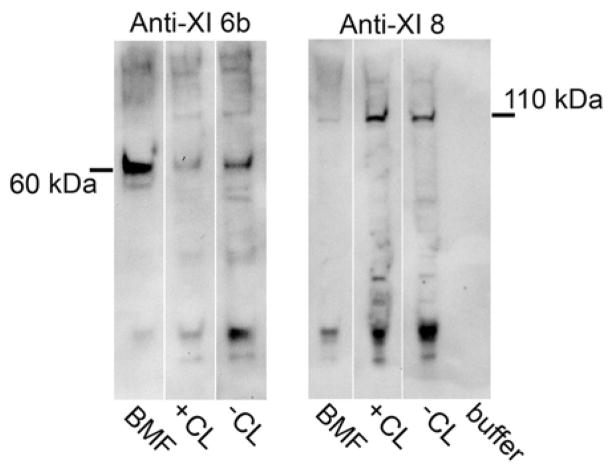
As shown in Figure 1, osteoblastic cells in culture initiate mineralization within spherical extracellular complexes we have termed biomineralization foci (BMF). These BMF are compositionally complex and contain matrix vesicles and larger sized vesicles in which the first mineral crystals are formed. We have used laser micro dissection microscopy to isolate and characterize the proteome of BMF. Interestingly, type XI collagen was found to be enriched within micro dissected BMF, specifically, a 60 kDa fragment containing the 6b alternative splice variant sequence (Figure 2). In contrast, another splice variant, 8, while expressed by UMR106-01 osteoblastic cells as a full length 105 kDa alpha 1 chain, is not enriched within BMF (Figure 2). Both the 6b and 8 sequences are part of the N-terminal domain of the type XI alpha 1 chain (58) (Figure 3). The charge character of the NTD can be changed dramatically by alternative splicing since the 6b sequence has an isoelectric point of 11.3 and unusual arginine triplet repeat sequences. In contrast, the alternating 6a sequence is acidic in character. The NTD is a large globular extension. The NTD variable region containing the 6b and 8 sequences survives BMP-1 processing and exists as part of the extracellular, mature type XI collagen trimer (Figure 3) (59).
Figure 1.
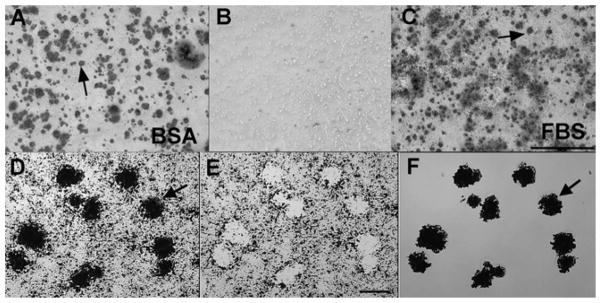
Biomineralization foci can be isolated from total cell layers by laser capture microscopy. A and B, UMR-106-01 osteoblastic cells were cultured in serum-depleted conditions (BSA), or C, the presence of serum (FBS). Cultures were stained with Alizarin red S to detect hydroxyapatite crystals. B, both conditions failed to mineralize in the absence of BGP. Arrows point to mineralized BMF (A and C). Scale bar: 500 μm. D–F, laser capture microscopy of Alizarin red S stained BMF from UMR-106-01 culture. Arrows refer to the same BMF structures in all panels. D, microscopic view of field to be laser-captured. E, appearance of the residual cell layer left behind after laser dissection of mineralized BMF. F, purified BMF temporarily affixed to the “cap” used for laser capture. Scale bar: 25 μm. (Reproduced with permission of the J. of Biological Chemistry).
Figure 2.
Type XI collagen 6b splice variant is enriched in biomineralization foci. Fractions from osteoblastic cultures in Figure 1 were extracted and solubilized and subjected to Western blotting. A 60 kDa N-terminal 6b immunoreactive fragment of the COL11A1 chain was enriched in mineralized BMF (see F, Figure 1) compared to that from the total cell layer from mineralized (see D, Figure 1) or un-mineralized cultures (see B, Figure 1). In contrast, immunoreactive splice variant 8 was not enriched in BMF. KEY:BMF, extract of laser micro dissected biomineralization foci; +CL, extract from cell layer from mineralized culture; −CL, extract from cell layer from un-mineralized culture. Antibodies kindly provided by Dr. J. T. Oxford.
Figure 3.
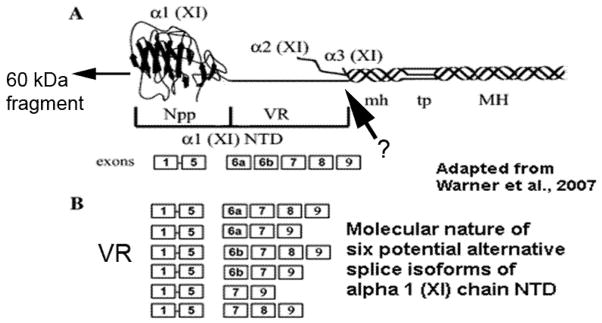
Model of type XI collagen structure [adapted from Warner et al. (58)]. A, Proposed trimeric structure showing large non-helical N-terminal domain for alpha 1 chain. Arrow demarks approximate position of proteolytic cleavage by unknown protease which generates 60 kDa N-terminal fragment found enriched within biomineralization foci (see Figure 2). B, Description of the composition of six different possible splice variant sequences for the N-terminal domain. KEY: VR, variable region; NTD, N-terminal domain; Npp, N-terminal propeptide.
6b sequence: KKKSNYTKKKRTLATNSKKKSKMSTTPKSEKFASKKKKRNQATAKAKLGVQ
6a sequence: YAPEDIIEYDYEYGETDYKEAESVTEMPTFTEETVAQT
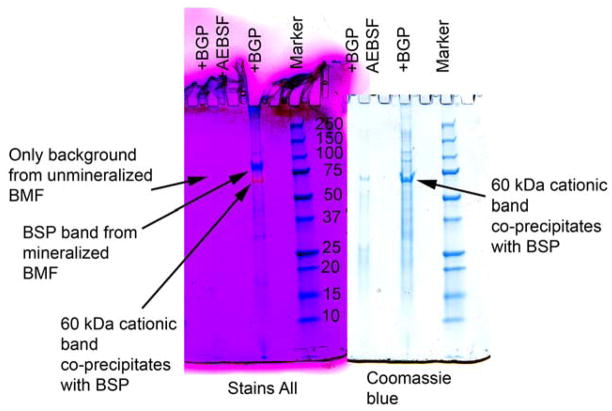
Alternatively, as shown by our culture data, the 6b region can be cleaved off as a 60 kDa fragment (Figures 2 and 3). As illustrated in Figure 4, the 60 kDa 6b containing type XI NTD fragment associates specifically with bone sialoprotein. Mineralized osteoblastic cell cultures were extracted with EDTA, which removes predominantly material associated with BMF (11), and then immunoprecipitated with anti-BSP antibodies. Interestingly, BSP and the 60 kDa type XI NTD region were only recovered from cultures which mineralized, not those inhibited with AEBSF (Figure 4). Other studies show that the 60 kDa band reacts with anti-6b type XI collagen antibodies and anti-Npp antibodies establishing it as the complete NTD domain (data not shown) (Figure 3).
Figure 4.
Bone sialoprotein is immunoprecipitated together with 60 kDa cationic type XI N-terminal fragment only from mineralized osteoblastic cultures. Extracts of mineralized and un-mineralized osteoblastic cultures were immunoprecipitated with anti-bone sialoprotein antibodies. Immunoprecipitates were subjected to SDS-PAGE and the same gel was stained sequentially with Stains All (left) and then with Coomassie blue dye (right). KEY: +BGP +AEBSF, extract from culture where mineralization was inhibited by 4-(2-aminoethyl)-benzenesulfonyl fluoride hydrochloride; +BGP, extract from mineralized culture; marker, molecular weight standards.
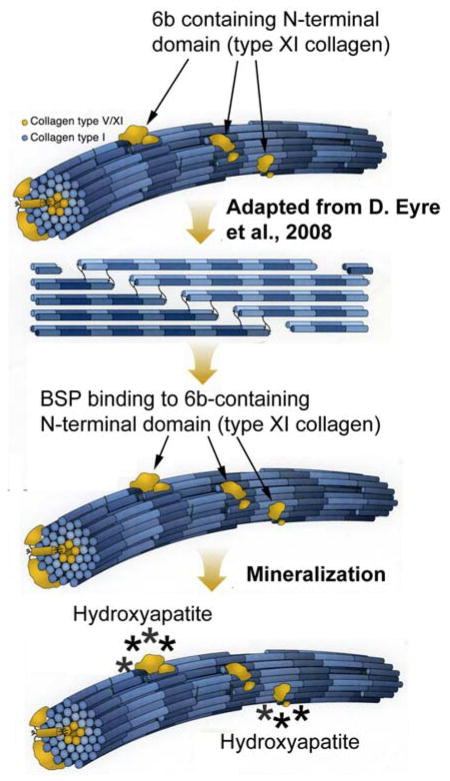
Taken together we believe these results provide a new novel mechanism by which to localize mineral nucleator protein BSP to the surface of collagen fibrils. Specifically, type XI collagen has been shown to play a critical role in the initiation of type I and II collagen fibrillogenesis, and, in the regulation of fibril diameter (60). As indicated by the model in Figure 5, the 6b sequence of the variable region of the COL11 A1 chain is believed to be expressed on the surface of type I collagen fibrils (61). We propose that BSP can bind to the cationic 6b sequence either as a 60 kDa fragment within mineralizing BMF (Figures 2 and 4) or as present in the BMP-1 processed COL A1 chain exposed on the surfaces of type I collagen fibrils (Figure 5). In this way, BSP/type XI collagen complexes could act as local nucleators to initate mineralization of collagen fibrils. The fact that BSP and the 6b containing 60 kDa type XI collagen fragment are also enriched within BMF suggests that BMF and collagen mineralization may share in part a common mechanism for mineral crystal nucleation.
Figure 5.
Model for mineralization of collagen fibrils involving 6b splice variant containing type XI collagen and phosphoprotein BSP. Adapted from image by D. Eyre et al. (61), cationic 6b containing N-terminal domains are positioned on the surface of type I fibrils where they can bind mineralization nucleator BSP. We propose this mechanism may fulfill the 1964 phosphoprotein-collagen hypothesis of Glimcher, and, Veis and Schleuter.
4.2. Noggin
Noggin, along with Chordin, is a bone morphogenetic protein (BMP) antagonist. During mouse embryogenesis, Noggin is expressed at multiple sites, including developing bones. Interestingly, Nog (−/−) mice die at birth from multiple defects that include bony fusion of the appendicular skeleton (62, 63). Groppe J et al. (64) reported the crystal structure of the antagonist noggin bound to BMP-7, which showed that noggin blocks the molecular interfaces of the binding epitopes for both type I and type II receptors. Importantly, the affinity of site-specific variants of noggin for BMP-7 correlated with changes in bone formation and apoptosis in chick limb development, showing that these complexes are biologically inactive.
4.3. Chordin
Chordin dorsalizes early vertebrate embryonic tissues by binding to bone morphogenetic proteins and, like noggin, sequestering them in latent complexes. Scott et al. (65) showed that over-expression of BMP1 and TLL1 in Xenopus embryos counteracted the developmental effects of chordin. Mechanistically, BMP1 and mTLL-1 cleave chordin at sites similar to procollagen C-propeptide cleavage sites. Other BMP-1 family members mTLD and mTLL-2 do not, however, cleave chordin. Interestingly, Wardle et al. (66) showed that noggin was not affected by over-expression of BMP-1 (and presumably not cleaved by it). Based on expression and its enzymatic activity, BMP-1 is the major chordin antagonist in early mammalian embryogenesis and in pre- and postnatal skeletogenesis.
4.4. Osteopontin (OPN)
OPN is an RGD-containing, variably phosphorylated member of the SIBLING family of non-collagenous matrix proteins. It is expressed in bone and bone marrow by osteoblastic and osteoclastic precursor cells, multi-nucleated osteoclasts, osteocytes, and osteoblasts (67–69). Franzen et al. (70) showed that OPN null mice did not experience age related bone loss. Specifically, measures of osteoclast function were several fold lower, despite an increase in the volume and number of osteoclastic cells in the lower tibial metaphysis which was hypothesized as a compensatory change to overcome the observed inactivity of osteoclasts. These findings indicate that OPN is required for osteoclastic resorption of bone (70). Asou et al. (71) also found that osteopontin deficient calvarial bone was inefficiently resorbed when implanted in osteopontin null mice compared with controls. The osteoclast deficiency observed here seemed to be due to reduced angiogenesis in the OPN null mice since it could be reversed by applying OPN protein to the deficient calvarial bone surface prior to its implantation in OPN deficient mice. These results demonstrate that OPN is required both for vascularization and subsequent osteoclastic resorption of bone (71).
A central role for OPN in the metabolic regulation of bone is also supported by the finding that PTH induced bone resorption does not occur in the absence of OPN (72). OPN deficiency also enhanced the formative response of long bones of null mice to intermittent PTH injections relative to that of control mice (73).
OPN has long been believed to function as an inhibitor of calcification reactions (74) by binding specifically to the growing faces of mineral crystals. However, the extent of phosphorylation is a critical modulator of OPN-mineral interactions (74, 75). For example, recombinant OPN and dephosphorylated OPN had no effect on hydroxyapatite crystal formation or growth. In contrast, milk OPN, which contains >30 phosphates/mole, promotes hydroxyapatite formation in static assays (74). In this context, Hunter et al. (76) has recently reviewed the topic of non-collagenous protein mediated biomineralization, e.g., the interaction of nucleator proteins with calcium and phosphate ions. They conclude that the widely held concept of epitaxic crystal initiation may not fit current evidence. Their review of existing data and new energy minimization calculations indicates that most known crystal-bone acidic protein interactions are electrostatic in nature and depend primarily upon the inherent high electronegative charge density of these interactions rather than upon a particular stereochemical conformation. As a result, Hunter et al. (76) have put forth a thoughtful alternate hypothesis, e.g., the flexible polyelectrolyte hypothesis of protein-biomineral interaction.
4.5. Osteopontin, bone sialoprotein, and DMP1 form individual complexes with MMPs
Fedarko and colleagues have shown that three SIBLING proteins form specific high affinity complexes with several individual proMMPs (pro-metalloproteinases) and their active forms expressed in skeletal tissues (77). BSP complexes with proMMP-2 and active MMP-2, while osteopontin binds proMMP-3 and active MMP-3, and DMP1 binds proMMP-9 and active MMP-9. For MMP-2, BSP is also able to induce catalytic activity in latent pro-MMP-2 as well as to rescue active enzyme from TIMP2-MMP-2 inhibitory complexes (78, 79). All complexes were disrupted by competition with serum complement factor H suggesting that analogous physiological SIBLING complexes are likely to be limited to sequestered areas near cells. Interestingly, bound MMPs displayed a diminished susceptibility to their respective natural and synthetic inhibitors (77) which may be particularly relevant to the invasive behavior of cancer cells which jointly express high levels of MMPs and these SIBLING proteins (80).
4.6. Bone acidic glycoprotein-75
BAG-75 is defined by its N-terminal sequence (LPVARYQNTEEEE) and its immunoreactivity with an anti-protein antibody, an anti-peptide antibody against its N-terminal sequence, and a monoclonal antibody (21, 81, 82). A highly acidic, phosphoprotein isolated from and enriched in primary or intramembranous bone (3), BAG-75 binds more calcium ions (133 atoms/mole) than either BSP or OPN on a molar basis. Functionally, when added to differentiated osteoclasts or to differentiating osteoclast culture models, BAG-75 inhibits bone resorption (55). In contrast to BSP or OPN, BAG-75 displays a propensity to self-associate into macromolecular complexes which have the capacity to also trap or sequester large numbers of phosphate ions (83). Operationally, BAG-75 has served as a useful immunoreactive biomarker for areas in osteoblastic cultures and in healing bone which will subsequently become mineralized (11, 19).
4.7 Dentin matrix protein 1
Originally thought to be expressed only in cementoblasts, ameloblasts, osteoblasts, and odontoblasts (84), DMP1 is now known to be also enriched in osteocytes (85). DMP1 is cleaved by BMP-1 into a 55 kDa C-terminal fragment and 35 kDa N-terminal fragment (86, 87). Using furin-deficient cells, von Marschall and Fisher (86) compared three different isoforms of BMP1 and each displayed different activities. In contrast to collagen cleavage by BMP-1, DMP1 cleavage showed no enhancement by procollagenase enhancer protein. DMP1 mediates cell adhesion via its RGD site with alphavbeta3 receptors on cells. RGD binding was the same for full length and C-terminal fragment, however, a proteoglycan form of DMP1 was much less active in cell adhesion assays. (88).
Tartaiz et al. (89) showed that non-phosphorylated recombinant DMP1 acted as a hydroxyapatite nucleator in a gelatin gel static diffusion system while DMP1 phosphorylated in vitro has no effect on crystal growth or formation. Phosphorylated DMP1 synthesized by marrow stromal cells (30 phosphates/mole) however acted as an inhibitor of hydroxyapatite crystal growth and formation. These results seem to indicate that the position of phosphorylation is important for DMP1 to function as a mineralization inhibitor. In vivo, DMP1 exists predominantly in tissues as two highly phosphorylated fragments of 57 kDa and 35 kDa. The 57 kDa fragment also acted as a hydroxyapatite nucleator. Gajjeraman et al. (90) has also shown that the N-terminal fragment of DMP1 acted as a mineralization inhibitor. However, in contrast to Tartaiz et al. (89), they also found that both full-length recombinant DMP1 and post-translationally modified native DMP1 were able to nucleate hydroxyapatite in the presence of type I collagen. This disparity may be due in part to the purity of the DMP1 fragment tested since it is now appreciated that two fragments are derived from the N-terminus, a chondroitin sulfate linked fragment and a 37 kDa polypeptide (91). The proteoglycan fragment acts as a mineralization inhibitor while the remaining 57 kDa and 37 kDa fragments both promoter hydroxyapatite crystal formation and growth in vitro.
Physiologically, the function of DMP1 in mineralized tissues is closely tied to systemic phosphate regulation. DMP1 null mice and patients with DMP1 mutations exhibit autosomic hypophosphatemic rickets (92, 93). Over-expression of either full-length DMP1 or the 57 kDa C-terminal fragment in the DMP1-null strain largely rescued the skeletal phenotype in mice suggesting that the latter fragment is able to recapitulate the function of full-length DMP1 (94). It can be assumed that the reduced mineralization detected in DMP1 null bone (95) may be largely due to the hypophosphatemia which is a characteristic of the skeletal phenotype (96). The hypophosphatemia of DMP1 null mice is associated with elevated levels of FGF23, a well known negative regulator of systemic phosphate concentration, although the exact mechanism remains unclear (96).
4.8. Osteocalcin
Thought to be the most bone restricted protein produced by osteoblastic cells, osteocalcin is unusual in that it is post-translationally modified at up to three glutamic acid residues (GLA)/mole by gamma-carboxylation in a vitamin K dependent enzymatic mechanism (97). Due to its acidic character and GLA residues osteocalcin was initially presumed to be an important regulator of bone mineralization. However, the phenotype of osteocalcin knockout mice is mild and displays no major bone abnormalities (98). These results raised serious questions regarding the function of osteocalcin in bone. Recently, Karsenty and colleagues have suggested that osteocalcin functions as a hormone released from bone into the blood to regulate insulin secretion by the beta-cells of the pancreas and to control insulin sensitivity by adipocytes (99). Part of a new complex metabolic feedback loop controlling bone, pancreas, and fat tissue, the active form of osteocalcin is undercarboxylated. In this way, the content of active, undercarboxylated osteocalcin in blood is believed to be derived via osteoclastic bone resorption of bone matrix. The process of osteoclastic resorption is believed to expose bone matrix derived osteocalcin to low pH conditions (<4.5) which lead to its chemical decarboxylation and release to the blood (99). The full pathway remains to be completely elucidated since the receptor on pancreatic and fat cells which recognizes undercarboxylated osteocalcin has not yet been identified. Also, the mechanism requires validation to show that acid mediated decarboxylation of osteocalcin is compatible with the temporal sequence of osteoclastic resorption in vivo. While this pathway has been largely worked out through genetic ablation of various genes in mice, a growing body of supporting evidence indicates that osteocalcin serves as a metabolic hormone in humans as well (100). Thus, this novel osteocalcin paradigm raises the intriguing question whether other bone matrix proteins may also possess hormonal functions regulating other metabolic systems, e.g., systemic calcium and phosphorus ion concentrations, fat storage, etc.
4.9. Fetuin (alpha2HS-glycoprotein)
Fetuin is produced by the liver and by osteoblastic cells (101). Highly acidic, fetuin has the capacity to bind large numbers of calcium ions. As such, fetuin is believed to be a major systemic inhibitor of ectopic calcification in the blood plasma and in tissue fluids (102). As a consequence, fetuin null mice develop micro-calcifications in small blood vessels and increased soft tissue calcification deposits. Interestingly, fetuin also forms a colloid or suspension of fine particles with insoluble calcium phosphate crystals which have been termed calciprotein particles or CPPs (102–105). Jahnen-Dechent and McKee (106) have suggested that CPPs participate in a circulating pathway in plasma to solubilize crystalline calcium deposits and transport finely divided crystal particles among sites of calcium loss (the intestine, kidney, bone). In this sense, recent stopped flow experimental data from this group suggests that the ability of fetuin to inhibit calcification is due to its ability to shield and stabilize early stage protein-mineral complexes as small CPP-like particles rather than permit aggregation of nuclei with subsequent mineral crystal precipitation (107). While very intriguing, the physiological significance CPPs in the process of osteoblast mediated bone formation and mineralization remains to be established. Although likely to be involved, the capacity of fetuin to form and stabilize particulate microcrystals of calcium phosphate (hydroxyapatite) has not been formally tested in the “mineralization by inhibitor exclusion” model proposed by Price et al. (17) (see above). Furthermore, the “calcification activity” detected in serum from humans to fish and sharks and partially characterized by Price and colleagues (108) also seems likely to be due, at least in part, to the fetuin in serum and its associated finely suspended colloidal particles of calcium phosphate.
4.10. Periostin
Kashima et al. (109) showed that periostin is a biomarker for intramembranous bone and is preferentially expressed in the bone produced by individuals with fibrous dysplasia, a benign condition, while not expressed in endochondral bone. Periostin is a disulfide linked GLA-containing 90 kDa protein secreted by osteoblasts and osteoblastic cell lines whose expression is down regulated by phosphate ion and is restricted to the periosteum and periodontal ligament (109, 110). Periostin shows some homology to Betaig-h3, a protein that promotes adhesion and spreading of fibroblasts (111). Betaig-h3 also contains GLA residues. Gamma-carboxylated periostin localizes to mineralized bone nodules formed by mesenchymal stem cells. Periostin knockout mice expressing LacZ were grossly normal at birth, however, 14% of the deficient mice died before weaning. Since all of remaining LacZ positive mice were growth retarded, periostin is required for normal postnatal bone growth (112). In this regard, Bonnet et al. (113) showed that periostin is required for the anabolic response of bone to mechanical loading and physical activity. In its absence, long bones in periostin null mice do not respond anabolically to axial compression loading, whereas periostin (+/+) mice respond normally by increasing bone formation activity especially in the periosteum. Importantly, the biomechanical response of periostin null mice was rescued by injection of sclerostin-blocking antibody. These results indicate that periostin acts to block the actions of sclerostin on bone formation. When combined with the skeletal retardation observed in periostin null mice (112), these results show that periostin is a regulator of bone mass and microstructure in response to loading.
Maruhashi et al. (114) also showed that periostin stimulated the proteolytic activation of lysyl oxidase. Lysyl oxidase mediates the formation of reactive aldehydes on collagen chains which participate in formation of chemical cross-links. However, lysyl oxidase requires proteolytic activation by BMP-1. Interestingly, the amount of active lysyl oxidase was reduced in calvarial osteoblasts from periostin null mice, while over-expression of periostin promoted release of its propeptide and enzymatic activation. Since direct binding studies showed BMP-1 binds to periostin, these results indicate that periostin acts in analogous way to PCOLCE-1, an enhancer protein which increases by up to 20-fold the rate of procollagen processing by BMP-1 (115).
4.11. Tissue Nonspecific Alkaline Phosphatase
While its expression is not restricted to bone, alkaline phosphatase has long been a prominent component of bone mineralization mechanisms due to its high expression in mineralizing tissues, its enrichment within matrix vesicles, and the ease of its functional analysis. TNAP is associated with the external face of surfaces of osteoblastic and chondrocytic cells via a phosphatidylinositol anchor (116, 117) which can be released by a specific phospholipase D (118). The functional role of alkaline phosphatase in mineralization has become somewhat refocused by very recent studies combining the TNAP knockout with that for Phospho 1.
4.12. Phospho 1 phosphatase
Phospho 1 is a phosphatase whose actions are restricted enzymatically to mineralizing cells where it displays a catalytic preference for substrates phosphatidylethanolamine and phosphatidylcholine (119). Phospho 1 is expressed specifically at sites of mineralization of cartilage and bone in osteoblasts and newly formed osteocytes (120). Phospho 1 null mice display growth plate abnormalities, spontaneous fractures, bowed long bones, osteomalacia, and scoliosis in early life. The fact that over-expression of TNAP does not prevent the development of skeletal abnormalities in the Phospho1 null mice, despite correction of plasma PPi levels and of greatly elevated TNAP levels, suggests that Phospho 1 functions through a pathway that is distinct from that of TNAP (8).
As a component of matrix vesicles, it has been suggested that Phospho 1 could produce free phosphate for initiation of matrix mineralization using phosphatidylethanolamine and phosphatidylcholine lipids in matrix vesicles (121). Previous models of mineralization in bone and cartilage have emphasized a role for alkaline phosphatase. However, while deficiency of alkaline phosphatase does diminish the mineral density of bone and growth plate cartilage in young TNAP knockout mice, careful transmission electron microscopic analyses revealed that initiation of mineralization still occurred within vesicles of TNAP deficient bone (32). These findings suggested that hypo-mineralization in TNAP-deficient mice results primarily from an inability of mineral crystals to proliferate beyond the limits of the vesicular membrane. This failure of the second (proliferation) stage of mineralization may be caused by an excess of pyrophosphate inhibitor in the surrounding extracellular fluid. As proposed by Millan and Terkeltaub and colleagues, the concentration of mineralization inhibitor pyrophosphate is regulated by the balance between its breakdown by alkaline phosphatase and its production by PC-1 (ENPP1) (122).
Functionally, Phospho 1 is required to initiate de novo mineral crystals within matrix vesicles. For example, treatment of mesenchymal micromass cultures with lansoprazole, a small inhibitor of Phospho 1 resulted in reduced Alizarin red staining. In situ hybridization showed that expression of Phospho 1 preceded mineralization (and correlated with TNAP). Importantly, treatment of developing embryos for 5 days with lansoprazole completely inhibited mineralization of all leg and wing long bones as assessed by Alcian blue/Alizarin red staining (123). Yadav et al. (8) simultaneously knocked out Phospho 1 and TNAP and showed that the double knockout was completely devoid of skeletal mineralization and exhibited perinatal lethality, despite relatively normal systemic phosphate levels. They concluded that Phospho 1 has a non-redundant functional role during endochondral ossification and proposed an inclusive model of calcification that places Phospho 1 phosphatase action within vesicles in the initiation of mineralization and the functions of TNAP, nucleotide pyrophosphatase phosphodiesterase-1 and collagen in the extravesicular progression (propagation) of mineralization. The reader is
4.13. Ectonucleotide Pyrophosphatase/Phosphodiesterase (ENPP1)
A transmembrane glycoprotein with a short cytoplasmic tail, single transmembrane segment, and an extracellular C- terminal domain, ENPP1 helps to regulate bone and cartilage mineralization by producing inorganic pyrophosphate. The enzyme exhibits both 5′-nucleotide phosphodiesterase I and nucleotide pyrophosphohydrolase activities (124). Based on the results of a double knockout of alkaline phosphatase and ENPP1, the Millan and Terkeltaub labs suggested that these two enzymes each served to balance the activity of the other. Specifically, the double knockout corrects bone mineralization abnormalities which occur in single deletions of each individual enzyme (122). As noted above, recent work with Phospho 1 has clarified the potential roles of ENPP1 both as a 1) producer of pyrophosphate mineralization inhibitor and 2), under conditions of limiting alkaline phosphatase activity, as a producer of extracellular inorganic phosphate via its phosphodiesterase activity to feed the growth and propagation of mineral crystals (8).
4.14. Biological Effects of Hydroxyapatite on Bone Matrix Proteins
Hydroxyapatite is the major crystalline form of calcium phosphate in bone and cartilage tissues. However, hydroxyapatite is thought to be biologically inert. Several studies raise the prospect that hydroxyapatite crystals can modify cellular MMP production and function in bone. First, exposure of fibroblasts or breast cancer cells to hydroxyapatite, calcium pyrophosphate, or basic calcium phosphate crystals induces mitogenesis as well as increases the transcription and expression of MMP-1 and MMP-3 (125–127). In all cases, endocytosis is required for crystals to induce synthesis of MMPs. The effect of calcium phosphate crystals could be abrogated by phosphocitrate, although the mechanism is unclear. Second, we have shown that hydroxyapatite crystals are able to alter dramatically the functional activity of MMP-1 and MMP-3 (83). Specifically, these MMPs, when bound, changed their substrate specificity such that each catalyzed their own autolytic degradation in an intermolecular, calcium-dependent process. Interestingly, other proteases including MMP-2, trypsin, chymotrypsin, papain, and zinc-dependent thermolysin were all unaffected by exposure to hydroxyapatite crystals (83).
Recent work with PHEX (phosphate regulating endoprotease homolog, x-linked) further supports an active role for hydroxyapatite crystals in bone function. PHEX is a single pass transmembrane endoprotease which regulates systemic phosphate metabolism since its mutational inactivation causes x-linked hypophosphatemia (128). Specifically, we have found that a 60 kDa, catalytically active fragment of PHEX is enriched within mineralized biomineralization foci purified by laser micro dissection from osteoblastic cultures (Figure 6). BMF are the initial sites of mineral nucleation within at least three different osteoblastic culture models (9–11, 18, 19). Initial proteomic characterization revealed that mineralized BMF were physically enriched in phosphoproteins BSP and BAG-75 (2–4). However, we have continued to investigate over 35 other matrix proteins and calcium or phosphate channels/transporters as candidate constituents of BMF. Thus far, only four proteins have been found to be physically enriched within BMF complexes, e.g., BSP (9–11, 18), BAG-75 (9–11, 18), Phex, and type XI collagen (see Figure 2 above). This point is illustrated in Figure 6, which shows the specificity of this enrichment through comparisons with 110 kDa full length MEPE and a 57 kDa fragment of DMP1, which are notably not enriched in mineralized BMF. Because BMF are detectable before mineralization and become the exclusive sites of initial mineral production/deposition in osteoblastic cultures, we hypothesize that these enriched proteins and their fragments work together to mediate this unique function of BMF complexes, e.g., mineral crystal nucleation.
Figure 6.
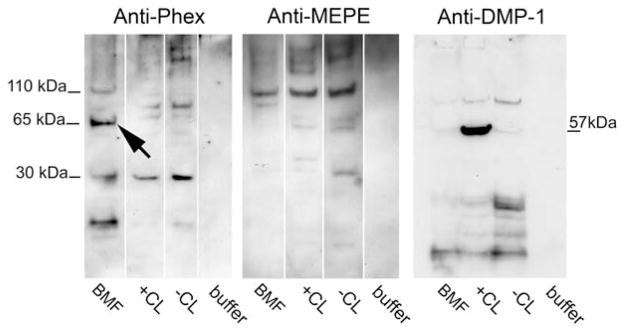
Immunoblotting shows that a 60 kDa fragment of PHEX is specifically enriched in biomineralization foci whereas MEPE and DMP1 are not. BMF were isolated from Alizarin red stained cell layers using laser micro dissection (see Figure 1), and along with total cell layer fractions, were extracted as described by Huffman et al. (11). Six micrograms of total protein were applied to each lane and Western blotted with monospecific antibodies using chemiluminescent detection. Large arrow denotes band specifically enriched in purified BMF. KEY: BMF, extract of laser micro dissected biomineralization foci; +CL, extract from cell layer from mineralized culture; −CL, extract from cell; and, buffer alone, extraction buffer only. Antibodies were kindly provided by Drs. P.S. Rowe and L.D. Bonewald. referred to the publication by Yadav et al. (8) for a more complete description of their “unified model of mineralization”.
PHEX is an endoprotease which is structurally related to the M13 family of proteases. We have found that PHEX also exhibits a limited homology with MMP-3 (matrix metalloproteinase-3 or stromelysin), another zinc-dependent metalloprotease. A two way BLAST comparison of these sequences
Phex: 538-NAFYSASTNQ I R - - FPAG-553 (identities noted with bold symbols)
MMP-3: 358-NQFWAIRGNEVRAGYPRG-375
reveals a statistically significant homology of 33% with the #538–553 PHEX peptide region depicted; four other peptides were also found to share a 30–40% identity with MMP-3 (not shown). Homologies >25–30% are generally considered “real” and likely reflect at least a partial shared evolutionary history. The homology with the #538–553 peptide may be particularly important because this region is believed to represent part of the PHEX substrate binding domain (10), whereas in MMP-3 it comprises part of the C-terminal hemopexin domain.
This newly identified structural relationship suggests that PHEX may share functional properties with MMP-3. Because PHEX is localized preferentially to mineralized BMF (Figure 6) and because the substrate binding domain of PHEX exhibits a limited structural homology with MMP-3 (see above), we asked whether the 60 kDa fragment of PHEX observed in BMF was produced by autolytic cleavage induced by exposure to hydroxyapatite crystals. Figure 7 shows that 60 kDa PHEX is preferentially produced only when recombinant 98 kDa PHEX is incubated with hydroxyapatite crystals in the presence of ASARM peptide. Recombinant 98 kDa PHEX is missing its single transmembrane spanning domain near the N-terminus and a small intracellular domain at the N-terminus of the native protein (129, 130). We conclude that PHEX was induced to cleave itself, as seen for MMP-1 or MMP-3 (83), upon exposure to hydroxyapatite crystals. Since a similar sized 60 kDa fragment was detected by Western blotting of both recombinant PHEX (Figure 7) and of laser micro dissected BMF complexes (Figure 6), we hypothesize that a single hydroxyapatite induced autolytic cleavage site for PHEX is located in the N-terminal half of the sequence yielding a 60 kDa fragment sharing the same C-terminus for both. This is further supported by the fact that Western blots used anti-PHEX antibodies specific for a peptide very near the zinc-binding domain of PHEX (gift of Dr. P. S. Rowe, Univ. of Kansas). Second, we hypothesized that the 60 kDa fragment contained the catalytic region (zinc binding domain and substrate binding site) of PHEX (130), e.g., the 60 kDa fragment was catalytically active.
Figure 7.
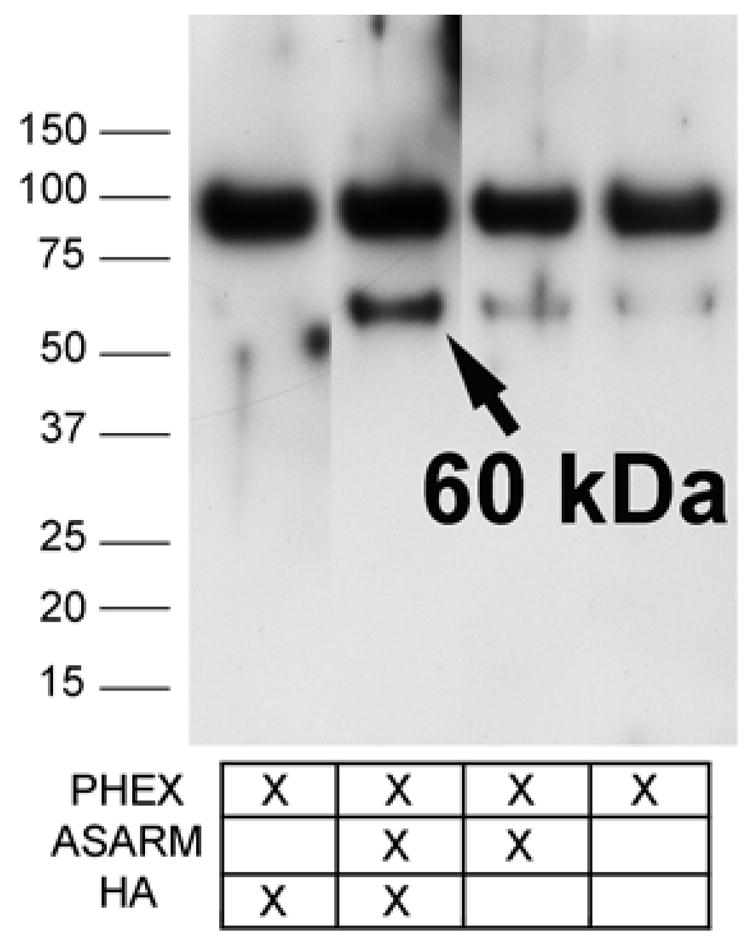
Hydroxyapatite induces autolytic cleavage of PHEX in presence of ASARM peptide. KEY: PHEX, recombinant 98 kDa secPHEX; ASARM, ASARM peptide; and HA, hydroxyapatite crystals. Proteins were kindly provided by Dr. Peter S. Rowe.
As shown in Figures 8A and 8B, both PHEX activity and the 60 kDa PHEX fragment content are both 2–3 fold higher in crude osteoblast plasma membrane preparations isolated from mineralized UMR106-01 osteoblastic cultures exposed to hydroxyapatite crystals as compared with that where mineralization was blocked by serine protease inhibitor AEBSF (11) or by omission of exogenous phosphate. Our results strongly suggest that an active 60 kDa PHEX fragment is produced by exposure to hydroxyapatite crystals during the process of mineralization occurring within biomineralization foci. We speculate that the 60 kDa PHEX could exhibit altered enzymatic properties relative to the 98 kDa recombinant isoform and/or the 105 kDa full-length membrane bound isoform. In this way, 60 kDa PHEX could provide a local or systemic feedback signal marking the initiation of mineralization, e.g., to mobilize additional phosphate ions to feed the growth of the new mineral phase.
Figure 8.
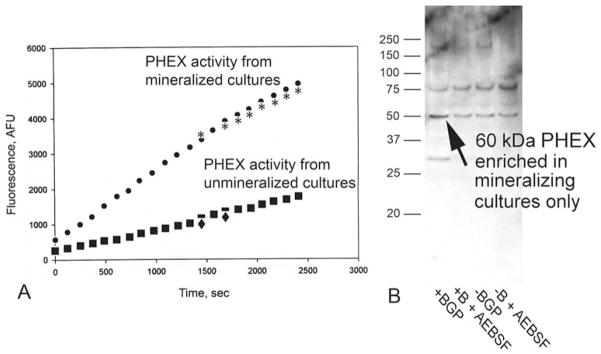
PHEX endoprotease activity and 60 kDa PHEX fragment content are higher in membranes from mineralized osteoblastic cultures versus un-mineralized controls. A, Crude membranes were isolated and assayed for PHEX endopeptidase activity with a specific fluorescent substrate assay as previously described (147). Cultures assayed were either mineralized by addition of BGP (● and *) or treated with AEBSF to block mineralization (■ and ◆) (188). Individual symbols on the graph represent duplicate analyses. Activity was normalized based on the protein content of membrane preparations. (Data obtained in collaboration with Drs. S. Liu and D. Quarles). B, Crude membranes from mineralizing cultures are enriched in the 60 kDa PHEX band. Comparing the data in Figures 8A and 8B leads to the conclusion that the 60 kDa PHEX band is catalytically active. KEY: +BGP, mineralized; +B + AEBSF, mineralization blocked with AEBSF; −BGP, unmineralized (without BGP); −BGP + AEBSF (without BGP but with AEBSF). (Data obtained in collaboration with Drs. S. Liu and D. Quarles.)
4.15. Sclerostin
Encoded by its gene Sost, Sclerostin is a secreted glycoprotein highly expressed by osteocytes. Inactivating mutations in humans leads to a rare high bone mass condition called sclerosteosis. Transgenic knockout of Sclerostin in mice also leads to increased bone formation and bone strength (131). These findings demonstrate that Sclerostin functions as an anabolic inhibitor, a blocker of bone formation. Its mechanism of action on osteoblasts/osteocytes appears to involve strong protein-protein interactions/complexes with Periostin, Noggin, and cysteine-rich protein 61. Cysteine-rich protein 61 (Cyr 61) regulates mesenchymal stem cell proliferation and differentiation, osteoblast and osteoclast function, and angiogenesis (132). Bonnet et al. (113) used mechanical stimuli to show that periostin is required for the anabolic response to mechanical loading and physical activity. Administration of anti-Sclerostin blocking antibody to periostin (−/−) null mice rescued the anabolic bone formation response to biomechanical stimuli which had previously been blocked by this transgenic deletion (131). Availability of Sclerostin is also regulated by its interactions with Noggin, with whom it forms tight, mutually inhibitory complexes (2.9 × 10−9 M) (133). The formation of the Sclerostin-Noggin complex directly neutralizes BMP binding activity and attenuates the effects of these two BMP antagonists. As a result, the net biological effect of Sclerostin may depend upon its balance between various binding partners in the extracellular matrix.
4.16. Tenascin C
A large oligomeric extracellular matrix glycoprotein, Tenascin is also known as hexabrachion or cytotactin due to its hexameric quaternary structure with six arms linked to a central globular domain (134). Its expression is restricted to vertebrate development where it appears during neural crest cell migration and later at sites of cartilage, bone, and tendon formation. Tenascin can function to promote neurite outgrowth in vitro and can inhibit cell interactions with fibronectin, where each of these functional interactions is mediated by distinct cell binding sites. Tenascin-C has been proposed to be involved in the development of articular cartilage and in the mechanism by which articular chondrocytes avoid the endochondral ossification process (which occurs with bulk of metaphyseal and diaphyseal chondrocytes) (135). Condensation is a crucial stage in skeletal development. It occurs when a previously dispersed population of cells gathers together to differentiate into a single cell or tissue type (136). Extracellular matrix molecules, receptors and adhesion molecules such as fibronectin, tenascin-C, syndecan, and N-CAM initiate condensation and set condensation boundaries. Hox genes and other factors like BMPs regulate the proliferation of cells within condensations. Growth of a condensation ceases when Noggin inhibits BMP signaling—which sets up the next stage in development which is overt cell differentiation (135). However, tenascin-C deficient mice generated by two groups (137, 138) show no apparent skeletal phenotype, e.g., normal development and adulthood, life span, and fecundity, as well as normal healing of skin and nerves.
4.17. Phosphate-regulating neutral endopeptidase (PHEX)
Identification of the genetic targets of X-linked hypophosphatemia, autosomal dominant hypophosphatemic rickets (ADHR), X-linked hypophosphatemia (XLH), autosomal recessive hypophosphatemia (ARHP), and hereditary hypophosphatemic rickets with hypercalciuria (HHRH) has identified at least eight genes whose function is required either indirectly or directly to control the systemic phosphate concentration in blood (139–141). One of these is zinc endoprotease PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) (142). Loss of function mutations in the Pex gene cause X-linked hypophosphatemic rickets. A Pex mutation is also responsible for the ‘hyp’ mouse skeletal phenotype: hypophosphatasia and elevated FGF23 expression (143). The precise function of PHEX is unclear although two genetic rescue studies using bone restricted promoters have demonstrated it has a direct, local effect on bone mineralization as well suggest it has an indirect effect on systemic phosphate homeostasis (129, 144). This conclusion is also supported by results of transplantation of mutant Hyp or control periostea into wild type or phosphate supplemented Hyp mice and into control or phosphate-depleted normal mice (145). Also, over-expression of human PHEX under the beta-actin promoter seemed to rescue the bone mineralization defect, but did not affect phosphate homeostasis suggesting again independent physiological mechanisms control hyperphosphaturia and bone mineralization in XLH and Hyp (146). Since all three transgenic attempts to replace mutant PHEX in the Hyp mouse were able to at least partially rescue bone mineralization, it is widely believed that PHEX has a role in mineralization of bone.
Using a small peptide substrate, Liu et al (147) showed that MEPE binding to PHEX inhibits the peptidase activity of the latter enzyme. Subsequently, McKee and co-workers showed that PHEX can cleave and inactivate the mineralization inhibitory activity of some ASARM peptide isoforms derived from MEPE (148). However, the most highly phosphorylated forms, which are also the predicted to be the most effective minhibins (mineral crystal growth inhibitors), were not effectively cleaved by Phex. Minhibins is the term coined by Rowe and colleagues to refer to the ASARM peptides representing highly conserved, acidic, protease-resistant C-terminal regions of SIBLING proteins DMP1, BSP and MEPE (149). This latter group has shown that degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins) is dramatically increased in the HYP (Phex-deficient) mouse and that ASARM-peptide(s) are directly responsible for defective mineralization in the HYP skeleton (148, 149). It appears that cathepsin B, a neutral protease, may mediate the accelerated release of ASARM peptides in HYP mice since cathepsin inhibitors rescue the defect (150). While these findings explain a number of features of the bone phenotype associated with loss of PHEX in man and mice, they do not clarify the role of PHEX in normal mineralization of bone.
4.18. Matrix extracellular phosphoglycoprotein (MEPE, OF45)
MEPE, matrix extracellular phosphoglycoprotein, was first isolated from tumor tissue from a patient with oncogenic hypophosphatemia and is capable of inhibiting kidney tubular phosphate re-absorption (151) and absorption by the intestine (152)—features ascribed to phosphatonins, substances acting on multiple organs to regulate phosphate metabolism. It is also highly expressed in osteocytes as compared to osteoblasts. Deletion of this gene in mice results in increased bone formation and bone mass and less trabecular bone loss with age (153) whereas over-expression of MEPE caused a growth and mineralization defect with altered vascularization of bone and kidney (154). MEPE contains a highly phosphorylated C-terminal region called the ASARM peptide. Based on work with recombinant proteins, PHEX binding to MEPE regulates the proteolytic release of ASARM peptides which act as potent inhibitors of mineralization in the HYP mouse (147) and in X-linked hypophosphatasia (155).
4.19. Functional importance of proteolysis in activation of transglutaminase and PCOLCE
Transglutaminase and PCOLCE [procollagen C-proteinase enhancer] are both required for normal bone formation and mineralization. PCOLCE is a 55 kDa precursor with an elongated structure that enhances the C-terminal processing of type I, II, and III collagen precursors catalyzed by BMP-1 (procollagen C-terminal propeptidase) (156). Following proteolytic activation, a 36 kDa active PCOLCE fragment mediates the effect on BMP-1 via its two CUB domains (157, 158). The C-terminal netrin-like domain of PCOLCE shares sequence homology with a similar domain in metalloproteinases. Although inactive with MMP-2, BMP-1, and ADAMTS, the netrin-like domain can accelerate procollagen processing in the presence of heparin or heparan sulfate glycosaminoglycan chains (159). Thus, PCOLCE alone can increase collagen processing about 10-fold (157), whereas a further increase in rate can be accomplished in association with cell surface heparan sulfate proteoglycans (159). Heparan sulfate proteoglycans are common components of most cell surfaces including osteoblasts.
Interestingly, PCOLCE (−/−) male mice possess larger long bones with altered geometries that increase resistance to loading compared with wild type bones. Female (−/−) long bones did not show this change. Under mechanical testing, null male long bones revealed diminished material properties which apparently were compensated for by adapative changes in bone geometry, e.g., making the bones bigger. For PCOLCE (−/−) vertebrae, both sexes exhibited diminished mechanical properties relative to controls. Similar to the male long bones, knockout vertebrae were thickened and had more numerous trabeculae with abnormal mineral crystal morphology. All of these findings appear consistent with the observed presence of abnormal collagen fibrils in both mineralized and non-mineralized tissues (160). Thus, PCOLCE is also a determinant of the mechanical and geometrical properties of bone in mammals mediating its effects through fibrillar collagens I, II, and III. It is particularly interesting that treatment of mineralizing osteoblastic cells with covalent serine protease inhibitor AEBSF blocks the activation of PCOLCE-1, while also blocking mineralization (11). Based on these results, activation of PCOLCE represents a protease regulated step in the mineralization pathway resulting in increased collagen fibrillogenesis and deposition.
Since transglutaminases, of which seven TGase genes have been cloned thus far, also require proteolytic activation, we questioned whether this step is also regulated similarly to that for PCOLCE. All TGase enzymes possess a conserved cys-his-asp catalytic triad and a calcium-binding domain although individual differences exist, e.g., TGM1 is a transmembrane enzyme and TGM2 contains a unique C-terminal GTPase/ATPase signaling region (161). The properties and enzymatic functions of TGase I, II, III, and Factor XIII are the most thoroughly studied. Individual TGases are the target genes in several human syndromes: Lamellar Ichthyosis of the skin (TGM1); Peeling Skin Syndrome (TGM5); and unstable blood clots and increased bleeding tendency (Factor XIII). However, at present, a requirement for transglutaminase for mineralization of bone is yet to be firmly established since the TGase II (−/−) mouse exhibits no developmental or postnatal skeletal defects (162, 163). In view of the multiple copies of TGase and their wide distribution, it is likely that functional redundancy could be operative particularly since Factor XIII is expressed by osteoblast lineage cells (164) and is present in plasma.
Despite this, the work of Kartinen and colleagues suggests that transglutaminase II is functionally important for bone formation. They showed that mineralization of MC3T3-E1 (subclone 14) pre-osteoblastic cultures could be blocked completely by treatment with cystamine, a TGase inhibitor (165). Osteoblast differentiation was also blocked as expression of bone sialoprotein, osteocalcin and alkaline phosphatase was diminished. Within bone and in osteoblastic cultures, osteopontin, collagen, and osteocalcin have been consistently shown to be substrates for transglutaminase catalyzed crosslinking (166, 167). Intramolecular crosslinking of osteopontin increases its collagen binding properties five-fold (168). While these, and other (169), studies imply TGase activity may influence bone cell signaling directly or indirectly, supporting conclusive mechanistic evidence is not available. Furthermore, it is unclear whether other transglutaminase isoforms, e.g., TGase III, could also be expressed by osteoblastic cells in vivo. Finally, other than for Factor XIII and thrombin, the identity of the cleavage site and of the physiological protease mediating initial activation of bone transglutaminases remains unknown.
4.20. Neutral Proteases in Bone
While specific examples of protease actions in bone development and mineralization are given above, it is likely that other functions for proteases in bone will be identified, aside from those associated with osteoclastic bone resorption. For example, during in vitro differentiation of tibial derived rat osteoblasts, expression is increased for MMP-2 and MMP-9 and membrane gelatinase MMP-14. These proteases, along with TIMP-1 and TIMP-2, are developmentally regulated in accordance with the maturation stage of the osteoblastic cells. MMP-14 is expressed in differentiating osteoblastic cells and is localized in cells forming nodules. MMP-14 expression in vivo in rat tibias (day 7) also shows the highest levels among osteogenic cells, in lining osteoblasts on the newly formed trabeculae under the growth plate and on the endosteal surface of cortical bone (170). MMP-2 (gelatinase-A) and MMP-9, also known as 72 kDa and 92 kDa type IV collagenase, respectively, specifically cleave type IV collagen a major component of basement membranes. No bone phenotype has yet been reported in MMP-2 or MMP-9 null mice (171, 172). BMP-1, a Zn+2 metalloproteinase, catalyzes biosynthetic processing of most collagens expressed by bone cells as well as other extracellular proproteins. Actually, BMP-1 comprises a family of four closely related enzymes which may display a redundancy in function (65). This latter point may explain the absence of a dramatic phenotype in BMP-1 null mice (173). Interestingly, transgenic deletion of caspase-3 leads to delayed ossification and decreased bone mineral density (174) due in part to impaired differentiation of bone marrow stromal stem cells. MT1-MMP is a transmembrane protease which acts on extracellular matrix components in the pericellular environment. Its deletion causes no detectable embryonic abnormality, but rather brings about progressive postnatal changes, e.g., craniofacial dysmorphism, osteopenia, arthritis, and dwarfism as well as fibrosis of soft tissues (175, 176). Clarification of a potential mechanism for these changes was provided by Holmebeck K et al (177). They showed that collagen cleavage in osteocytes from mice deficient in MT1-MMP was disrupted and ultimately led to the loss of formation of osteocyte cell processes called dendrites important in inter-cellular communication. A potential implication from these static studies is that MTI-MMP mediates an active invasive collagen degradation process during osteocyte embedding.
SKI-1 is a member of the proprotein convertase family of serine proteases (178). PCs are required to process or mature the structure of growth factors, neuropeptides, toxins, glycoproteins, viral capsid proteins, and transcription factors. Transmembrane transcription factor precursors SREBP-1 and -2 are activated in a sequential process involving first SKI-1 cleavage and then S2P protease cleavage (179). After activation, the N-terminal fragments of SREBP-1 and SREBP-2 can be imported into the nucleus where they regulate gene expression by binding to transcriptional promoters containing consensus SRE sequences (180). CREB/ATF family transcription factors [ATF-6, LUMAN (CREB3), OASIS/BBF2H7 (CREB3L2), CREB-H, CREB-4, and AIbZIP/Tisp40 (CREB3L4)] also include transmembrane proteins which require SKI-1 catalyzed release of an N-terminal transcriptionally active fragment. SKI-1 is also an initiator of the unfolded protein response and acts generally to decrease cellular stress by increasing chaperone production, influencing ER-associated degradation of proteins, and regulating membrane remodeling (181).
Recent studies suggest a functional role for SKI-1 in normal bone formation (22). First, the skeletons of mice deficient in SKI-1 activatable transcription factor OASIS exhibit a severe osteopenia characterized by a type I collagen-deficient bone matrix and reduced osteoblastic activity (182, 183). Second, mice over-expressing ICER, a dominant negative effector of CREB/ATF transcription factors, displayed dramatically reduced trabecular bone and a markedly reduced femoral bone formation rate (184). Third, SKI-1 is required for normal cartilage morphogenesis in zebra fish (185). In a similar way, conditional inactivation of SKI-1 in mice using a type II collagen Cre recombinase transgene displayed abnormal calcification of the growth plate (186). Homozygous germ-line deletion of SKI-1 is embryonic lethal in mice (187). Finally, we have recently shown that inhibition of SKI-1 in osteoblastic cultures blocks the deposition of mineral crystals by blocking the expression of mineralization related proteins PHEX, type XI collagen, fibronectin, and DMP1 (188).
5. SUMMARY AND PERSPECTIVE
The role of extracellular non-collagenous proteins, cell surface and secreted, in biomineralization has evolved dramatically from initial considerations of calcium binding and cell adhesion. While detailed structure/function relationships are still under investigation, it is now clear that some proteins, such as osteocalcin, act as systemic hormones which serve to integrate the metabolic function of bone, fat and nervous tissues. Others, such as sclerostin, periostin, act as local regulators of the Wnt pathway regulating the osteocyte’s response to mechanical loading. Fetuin is now well established as a product of bone cells as well as hepatocytes and plays a role in vascular calcium particle transport. Interestingly, it is now possible to assign roles during biomineralization. Phosphatase Phospho 1, a vesicular component, is required to form the initial mineral crystals in vitro and in vivo, while alkaline phosphatase and ENPP1 appear to be needed to propagate or grow these initial crystals. Phex, DMP1, and MEPE all participate in the regulation of systemic phosphate, an ion critical for forming calcium hydroxyapatite. Newly identified protein-protein interactions like that proposed for bone sialoprotein and a specific, cationic, alternative splice variant of type XI collagen allow us to localize phosphoprotein nucleators at the gap regions of collagen fibrils within mineralizing osteoid. Also, bone sialoprotein is capable of rescuing catalytically active MMP-2 from previously inactive TIMP-MMP-2 complexes. Recent studies also suggest that hydroxyapatite crystals themselves may directly influence the catalytic function of metalloproteinases present within mineralized bone and cartilage. Finally, it now appears that expression of a number of mineralization related extracellular matrix proteins may be regulated in a common pathway. However, further work is needed to determine the extent to which the catalytic and inhibitor specificities of individual enzymes such as PHEX can be modified. Conceptually as well, the model of bone biomineralization proposed by Hunter et al. (76) has moved from a fixed epitaxic view of protein-mineral crystal interactions consistent with invertebrate shells to a less organized “flexible polyelectrolyte” model which reflects two decades of structural studies on acidic phosphoproteins such as osteopontin and bone sialoprotein.
Acknowledgments
JPG wishes to thank Mrs. Nichole T. Huffman for her excellent work in performing experimental work described here as well as the ongoing support of the UMKC Bone Group, the Center of Excellence in Mineralized Tissues, and the faculty of the Oral Biology Department of UMKC. Special thanks also to collaborators Drs. R.J. Midura, Julie T. Oxford, Peter Rowe, L. Darryl Quarles, and Nabil G. Seidah. Supported by NIH grant AR052775, UMKC Center of Excellence in Mineralized Tissues, the Patton Trust and the Kansas City Life Science Board, and the University of Missouri Research Board.
Abbreviations
- BMF
biomineralization foci
- BSP
bone sialoprotein
- AEBSF
4-(2-aminoethyl) benzenesulfonyl fluoride hydrochloride
- SKI-1
site-1, MBTPS1, or subtilisin/kexin isoenzyme-1
- PCOLCE1
procollagen C-terminal propeptidase enhancer1
- 1,25-vit.
D3-MARRS protein, membrane associated, rapid response steroid binding receptor
- BMP
bone morphogenetic protein
- TGase
transglutaminase
- ADHR
X-linked hypophosphatemia, autosomal dominant hypophosphatemic rickets
- XLH
X-linked hypophosphatemia
- ARHP
autosomal recessive hypophosphatemia
- HHRH
hereditary hypophosphatemic rickets with hypercalciuria
- ENPP1
PC-1, ectonucleotide pyrophosphatase/phosphodiesterase
- Fetuin
alpha2HS-glycoprotein
- TNAP
tissue nonspecific alkaline phosphatase
- DMP1
dentin matrix protein 1
- OPN
osteopontin
- NTD
N-terminal domain
- GLA
gamma-carboxyglutamic acid
- SIBLING
small integrin-binding ligand, N-linked glycoprotein
- BGP
beta-glycerolphosphate
- MMP
matrix metalloproteinase
- BAG-75
bone acidic glycoprotein-75
- PC
proprotein convertase
- Osx
osterix
- BSA
bovine serum albumin
- FBS
fetal bovine serum
References
- 1.Olsen Bjorn R. Bone Embryology. In: Favus Murry J., editor. Primer on the Metabolic Bone Diseases and Disorders of Mineral Metabolism. American Society for Bone and Mineral Research; Washington, DC: 2006. [Google Scholar]
- 2.Turner Charles H, Woltman TA, Belongia DA. Structural changes in rat bone subjected to long-term, in vivo mechanical loading. Bone. 1992;13:417–422. doi: 10.1016/8756-3282(92)90084-a. [DOI] [PubMed] [Google Scholar]
- 3.Gorski Jeffrey P. Is all bone the same? Distinctive distributions and properties of non-collagenous matrix proteins in lamellar vs. woven bone imply the existence of different underlying osteogenic mechanisms. Crit Rev Oral Biol Med. 1998;9:201–223. doi: 10.1177/10454411980090020401. [DOI] [PubMed] [Google Scholar]
- 4.Simmons Edward D, Jr, Pritzker Kenneth P, Grynpas Marc D. Age-related changes in the human femoral cortex. J Orthop Res. 1991;9:155–167. doi: 10.1002/jor.1100090202. [DOI] [PubMed] [Google Scholar]
- 5.Bonucci Ermanno. Comments on the ultrastructural morphology of the calcification process: an attempt to reconcile matrix vesicles, collagen fibrils, and crystal ghosts. Bone and Mineral. 1992;17:219–222. doi: 10.1016/0169-6009(92)90740-5. [DOI] [PubMed] [Google Scholar]
- 6.Clarke Anderson H. Molecular biology of matrix vesicles. Clin Orthop Relat Res. 1995;314:266–280. [PubMed] [Google Scholar]
- 7.Kirsch Theodore, Harrison Gerald, Golub Ellis E, Nah Hyun-Duck. The roles of annexins and types II and X collagen in matrix vesicle mediated mineralization of growth plate cartilage. J Biol Chem. 2000;275:35577–35583. doi: 10.1074/jbc.M005648200. [DOI] [PubMed] [Google Scholar]
- 8.Yadav Manisha C, Simão Ana M, Narisawa Sonoko, Huesa Carmen, McKee Marc D, Farquharson Colin, Millán Jose Luis. Loss of skeletal mineralization by the simultaneous ablation of PHOSPHO1 and alkaline phosphatase function - A unified model of the mechanisms of initiation of skeletal calcification. J Bone Miner Res. 2010 Aug 3; doi: 10.1002/jbmr.195. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gorski Jeffrey P, Wang Amin, Lovitch Dina, Law Douglas, Powell Kathyrn, Midura Ronald J. Extracellular bone acidic glycoprotein-75 defines condensed mesenchyme regions to be mineralized and localizes with bone sialoprotein during intramembranous bone formation. J Biol Chem. 2004;279:25455–25463. doi: 10.1074/jbc.M312408200. [DOI] [PubMed] [Google Scholar]
- 10.Midura Ronald J, Wang Amin, Lovitch Dina, Law Douglas, Powell Kathyrn, Gorski Jeffrey P. Bone acidic glycoprotein-75 delineates the extracellular sites of future bone sialoprotein accumulation and apatite nucleation in osteoblastic cultures. J Biol Chem. 2004;279:25464–25473. doi: 10.1074/jbc.M312409200. [DOI] [PubMed] [Google Scholar]
- 11.Huffman Nichole T, Keightley John A, Chaoying Cui, Midura Ronald J, Lovitch Dinah, Veno Patricia A, Dallas Sarah L, Gorski Jeffrey P. Association of specific proteolytic processing of bone sialoprotein and bone acidic glycoprotein-75 with mineralization within biomineralization foci. J Biol Chem. 2007;282:26002–26013. doi: 10.1074/jbc.M701332200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonucci Ermanno. Ultrastructural organic-inorganic relationships in calcified tissues: cartilage and bone versus enamel. Connect Tissue Res. 1995;33:157–162. doi: 10.3109/03008209509016996. [DOI] [PubMed] [Google Scholar]
- 13.Aaron JE, Oliver B, Clarke N, Carter DH. Calcified microspheres as biological entities and their isolation from bone. Histochem J. 1999;31:455–470. doi: 10.1023/a:1003707909842. [DOI] [PubMed] [Google Scholar]
- 14.Glimcher Melvin J. Mechanism of calcification: role of collagen fibrils and collagen-phosphoprotein complexes in vitro and in vivo. Anat Rec. 1989;224:139–153. doi: 10.1002/ar.1092240205. [DOI] [PubMed] [Google Scholar]
- 15.Veis Arthur, Schlueter RJ. The macromolecular organization of dentine matrix collagen. 1. Characterization of dentine collagen. Biochemistry. 1964;3:1650–1657. doi: 10.1021/bi00899a009. [DOI] [PubMed] [Google Scholar]
- 16.Glimcher Melvin J, Francois CJ, Richards L, Krane Stephen M. The presence of organic phosphorus in collagens and gelatins. Biochim Biophys Acta. 1964;93:585–602. doi: 10.1016/0304-4165(64)90342-3. [DOI] [PubMed] [Google Scholar]
- 17.Price Paul A, Toroian D, Lim JE. Mineralization by inhibitor exclusion: the calcification of collagen with fetuin. J Biol Chem. 2009;284:17092–17101. doi: 10.1074/jbc.M109.007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Aimin, Martin James A, Lembke Lois A, Midura Ronald J. Reversible Suppression of in vitro Biomineralization by Activation of Protein Kinase A. J Biol Chem. 2000;275:11082–11091. doi: 10.1074/jbc.275.15.11082. [DOI] [PubMed] [Google Scholar]
- 19.Wang Chianyi, Wang Yang, Huffman Nichole T, Cui Chaoying, Yao, Midura Sharon, Midura Ronald J, Gorski Jeffrey P. Confocal laser raman microspectroscopy of biomineralization foci in UMR 106 osteoblastic cultures reveals temporally synchronized protein changes preceding and accompanying mineral crystal deposition. J Biol Chem. 2009;284:7100–7113. doi: 10.1074/jbc.M805898200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bonucci Ermanno. Biological Calcification, Normal and Pathological Processes in the Early Stages. Springer-Verlag; New York: p. 195. [Google Scholar]
- 21.Gorski Jeffrey P, Griffin David, Dudley Gerry, Stanford Clarke, Thomas Randy, Huang Conway, Lai Eugene, Karr Bradley, Solursh Micheal. Bone acidic glycoprotein-75 is a major synthetic product of osteoblastic cells and localized as 75- and/or 50-kDa forms in mineralized phases of bone and growth plate and in serum. J Biol Chem. 1990;265:14956–14963. [PubMed] [Google Scholar]
- 22.Gorski Jeffrey P, Huffman Nichole T, Cui Chaoying, Henderson Ellen, Midura Sharon B, Seidah Nabil G. Potential role of proprotein convertase SKI-1 in the mineralization of primary bone. Cells Tissues Organs. 2009;189:25–32. doi: 10.1159/000151723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Elagoz Aram S, Benjannet Suzanne, Mammarbassi Aida, Wickham Louise, Seidah Nabil G. Biosynthesis and cellular trafficking of the convertase SKI-1/S1P: ectodomain shedding requires SKI-1 activity. J Biol Chem. 2002;277:11265–11275. doi: 10.1074/jbc.M109011200. [DOI] [PubMed] [Google Scholar]
- 24.Seidah Nabil G, Mowla Seyed J, Hamelin Josee, Mamarbachi Aida M, Benjannet Suzanne, Touré Barry B, Basak Ajoy, Munzer Jon Scott, Marcinkiewicz Jadwiga, Zhong Mei, Barale Jean-Christophe, Lazure Claude, Murphy Richard A, Chrétien Michel, Marcinkiewicz Mieczyslaw. Mammalian subtilisin/kexin isozyme SKI-1: A widely expressed proprotein convertase with a unique cleavage specificity and cellular localization. Proc Natl Acad Sci U S A. 1999;96:1321–1326. doi: 10.1073/pnas.96.4.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gorski Jeff P, Huffman Nichole T, Chittur Sridar, Midura Ronald J, Black Claudine, Oxford Julie, Seidah Nabil G. Inhibition of SKI-1 Proprotein Convertase Blocks Transcription of Key Extracellular Matrix Genes Regulating Osteoblastic Mineralization. J Biol Chem. 2010;285 doi: 10.1074/jbc.M110.151647. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boyde Alan, Sela Jona J. Scanning electron microscope study of separated calcospherites from the matrices of different mineralizing systems. Calcif Tissue Res. 1978;26:47–49. doi: 10.1007/BF02013233. [DOI] [PubMed] [Google Scholar]
- 27.Bonucci Ermanno. Comments on the ultrastructural morphology of the calcification process: an attempt to reconcile matrix vesicles, collagen fibrils, and crystal ghosts. Bone and Mineral. 1992;17:219–222. doi: 10.1016/0169-6009(92)90740-5. [DOI] [PubMed] [Google Scholar]
- 28.Aaron Jean E, Oliver Barry, Clarke Noel, Howard Carter D. Calcified microspheres as biological entities and their isolation from bone. Histochem J. 1999;31:455–470. doi: 10.1023/a:1003707909842. [DOI] [PubMed] [Google Scholar]
- 29.Midura Ronald J, Vasanji A, Su X, Wang A, Midura Sharon B, Gorski Jeff P. Calcospherulites isolated from the mineralization front of bone induce the mineralization of type I collagen. Bone. 2007;41:1005–1016. doi: 10.1016/j.bone.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Midura Ronald J, Vasanji A, Su X, Midura Sharon B, Gorski Jeff P. Isolation of calcospherulites from the mineralization front of bone. Cells Tissues Organs. 2009;189:75–9. doi: 10.1159/000152914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bonucci Ermanno. Fine structure of early cartilage calcification. J Ultrastruct Res. 1967;20:33–50. doi: 10.1016/s0022-5320(67)80034-0. [DOI] [PubMed] [Google Scholar]
- 32.Clarke Anderson H. Electron microscopic studies of induced cartilage development and calcification. J Cell Biol. 1967;35:81–101. doi: 10.1083/jcb.35.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clarke Anderson H, Sipe Joseph B, Hessle Lovisa, Dhanyamraju Rama, Atti Elisa, Camacho Nancy P, Millán Jose Luis. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol. 2004;164:841–847. doi: 10.1016/s0002-9440(10)63172-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xiao Zhen, Camalier Corinne E, Nagashima Kunio, Chan King C, Lucas David A, Jason de la Cruz M, Gignac Michelle, Lockett Stephen, Issaq Haleem J, Veenstra Timothy D, Conrads Thomas P, Beck Geoge R., Jr Analysis of the extracellular matrix vesicle proteome in mineralizing osteoblasts. J Cell Physiol. 2007;210:325–335. doi: 10.1002/jcp.20826. [DOI] [PubMed] [Google Scholar]
- 35.Boskey Adele L. Matrix proteins and mineralization: an overview. Connect Tissue Res. 1996;35:357–363. doi: 10.3109/03008209609029212. [DOI] [PubMed] [Google Scholar]
- 36.Robey Pam G. Vertebrate mineralized matrix proteins: structure and function. Connect Tissue Res. 1996;35:131–136. doi: 10.3109/03008209609029183. [DOI] [PubMed] [Google Scholar]
- 37.Qin Chunlin, Baba Otto, Bulter William T. Post-translational modifications of sibling proteins and their roles in osteogenesis and dentinogenesis. Crit Rev Oral Biol Med. 2004;15:126–136. doi: 10.1177/154411130401500302. [DOI] [PubMed] [Google Scholar]
- 38.Fisher Larry W, William Whitson S, Avioli Louis V, Termine John D. Matrix sialoprotein of developing bone. J Biol Chem. 1983;258:12723–12727. [PubMed] [Google Scholar]
- 39.Herring Geoffrey M. In: The Biochemistry and Physiology of Bone. Bourne GH, editor. Academic Press; New York: 1972. pp. 1pp. 127–189. [Google Scholar]
- 40.Hunter Graeme K, Goldberg Harvey A. Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci U S A. 1993;90:8562–8565. doi: 10.1073/pnas.90.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hunter Graeme K, Poitras Michael S, Michael Underhill T, Grynpas Marc D, Goldberg Harvey A. Induction of collagen mineralization by a bone sialoprotein--decorin chimeric protein. Biomed Mater Res. 2001;55:496–502. doi: 10.1002/1097-4636(20010615)55:4<496::aid-jbm1042>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 42.Gordon Jonathan AR, Tye Coralee E, Sampaio Arthur V, Michael Underhill T, Hunter Graeme K, Goldberg Harvey A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–473. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 43.Baht Gurpreet S, Hunter Graeme K, Goldberg Harvey A. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 2008;27:600–608. doi: 10.1016/j.matbio.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Baht Gurpreet S, O’Young Jason, Borovina Antonia, Chen Hong, Tye Coralee E, Karttunen Mikko, Lajoie Gilles A, Hunter Graeme K, Goldberg Harvey A. Phosphorylation of Ser136 is critical for potent bone sialoprotein-mediated nucleation of hydroxyapatite crystals. Biochem J. 2010;428:385–395. doi: 10.1042/BJ20091864. [DOI] [PubMed] [Google Scholar]
- 45.Mintz Keith P, Grzesik Wojciech J, Midura Ronald J, Robey Pamela G, Termine John D, Fisher Larry W. Purification and fragmentation of nondenatured bone sialoprotein: evidence for a cryptic, RGD-resistant cell attachment domain. J Bone Miner Res. 1993;8:985–995. doi: 10.1002/jbmr.5650080812. [DOI] [PubMed] [Google Scholar]
- 46.Stubbs John T, 3rd, Mintz Keith P, Eanes Edward D, Torchia Dennis A, Fisher Larry W. Characterization of native and recombinant bone sialoprotein: delineation of the mineral-binding and cell adhesion domains and structural analysis of the RGD domain. J Bone Miner Res. 1993;12:1210–1222. doi: 10.1359/jbmr.1997.12.8.1210. [DOI] [PubMed] [Google Scholar]
- 47.Malaval Luc, Monfoulet Laurent, Fabre Thierry, Pothuaud Laurent, Bareille Reine, Miraux Sylvain, Thiaudiere Eric, Raffard Gerard, Franconi Jean-Michel, Lafage-Proust Marie-Helene, Aubin Jane E, Vico Laurence, Amédée Joelle. Absence of bone sialoprotein (BSP) impairs cortical defect repair in mouse long bone. Bone. 2009;45:853–861. doi: 10.1016/j.bone.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Monfoulet Laurent, Malaval Luc, Aubin Jane E, Rittling Susan R, Gadeau Alain P, Fricain Jean-Christophe, Chassande Olivier. Bone sialoprotein, but not osteopontin, deficiency impairs the mineralization of regenerating bone during cortical defect healing. Bone. 2009;46:447–452. doi: 10.1016/j.bone.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 49.Wade-Gueye Ndeye M, Boudiffa Maya, Laroche Norbert, Vanden-Bossche Arnaud, Fournier Carole, Aubin Jane E, Vico Laurence, Lafage-Proust Marie-Helene, Malaval Luc. Mice Lacking Bone Sialoprotein (BSP) Lose Bone after Ovariectomy and Display Skeletal Site-Specific Response to Intermittent PTH Treatment. Endocrinology. 2010 Sep 15; doi: 10.1210/en.2010-0091. Epub ahead. [DOI] [PubMed] [Google Scholar]
- 50.Boudiffa Maya, Wade-Gueye Ndeye M, Guignandon Alain, Vanden-Bossche Arnaud, Sabido Odile, Aubin Jane E, Jurdic Pierre, Vico Laurence, Lafage-Proust Marie Helene, Malaval Luc. Bone Sialoprotein deficiency impairs osteoclastogenesis and mineral resorption in vitro. J Bone Miner Res. 2010 Sep 1; doi: 10.1002/jbmr.245. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 51.Malaval Luc, Wade-Guéye Ndeye M, Boudiffa Maya, Fei Jia, Zirngibl Ralph, Chen Frieda, Laroche Norbert, Roux Jean-Paul, Burt-Pichat Brigitte, Duboeuf Francois, Boivin Georges, Jurdic Pierre, Lafage-Proust Marie-Helene, Amédée Joelle, Vico Laurence, Rossant Janet, Aubin Jane E. Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med. 2008;205:1145–1153. doi: 10.1084/jem.20071294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gordon Jonathan A, Tye Coralee E, Sampaio Arthur V, Michael Underhill T, Hunter Graeme K, Goldberg Harvey A. Bone sialoprotein expression enhances osteoblast differentiation and matrix mineralization in vitro. Bone. 2007;41:462–473. doi: 10.1016/j.bone.2007.04.191. [DOI] [PubMed] [Google Scholar]
- 53.Gordon Jonathan A, Hunter Graeme K, Goldberg Harvey A. Activation of the mitogen-activated protein kinase pathway by bone sialoprotein regulates osteoblast differentiation. Cells Tissues Organs. 2009;189:138–143. doi: 10.1159/000151728. [DOI] [PubMed] [Google Scholar]
- 54.Curtin Paul, McHugh Kevin P, Zhou Hia-Yan, Flückiger Rudolf, Goldhaber Paul, Oppenheim Frank G, Salih Erdjan. Modulation of bone resorption by phosphorylation state of bone sialoprotein. Biochemistry. 2009;48:6876–6886. doi: 10.1021/bi900066b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sato Masahiko, Grasser William, Harm Sandy, Fullenkamp Colleen, Gorski Jeffrey P. Bone acidic glycoprotein 75 inhibits resorption activity of isolated rat and chicken osteoclasts. FASEB J. 1992;6:2966–2976. doi: 10.1096/fasebj.6.11.1644260. [DOI] [PubMed] [Google Scholar]
- 56.Tye Coralee E, Rattray, Warner Kevin R, Gordon Jonathan A, Sodek Jaro, Hunter Graeme K, Goldberg Harvey A. Delineation of the hydroxyapatite-nucleating domains of bone sialoprotein. J Biol Chem. 2003;278:7949–7955. doi: 10.1074/jbc.M211915200. [DOI] [PubMed] [Google Scholar]
- 57.Boskey Adele L. Osteopontin and related phosphorylated sialoproteins: effects on mineralization. Ann N Y Acad Sci. 1995;760:249–256. doi: 10.1111/j.1749-6632.1995.tb44635.x. [DOI] [PubMed] [Google Scholar]
- 58.Warner Lisa R, Blasick Christina M, Brown Raquel J, Oxford Julia T. Expression, purification, and refolding of recombinant collagen alpha1(XI) amino terminal domain splice variants. Protein Expr Purif. 2007;52:403–409. doi: 10.1016/j.pep.2006.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Morris Nicholas P, Oxford Julia Thom, Davies Gillian BM, Smoody Barbara F, Keene Douglas R. Developmentally regulated alternative splicing of the alpha1(XI) collagen chain: spatial and temporal segregation of isoforms in the cartilage of fetal rat long bones. J Histochem Cytochem. 2000;48:725–741. doi: 10.1177/002215540004800601. [DOI] [PubMed] [Google Scholar]
- 60.Hansen Uwe, Bruckner Peter. Macromolecular specificity of collagen fibrillogenesis: fibrils of collagens I and XI contain a heterotypic alloyed core and a collagen I sheath. J Biol Chem. 2003;278:37352–37359. doi: 10.1074/jbc.M304325200. [DOI] [PubMed] [Google Scholar]
- 61.Eyre David R, Weis Mary Ann, Wu Jiann-Jiu. Advances in collagen cross-link analysis. Methods. 2008;45:65–74. doi: 10.1016/j.ymeth.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brunet Lisa J, McMahon Jill A, McMahon Andrew P, Harland Richard M. Noggin, cartilage morphogenesis, and joint formation in the mammalian skeleton. Science. 1998;280:1455–1457. doi: 10.1126/science.280.5368.1455. [DOI] [PubMed] [Google Scholar]
- 63.McMahon Jill A, Takada Shinji, Zimmerman Lyle B, Fan Chen-Ming, Harland Richard M, McMahon Andrew P. Noggin-mediated antagonism of BMP signaling is required for growth and patterning of the neural tube and somite. Genes Dev. 1998;12:1438–1452. doi: 10.1101/gad.12.10.1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Groppe Jay, Greenwald Jason, Wiater Ezra, Rodriguez-Leon Joaquin, Economides Aris N, Kwiatkowski Witek, Affolter Markus, Vale Wylie W, Belmonte Juan Carlos, Choe Senyon. Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature. 2002;420:636–642. doi: 10.1038/nature01245. [DOI] [PubMed] [Google Scholar]
- 65.Scott Ian C, Blitz Ira L, Pappano William N, Imamura Yasutada, Clark Timothy G, Steiglitz Barry M, Thomas Christina L, Maas Sarah A, Takahara Kazuhiko, Cho Ken WY, Greenspan Daniel S. Mammalian BMP-1/Tolloid-related metalloproteinases, including novel family member mammalian Tolloid-like 2, have differential enzymatic activities and distributions of expression relevant to patterning and skeletogenesis. Dev Biol. 1999;213:283–300. doi: 10.1006/dbio.1999.9383. [DOI] [PubMed] [Google Scholar]
- 66.Wardle Fiona C, Welch Jennifer V, Dale Leslie. Bone morphogenetic protein 1 regulates dorsal-ventral patterning in early Xenopus embryos by degrading chordin, a BMP4 antagonist. Mech Dev. 1999;86:75–85. doi: 10.1016/s0925-4773(99)00114-8. [DOI] [PubMed] [Google Scholar]
- 67.Yamate Tomoo, Mocharla Hanna, Taguchi Yasuto, Igietseme Joseph U, Manolagas Stavros C, Abe Etsuko. Osteopontin expression by osteoclast and osteoblast progenitors in the murine bone marrow: demonstration of its requirement for osteoclastogenesis and its increase after ovariectomy. Endocrinology. 1997;138:3047–3055. doi: 10.1210/endo.138.7.5285. [DOI] [PubMed] [Google Scholar]
- 68.Ikeda Tohru, Nomura Shintaro, Yamaguchi Akira, Suda Tatsuo, Yoshiki Shusaku. In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J Histochem Cytochem. 1992;40:1079–1088. doi: 10.1177/40.8.1619274. [DOI] [PubMed] [Google Scholar]
- 69.Merry Karen, Dodds Robert, Littlewood Amanda, Gowen Maxine. Expression of osteopontin mRNA by osteoclasts and osteoblasts in modelling adult human bone. J Cell Sci. 1993;104:1013–1020. doi: 10.1242/jcs.104.4.1013. [DOI] [PubMed] [Google Scholar]
- 70.Franzén Ahnders, Hultenby Kjell, Reinholt Finn P, Onnerfjord Patrik, Heinegård Dick. Altered osteoclast development and function in osteopontin deficient mice. J Orthop Res. 2008;26:721–728. doi: 10.1002/jor.20544. [DOI] [PubMed] [Google Scholar]
- 71.Asou Yoshinori, Rittling Susan R, Yoshitake Hiroyuki, Tsuji Kunikazu, Shinomiya Kenichi, Nifuji Akira, Denhardt David T, Noda Masaki. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology. 2001;142:1325–1332. doi: 10.1210/endo.142.3.8006. [DOI] [PubMed] [Google Scholar]
- 72.Ihara Hideyo, Denhardt David T, Furuya Koichi, Yamashita Teruhito, Muguruma Yukari, Tsuji Kunikazu, Hruska Keith A, Higashio Kanji, Enomoto Shoji, Nifuji Akira, Rittling Susan R, Noda Masaki. Parathyroid hormone-induced bone resorption does not occur in the absence of osteopontin. J Biol Chem. 2001;276:13065–13071. doi: 10.1074/jbc.M010938200. [DOI] [PubMed] [Google Scholar]
- 73.Kitahara Keiichiro, Ishijima Muneaki, Rittling Susan R, Tsuji Kunikazu, Kurosawa Hisashi, Nifuji Akira, Denhardt David T, Noda Masaki. Osteopontin deficiency induces parathyroid hormone enhancement of cortical bone formation. Endocrinology. 2003;144:2132–2140. doi: 10.1210/en.2002-220996. [DOI] [PubMed] [Google Scholar]
- 74.Gericke Arne, Qin Chunlin, Spevak Lyudmila, Fujimoto, Butler William T, Sørensen Esben ESS, Boskey Adele L. Importance of phosphorylation for osteopontin regulation of biomineralization. Calcif Tissue Int. 2005;77:45–54. doi: 10.1007/s00223-004-1288-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jono Shuichi, Peinado Christopher, Giachelli Cecilia M. Phosphorylation of osteopontin is required for inhibition of vascular smooth muscle cell calcification. J Biol Chem. 2000;275:20197–20203. doi: 10.1074/jbc.M909174199. [DOI] [PubMed] [Google Scholar]
- 76.Hunter Graeme K, O’Young Jason, Grohe Bernd, Karttunen Mikko, Goldberg Harvey A. The Flexible Polyelectrolyte Hypothesis of Protein-Biomineral Interaction. Langmuir. 2010 Jun 8; doi: 10.1021/la100401r. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 77.Fedarko Neal S, Jain Alka, Karadag Abdullah, Fisher Larry W. Three small integrin binding ligand N-linked glycoproteins (SIBLINGs) bind and activate specific matrix metalloproteinases. FASEB J. 2004;18:734–736. doi: 10.1096/fj.03-0966fje. [DOI] [PubMed] [Google Scholar]
- 78.Jain Alka, Fisher Larry LWW, Fedarko Neal S. Bone sialoprotein binding to matrix metalloproteinase-2 alters enzyme inhibition kinetics. Biochemistry. 47:5986–5995. doi: 10.1021/bi800133n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jain Alka, Karadag Abdullah, Fisher Larry W, Fedarko Neal S. Structural requirements for bone sialoprotein binding and modulation of matrix metalloproteinase-2. Biochemistry. 2008;47:10162–10170. doi: 10.1021/bi801068p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bellahcène Akeila, Castronovo Vincent, Ogbureke Kalu UE, Fisher Larry W, Fedarko Neal S. Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Nat Rev. 2008;8:212–226. doi: 10.1038/nrc2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gorski Jeffrey P, Shimizu Katsuji. Isolation of new phosphorylated glycoprotein from mineralized phase of bone that exhibits limited homology to adhesive protein osteopontin. J Biol Chem. 1988;263:15938–15945. [PubMed] [Google Scholar]
- 82.Gorski Jeffrey P, Kremer Edward A, Chen Yan, Ryan Steve, Fullenkamp Colleen, Delviscio John, Jensen Karen, McKee Marc D. Bone acidic glycoprotein-75 self-associates to form macromolecular complexes in vitro and in vivo with the potential to sequester phosphate ions. J Cell Biochem. 1997;64:547–64. [PubMed] [Google Scholar]
- 83.Kremer Edward A, Chen Yan, Suzuki Ko, Nagase Hideaki, Gorski Jeffrey P. Hydroxyapatite induces autolytic degradation and inactivation of matrix metalloproteinase-1 and -3. J Bone Miner Res. 1998;13:1890–1902. doi: 10.1359/jbmr.1998.13.12.1890. [DOI] [PubMed] [Google Scholar]
- 84.MacDougall Mary, Gu Ting Tin, Luan Xinghong, Simmons Darrin, Chen Jinkun. Identification of a novel isoform of mouse dentin matrix protein 1: spatial expression in mineralized tissues. J Bone Miner Res. 1998;13:422–431. doi: 10.1359/jbmr.1998.13.3.422. [DOI] [PubMed] [Google Scholar]
- 85.Toyosawa Satoru, Satoru Shintani S, Fujiwara Taku, Ooshima Takashi, Sato Atsushige, Naokuni Ijuhin N, Komori Toshihisa. Dentin matrix protein 1 is predominantly expressed in chicken and rat osteocytes but not in osteoblasts. J Bone Miner Res. 2001;16:2017–2026. doi: 10.1359/jbmr.2001.16.11.2017. [DOI] [PubMed] [Google Scholar]
- 86.von Marschall Zofia, Fisher Larry W. Dentin sialophosphoprotein (DSPP) is cleaved into its two natural dentin matrix products by three isoforms of bone morphogenetic protein-1 (BMP1) Matrix Biol. 2010;29:295–303. doi: 10.1016/j.matbio.2010.01.002. Epub Jan 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Steiglitz Barry M, Ayala Melvin, Narayanan Karthikeyan, George Anne, Greenspan Daniel S. Bone morphogenetic protein-1/Tolloid-like proteinases process dentin matrix protein-1. J Biol Chem. 2004;279:980–986. doi: 10.1074/jbc.M310179200. [DOI] [PubMed] [Google Scholar]
- 88.von Marschall Zofia, Fisher Larry W. Dentin matrix protein-1 isoforms promote differential cell attachment and migration. J Biol Chem. 2008;283:32730–32740. doi: 10.1074/jbc.M804283200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tartaix Philippe H, Doulaverakis Marie, George Anne, Fisher Larry W, Butler William T, Qin Chunlin, Salih Erdjan, Tan Melin, Fujimoto Yukiji, Spevak Lyudmila, Boskey Adele L. In vitro effects of dentin matrix protein-1 on hydroxyapatite formation provide insights into in vivo functions. J Biol Chem. 2004;279:18115–18820. doi: 10.1074/jbc.M314114200. [DOI] [PubMed] [Google Scholar]
- 90.Gajjeraman Sivakumar, Narayanan Karthikeyan, Hao Jianjun, Qin Chunlin, George Anne. Matrix macromolecules in hard tissues control the nucleation and hierarchical assembly of hydroxyapatite. J Biol Chem. 2007;282:1193–1204. doi: 10.1074/jbc.M604732200. [DOI] [PubMed] [Google Scholar]
- 91.Gericke A, Qin C, Sun Y, Redfern R, Redfern D, Fujimoto Y, Taleb H, Butler WT, Boskey Adele L. Different forms of DMP1 play distinct roles in mineralization. J Dent Res. 2010;89:355–359. doi: 10.1177/0022034510363250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Feng Jian Q, Ward Leanne M, Liu Shiguang, Lu Yongbo, Xie Yixia, Yuan Baozhi, Yu Xijie, Rauch Frank, Davis Siobhan, Zhang Shubin, Rios Hector, Drezner Marc K, Darryl Quarles L, Bonewald Lynda F, White Kenneth E. Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet. 2006;38:1310–1315. doi: 10.1038/ng1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lorenz-Depiereux Bettina, Bastepe Murat, Benet-Pagès Anna, Amyere Mustapha, Wagenstaller Janine, Müller-Barth Ursula, Badenhoop Klaus, Kaiser Stephanie M, Rittmaster Roger S, Shlossberg Alan H, Olivares Jose L, Loris Cesar, Ramos Feliciano J, Glorieux Francis, Vikkula Miikka, Jüppner Harald, Strom Tim M. DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet. 38:1248–1250. doi: 10.1038/ng1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lu Yongbon, Qin Chunlin, Xie Yixia, Bonewald Lynda F, Feng Jian Q. Studies of the DMP1 57-kDa functional domain both in vivo and in vitro. Cells Tissues Organs. 2009;189:175–185. doi: 10.1159/000151727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ling Yunfeng, Rios Hector F, Myers Elizabeth R, Lu Yongbo, Feng Jian Q, Boskey Adele L. DMP1 depletion decreases bone mineralization in vivo: an FTIR imaging analysis. J Bone Miner Res. 20:2169–2177. doi: 10.1359/JBMR.050815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Shiguang, Zhou Jianping, Tang Wen, Menard Rochelle, Feng Jian Q, Darryl Quarles L. Pathogenic role of Fgf23 in Dmp1-null mice. Am J Physiol Endocrinol Metab. 2008;295:E254–E261. doi: 10.1152/ajpendo.90201.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Poser James W, Esch Fred S, Ling Nicholas C, Price Paul A. Isolation and sequence of the vitamin K-dependent protein from human bone. Undercarboxylation of the first glutamic acid residue. J Biol Chem. 1980;255:8685–8691. [PubMed] [Google Scholar]
- 98.Ducy Patricia, Desbois Christelle, Boyce Brendan, Pinero Gerald, Story Beryl, Dunstan Colin, Smith Erica, Bonadio Jeffrey, Goldstein Steven, Gundberg Caren, Bradley Allan, Karsenty Gerard. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- 99.Ferron Mathieu, Wei Jianwen, Yoshizawa Tatsuya, Del Fattore Andrea, DePinho Ronald A, Teti Anna, Ducy Patricia, Karsenty Gerard. Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell. 2010;142:296–308. doi: 10.1016/j.cell.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Foresta Carlo, Strapazzon Giacomo, De Toni Luca, Gianesello Lisa, Calcagno Alessandra, Pilon Catia, Plebani Mario, Vettor Roberto. Evidence for osteocalcin production by adipose tissue and its role in human metabolism. J Clin Endocrinol Metab. 2010;95:3502–3506. doi: 10.1210/jc.2009-2557. [DOI] [PubMed] [Google Scholar]
- 101.Ohnishi Tomokazu, Nakamura Osamu, Ozawa Masayuki, Arakaki Naokatu, Muramatsu Takashi, Daikuhara Yasushi. Molecular cloning and sequence analysis of cDNA for a 59 kD bone sialoprotein of the rat: demonstration that it is a counterpart of human alpha 2-HS glycoprotein and bovine fetuin. J Bone Miner Res. 1993;8:367–377. doi: 10.1002/jbmr.5650080314. [DOI] [PubMed] [Google Scholar]
- 102.Jahnen-Dechent Willi, Schäfer Cora, Heiss Alexander, Grötzinger Joachim. Systemic inhibition of spontaneous calcification by the serum protein alpha 2-HS glycoprotein/fetuin. Z Kardiol. 2001;90(Suppl 3):47–56. doi: 10.1007/pl00022849. [DOI] [PubMed] [Google Scholar]
- 103.Heiss Alexander, DuChesne Alexander, Denecke Bernd, Grötzinger Joachim, Yamamoto Kazuhiko, Renné Thomas, Jahnen-Dechent Willi. Structural basis of calcification inhibition by alpha 2-HS glycoprotein/fetuin-A. Formation of colloidal calciprotein particles. J Biol Chem. 2003;278:13333–13341. doi: 10.1074/jbc.M210868200. [DOI] [PubMed] [Google Scholar]
- 104.Heiss Alexander, Jahnen-Dechent Willi, Endo Hisako, Dietmar Schwahn D. Structural dynamics of a colloidal protein-mineral complex bestowing on calcium phosphate a high solubility in biological fluids. Biointerphases. 2007;2:16–20. doi: 10.1116/1.2714924. [DOI] [PubMed] [Google Scholar]
- 105.Heiss Alexander, Eckert Thomas, Aretz Anke, Richtering Walter, van Dorp Wim, Schäfer Cora, Jahnen-Dechent Willi. Hierarchical role of fetuin-A and acidic serum proteins in the formation and stabilization of calcium phosphate particles. J Biol Chem. 2008;283:14815–14825. doi: 10.1074/jbc.M709938200. [DOI] [PubMed] [Google Scholar]
- 106.Jahnen-Dechent Willi, Schäfer Cora, Ketteler Markus, McKee Marc D. Mineral chaperones: a role for fetuin-A and osteopontin in the inhibition and regression of pathologic calcification. J Mol Med. 2008;86:379–389. doi: 10.1007/s00109-007-0294-y. [DOI] [PubMed] [Google Scholar]
- 107.Rochette Christophe N, Rosenfeldt Sabine, Heiss Alexander, Narayanan Theyencheri, Ballauff Matthias, Jahnen-Dechent Willi. A shielding topology stabilizes the early stage protein-mineral complexes of fetuin-A and calcium phosphate: a time-resolved small-angle X-ray study. Chembiochem. 2009;10:735–740. doi: 10.1002/cbic.200800719. [DOI] [PubMed] [Google Scholar]
- 108.Hamlin Nicholas J, Ong Karen G, Price Paul A. A serum factor that recalcifies demineralized bone is conserved in bony fish and sharks but is not found in invertebrates. Calcif Tissue Int. 2006;78:326–334. doi: 10.1007/s00223-005-0205-6. [DOI] [PubMed] [Google Scholar]
- 109.Kashima Takeshi G, Nishiyama Takashi, Shimazu Kazuhiro, Shimazaki Masahi, Kii Isao, Grigoriadis Agamemnon E, Fukayama Masashi, Kudo Akira. Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol. 2008;40:226–237. doi: 10.1016/j.humpath.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 110.Beck George R, Jr, Moran Elizabeth, Knecht Nicole. Inorganic phosphate regulates multiple genes during osteoblast differentiation, including Nrf2. Exp Cell Res. 2003;288:288–300. doi: 10.1016/s0014-4827(03)00213-1. [DOI] [PubMed] [Google Scholar]
- 111.Horiuchi Keisuke, Amizuka Norio, Takeshita Sunao, Takamatsu Hiroyuki, Katsuura Mieko, Ozawa Hidehiro, Toyama Yoshiaki, Bonewald Lynda F, Kudo Akira. Identification and characterization of a novel protein, periostin, with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor beta. J Bone Miner Res. 1999;14:1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- 112.Rios Hector, Koushik Shrinagesh V, Wang Haiyan, Wang Jian, Zhou Hong-Ming, Lindsley Andrew, Rogers Rhonda, Chen Zhi, Maeda Manabu, Kruzynska-Frejtag Agnieszka, Feng Jian Q, Conway Simon J. Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol. 2005;25:11131–11144. doi: 10.1128/MCB.25.24.11131-11144.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bonnet Nicholas, Standley Kara N, Bianchi Estelle N, Stadelmann Vincent, Foti Michelangelo, Conway Simon J, Ferrari Serge L. The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem. 2009;284:35939–35950. doi: 10.1074/jbc.M109.060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Maruhashi Takumi, Kii Isao, Saito Mitsuru, Kudo Akira. Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem. 2010;285:13294–13303. doi: 10.1074/jbc.M109.088864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ricard-Blum Sylvie, Bernocco Simonetta, Font Bernard, Moali Catherine, Eichenberger Denise, Farjanel Jean, Burchardt Elmar R, van der Rest Michel, Kessler Efrat, Hulmes David JS. Interaction properties of the procollagen C-proteinase enhancer protein shed light on the mechanism of stimulation of BMP-1. J Biol Chem. 2002;277:33864–33869. doi: 10.1074/jbc.M205018200. [DOI] [PubMed] [Google Scholar]
- 116.Jemmerson Ronald, Low Martin G. Phosphatidylinositol anchor of HeLa cell alkaline phosphatase. Biochemistry. 1987;26:5703–5709. doi: 10.1021/bi00392a019. [DOI] [PubMed] [Google Scholar]
- 117.Howard Andrew D, Berger Joel, Gerber Louise, Familletti Philip, Udenfriend Sidney. Characterization of the phosphatidylinositol-glycan membrane anchor of human placental alkaline phosphatase. Proc Natl Acad Sci U S A. 1987;84:6055–6059. doi: 10.1073/pnas.84.17.6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Suzuki Atsushi, Kozawa Osamu, Shinoda Jun, Watanabe Yasuko, Saito Hidehiko, Oiso Yutaka. Thrombin induces proliferation of osteoblast-like cells through phosphatidylcholine hydrolysis. J Cell Physiol. 1996;168:209–216. doi: 10.1002/(SICI)1097-4652(199607)168:1<209::AID-JCP25>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 119.Roberts Scott J, Stewart Alan J, Sadler Peter J, Farquharson Colin. Human PHOSPHO1 exhibits high specific phosphoethanolamine and phosphocholine phosphatase activities. Biochem J. 2004;382:59–65. doi: 10.1042/BJ20040511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Houston Brian, Stewart Alan J, Farquharson Colin. PHOSPHO1-A novel phosphatase specifically expressed at sites of mineralisation in bone and cartilage. Bone. 2004;34:629–637. doi: 10.1016/j.bone.2003.12.023. [DOI] [PubMed] [Google Scholar]
- 121.Stewart Alan J, Roberts Scott J, Seawright Elaine, Davey Megan G, Fleming Robert H, Farquharson Colin. The presence of PHOSPHO1 in matrix vesicles and its developmental expression prior to skeletal mineralization. Bone. 2006;39:1000–1007. doi: 10.1016/j.bone.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 122.Hessle Lovisa, Johnson Kristen A, Clarke Anderson H, Narisawa Sonoko, Sali Adnan, Goding James W, Terkeltaub Robert, Millan Jose Luis. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc Natl Acad Sci U S A. 2002;99:9445–9449. doi: 10.1073/pnas.142063399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Macrae Vicky E, Davey Megan G, McTeir Lynn, Narisawa Sonoko, Yadav Manisha C, Millan Jose Luis, Farquharson Colin. Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick. Bone. 2010;46:1146–1155. doi: 10.1016/j.bone.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Rebbe Neil F, Tong Benton D, Finley Elizabeth M, Hickman Scot. Identification of nucleotide pyrophosphatase/alkaline phosphodiesterase I activity associated with the mouse plasma cell differentiation antigen PC-1. Proc Natl Acad Sci U S A. 1991;88:5192–5196. doi: 10.1073/pnas.88.12.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Cheung Herman S, Kurup Indira V, Sallis John D, Ryan Lawrence M. Inhibition of calcium pyrophosphate dihydrate crystal formation in articular cartilage vesicles and cartilage by phosphocitrate. J Biol Chem. 1996;271:28082–28085. doi: 10.1074/jbc.271.45.28082. [DOI] [PubMed] [Google Scholar]
- 126.Cheung Herman S, Devine TR, Hubbard William. Calcium phosphate particle induction of metalloproteinase and mitogenesis: effect of particle sizes. Osteoarthritis Cartilage. 1997;5:145–151. doi: 10.1016/s1063-4584(97)80009-x. [DOI] [PubMed] [Google Scholar]
- 127.Cooke Michelle M, McCarthy Geraldine M, Sallis John D, Morgan Maria P. Phosphocitrate inhibits calcium hydroxyapatite induced mitogenesis and upregulation of matrix metalloproteinase-1, interleukin-1beta and cyclooxygenase-2 mRNA in human breast cancer cell lines. Breast Cancer Res Treat. 2003;79:253–263. doi: 10.1023/a:1023908307108. [DOI] [PubMed] [Google Scholar]
- 128.A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- 129.Liu Shiguang, Guo Rong, Tu Qisheng, Darryl Quarles L. Over-expression of PHEX in osteoblasts fails to rescue the Hyp mouse phenotype. J Biol Chem. 2002;277:3686–3697. doi: 10.1074/jbc.M107707200. [DOI] [PubMed] [Google Scholar]
- 130.Guo Rong, Darryl Quarles L. Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J Bone Miner Res. 1997;12:1009–1017. doi: 10.1359/jbmr.1997.12.7.1009. [DOI] [PubMed] [Google Scholar]
- 131.Li Xiaodong, Ominsky Michael S, Niu Qing-Tian, Sun Ning, Daugherty Betsy, D’Agostin Diane, Kurahara Carole, Gao Yongming, Cao Jin, Gong Jianhua, Asuncion Frank, Barrero Mauricio, Warmington Kelly, Dwyer Denise, Stolina Marina, Morony Sean, Sarosi IIdiko, Kostenuik Paul J, Lacey David L, Simonet W Scott, Ke Hua Zhu, Paszty Chris. Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res. 2008;23:860–869. doi: 10.1359/jbmr.080216. [DOI] [PubMed] [Google Scholar]
- 132.Craig Theodore A, Bhattacharya Resham, Mukhopadhyay Debabrata, Kumar Rajiv. Sclerostin binds and regulates the activity of cysteine-rich protein 61. Biochem Biophys Res Commun. 2009;392:36–40. doi: 10.1016/j.bbrc.2009.12.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Winkler David G, Yu Changpu, Geoghegan James C, Ojala Ethan W, Skonier John E, Shpektor Diana, Sutherland May K, Latham John A. Noggin and sclerostin bone morphogenetic protein antagonists form a mutually inhibitory complex. J Biol Chem. 2004;279:36293–36298. doi: 10.1074/jbc.M400521200. [DOI] [PubMed] [Google Scholar]
- 134.Erickson Harold HPP, Inglesias JL. A six-armed oligomer isolated from cell surface fibronectin preparations. Nature. 1984;311:267–269. doi: 10.1038/311267a0. [DOI] [PubMed] [Google Scholar]
- 135.Pacifici Maurizio. Tenascin-C and the development of articular cartilage. Matrix Biol. 1995;14:689–698. doi: 10.1016/s0945-053x(05)80011-3. [DOI] [PubMed] [Google Scholar]
- 136.Hall Brian K, Miyake Tsutomo. All for one and one for all: condensations and the initiation of skeletal development. Bioessays. 2000;22:138–147. doi: 10.1002/(SICI)1521-1878(200002)22:2<138::AID-BIES5>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 137.Forsberg Erick, Hirsch Emilio, Fröhlich Leopold, Meyer Michael, Ekblom Peter, Aszodi Attila, Werner Sabine, Fässler Reinhard. Skin wounds and severed nerves heal normally in mice lacking tenascin-C. Proc Natl Acad Sci U S A. 1996;93:6594–6599. doi: 10.1073/pnas.93.13.6594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Saga Yumiko, Yagi Takeshi, Ikawa Yoji, Sakakura Teruyo, Aizawa Shinichi. Mice develop normally without tenascin. Genes Dev. 1992;6:1821–1831. doi: 10.1101/gad.6.10.1821. [DOI] [PubMed] [Google Scholar]
- 139.Rowe Peter S, Oudet Claudine L, Francis Fiona, Sinding Christiane, Pannetier Solange, Econs Mike J, Strom Tim M, Meitinger Thomas, Garabedian Michele, David Albert, Macher Marie-Alice, Questiaux Elisabeth, Popowska Ewa, Pronicka Ewa, Read Andrew P, Mokrzycki Agnes, Glorieux Francis H, Drezner Marc K, Hanauer Andre, Lehrach Hans, Goulding Johnathan N, O’Riordan Jeffrey LH. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP) Hum Mol Genet. 1997;6:539–549. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]
- 140.Strom Tim M, Jüppner Harald. PHEX, FGF23, DMP1 and beyond. Curr Opin Nephrol Hypertens. 2008;17:357–362. doi: 10.1097/MNH.0b013e3282fd6e5b. [DOI] [PubMed] [Google Scholar]
- 141.Kiela Pawel R, Ghishan Fayez K. Recent advances in the renal-skeletal-gut axis that controls phosphate homeostasis. Lab Invest. 2009;89:7–14. doi: 10.1038/labinvest.2008.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ruchon Andrea F, Tenenhouse Harriet S, Marcinkiewicz Mieczyslaw, Siegfried Geraldine, Aubin Jane E, DesGroseillers Luc, Crine Philippe, Boileau Guy. Developmental expression and tissue distribution of PHEX protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000;15:1440–1450. doi: 10.1359/jbmr.2000.15.8.1440. [DOI] [PubMed] [Google Scholar]
- 143.Liu Shiguang, Tang Wen, Zhou Jianping, Vierthaler Luke, Darryl Quarles L. Distinct roles for intrinsic osteocyte abnormalities and systemic factors in regulation of FGF23 and bone mineralization in Hyp mice. Am J Physiol Endocrinol Metab. 2007;293:E1636–1644. doi: 10.1152/ajpendo.00396.2007. [DOI] [PubMed] [Google Scholar]
- 144.Bai Xiuying, Miao Dengshun, Panda Dibiyendu, Grady Scott, McKee Marc D, Goltzman David, Karaplis Andrew C. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (phosphate-regulating gene with homologies to endopeptidases on the X chromosome) expression. Mol Endocrinol. 2002;16:2913–2925. doi: 10.1210/me.2002-0113. [DOI] [PubMed] [Google Scholar]
- 145.Ecarot Brigitte, Glorieux Francis H, Desbarats Marguerite, Travers Rose, Labelle L. Effect of dietary phosphate deprivation and supplementation of recipient mice on bone formation by transplanted cells from normal and X-linked hypophosphatemic mice. J Bone Miner Res. 1992;7:523–530. doi: 10.1002/jbmr.5650070508. [DOI] [PubMed] [Google Scholar]
- 146.Erben Reinhold, Mayer Dagmar, Weber Karin, Jonsson Kenneth, Jüppner Harald, Lanske Beate. Over-expression of human PHEX under the human beta-actin promoter does not fully rescue the Hyp mouse phenotype. J Bone Miner Res. 2005;20:1149–1160. doi: 10.1359/JBMR.050212. [DOI] [PubMed] [Google Scholar]
- 147.Liu Shiguang, Rowe Peter S, Vierthaler Luke, Zhou Jianping, Darryl Quarles L. Phosphorylated acidic serine-aspartate-rich MEPE-associated motif peptide from matrix extracellular phosphoglycoprotein inhibits phosphate regulating gene with homologies to endopeptidases on the X-chromosome enzyme activity. J Endocrinol. 2007;192:261–267. doi: 10.1677/joe.1.07059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Addison William N, Nakano Yukiko, Loisel Thomas, Crine Phillippe, McKee Marc D. MEPE-ASARM peptides control extracellular matrix mineralization by binding to hydroxyapatite: an inhibition regulated by PHEX cleavage of ASARM. J Bone Miner Res. 2008;23:1638–1649. doi: 10.1359/jbmr.080601. [DOI] [PubMed] [Google Scholar]
- 149.Martin Aline, David Valentin, Laurence Jennifer S, Schwarz Patricia M, Lafer Eileen M, Hedge Anne-Marie, Rowe Peter S. Degradation of MEPE, DMP1, and release of SIBLING ASARM-peptides (minhibins): ASARM-peptide(s) are directly responsible for defective mineralization in HYP. Endocrinology. 2008;149:1757–1772. doi: 10.1210/en.2007-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Rowe Peter S, Matsumoto Naoko, Jo Osk D, Shih Remi N, Oconnor Jeannine, Roudier Martine P, Bain Steve, Liu Shiguang, Harrison Jody, Yanagawa Norimoto. Correction of the mineralization defect in hyp mice treated with protease inhibitors CA074 and pepstatin. Bone. 2006;39:773–786. doi: 10.1016/j.bone.2006.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dobbie Hamish, Unwin Robert J, Faria Nuno JR, Shirley David G. Matrix extracellular phosphoglycoprotein causes phosphaturia in rats by inhibiting tubular phosphate reabsorption. Nephrol Dial Transplant. 2008;23:730–733. doi: 10.1093/ndt/gfm535. [DOI] [PubMed] [Google Scholar]
- 152.Marks Joanne, Churchill Linda J, Debnam Edward S, Unwin Robert J. Matrix extracellular phosphoglycoprotein inhibits phosphate transport. J Am Soc Nephrol. 2008;19:2313–2320. doi: 10.1681/ASN.2008030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Liu Shiguang, Brown Thomas A, Zhou Jianping, Xiao Zhou-Sheng, Awad Hani, Guilak Farshid, Darryl Quarles L. Role of matrix extracellular phosphoglycoprotein in the pathogenesis of X-linked hypophosphatemia. J Am Soc Nephrol. 2005;16:1645–1653. doi: 10.1681/ASN.2004121060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.David Valentin, Martin Aline, Hedge Anne-Marie, Rowe Peter S. Matrix extracellular phosphoglycoprotein (MEPE) is a new bone renal hormone and vascularization modulator. Endocrinology. 2009;150:4012–4023. doi: 10.1210/en.2009-0216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bresler Doron, Bruder Jan, Mohnike Klaus, Fraser William D, Rowe Peter SN. Serum MEPE-ASARM-peptides are elevated in X-linked rickets (HYP): implications for phosphaturia and rickets. J Endocrinol. 2004;183:R1–9. doi: 10.1677/joe.1.05989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Bernocco Simonetta, Steiglitz Barry M, Svergun Dmitri I, Petoukhov Maxim V, Ruggiero Florence, Ricard-Blum Sylvie, Ebel Christine, Geourjon Christophe, Deleage Gilbert, Font Bernard, Eichenberger Denise, Greenspan Daniel S, Hulmes David JS. Low resolution structure determination shows procollagen C-proteinase enhancer to be an elongated multidomain glycoprotein. J Biol Chem. 2002;278:7199–7205. doi: 10.1074/jbc.M210857200. [DOI] [PubMed] [Google Scholar]
- 157.Hulmes David JS, Paul Mould A, Kessler Efrat. The CUB domains of procollagen C-proteinase enhancer control collagen assembly solely by their effect on procollagen C-proteinase/bone morphogenetic protein-1. Matrix Biol. 1997;16:41–45. doi: 10.1016/s0945-053x(97)90115-3. [DOI] [PubMed] [Google Scholar]
- 158.Moali Catherine, Font Bernard, Ruggiero Florence, Eichenberger Denise, Rousselle Patricia, François Vincent, Oldberg Ake, Bruckner-Tuderman Leena, Hulmes David JS. Substrate-specific modulation of a multisubstrate proteinase. C-terminal processing of fibrillar procollagens is the only BMP-1-dependent activity to be enhanced by PCPE-1. J Biol Chem. 2005;280:24188–24194. doi: 10.1074/jbc.M501486200. [DOI] [PubMed] [Google Scholar]
- 159.Bekhouche Mourad, Kronenberg Daniel, Goff Sandrine Vadon-Le, Bijakowski Cecile, Lim Ngee H, Font Bernard, Kessler Efrat, Colige Alain, Nagase Hideaki, Murphy Gillian, Hulmes David JS, Moali Catherine. Role of the netrin-like domain of procollagen C-proteinase enhancer-1 in the control of metalloproteinase activity. J Biol Chem. 2010;285:15950–15959. doi: 10.1074/jbc.M109.086447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Steiglitz Barry M, Kreider Jaclynn M, Frankenburg Elizabeth P, Pappano William N, Hoffman Guy G, Meganck Jeffrey A, Liang Xiaowen, Höök Magnus, Birk David E, Goldstein Steven A, Greenspan Daniel S. Procollagen C proteinase enhancer 1 genes are important determinants of the mechanical properties and geometry of bone and the ultrastructure of connective tissues. Mol Cell Biol. 2006;26:238–49. doi: 10.1128/MCB.26.1.238-249.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nakano Yukiko, Forsprecher Jennifer, Kaartinen Mari T. Regulation of ATPase activity of transglutaminase 2 by MT1-MMP: implications for mineralization of MC3T3-E1 osteoblast cultures. J Cell Physiol. 2010;223:260–269. doi: 10.1002/jcp.22034. [DOI] [PubMed] [Google Scholar]
- 162.De Laurenzi Vincenzo, Melino Gerry. Gene disruption of tissue transglutaminase. Mol Cell Biol. 2001;21:148–155. doi: 10.1128/MCB.21.1.148-155.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nanda Nisha, Iismaa Siiri E, Andrew Owens W, Husain Ahsan, Mackay Fabienne, Graham Robert M. Targeted inactivation of Gh/tissue transglutaminase II. J Biol Chem. 2001;276:20673–20678. doi: 10.1074/jbc.M010846200. [DOI] [PubMed] [Google Scholar]
- 164.Nakano Yukiko, Al-Jallad Hadil F, Mousa Aisha, Kaartinen Mari T. Expression and localization of plasma transglutaminase factor XIIIA in bone. J Histochem Cytochem. 2007;55:675–685. doi: 10.1369/jhc.6A7091.2007. [DOI] [PubMed] [Google Scholar]
- 165.Al-Jallad Hadil F, Nakano Yukiko, Chen Jeff LY, McMillan Erin, Lefebvre Celine, Kaartinen Mari T. Transglutaminase activity regulates osteoblast differentiation and matrix mineralization in MC3T3-E1 osteoblast cultures. Matrix Biol. 2006;25:135–148. doi: 10.1016/j.matbio.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 166.Sørensen Esben S, Petersen Torben E. Phosphorylation, glycosylation, and transglutaminase sites in bovine osteopontin. Ann N Y Acad Sci. 1995;760:363–366. doi: 10.1111/j.1749-6632.1995.tb44658.x. [DOI] [PubMed] [Google Scholar]
- 167.Kaartinen Mari T, Pirhonen Arja, Linnala-Kankkunen Annikka, Mäenpää Pekka H. Transglutaminase-catalyzed cross-linking of osteopontin is inhibited by osteocalcin. J Biol Chem. 1997;272:22736–22741. doi: 10.1074/jbc.272.36.22736. [DOI] [PubMed] [Google Scholar]
- 168.Kaartinen Mari T, Pirhonen Arja, Linnala-Kankkunen Annikka, Mäenpää Pekka. Cross-linking of osteopontin by tissue transglutaminase increases its collagen binding properties. J Biol Chem. 1999;274:1729–1735. doi: 10.1074/jbc.274.3.1729. [DOI] [PubMed] [Google Scholar]
- 169.Nurminskaya Maria, Magee Cordula, Faverman Lidia, Linsenmayer Thomas F. Chondrocyte-derived transglutaminase promotes maturation of preosteoblasts in periosteal bone. Dev Biol. 2003;263:139–152. doi: 10.1016/s0012-1606(03)00445-7. [DOI] [PubMed] [Google Scholar]
- 170.Filanti C, Dickson GR, Di Martino D, Ulivi V, Sanguineti C, Romano P, Palermo C, Manduca P. The expression of metalloproteinase-2, -9, and -14 and of tissue inhibitors-1 and -2 is developmentally modulated during osteogenesis in vitro, the mature osteoblastic phenotype expressing metalloproteinase-14. J Bone Miner Res. 2000;15:2154–2168. doi: 10.1359/jbmr.2000.15.11.2154. [DOI] [PubMed] [Google Scholar]
- 171.Itoh Takeshi, Tanioka Masatoshi, Yoshida Hiroshi, Yoshioka Takayuki, Nishimoto Hirofumi, Itohara Shigeyoshi. Reduced angiogenesis and tumor progression in gelatinase A-deficient mice. Cancer Res. 1998;58:1048–1051. [PubMed] [Google Scholar]
- 172.Pyo Robert, Lee Jason K, Michael Shipley J, Curci John A, Mao Dongli, Ziporin Scott J, Ennis Terri L, Shapiro Steven D, Senior Robert M, Thompson Robert W. Targeted gene disruption of matrix metalloproteinase-9 (gelatinase B) suppresses development of experimental abdominal aortic aneurysms. J Clin Invest. 2000;105:1641–1649. doi: 10.1172/JCI8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Rattenholl Anke, Pappano William N, Koch Manuel, Keene Douglas R, Kadler Karl E, Sasaki Takako, Timpl Rupert, Burgeson Robert E, Greenspan Daniel S, Bruckner-Tuderman Leena. Proteinases of the bone morphogenetic protein-1 family convert procollagen VII to mature anchoring fibril collagen. J Biol Chem. 2002;277:26372–26378. doi: 10.1074/jbc.M203247200. [DOI] [PubMed] [Google Scholar]
- 174.Miura Masako, Chen Xiao-Dong, Allen Matthew R, Bi Yanming, Gronthos Stan, Seo Byoung-Moo, Lakhani Saquib, Flavell Richard A, Feng Xin-Hua, Robey Pamela G, Young Marian M, Shi Songtao. A crucial role of caspase-3 in osteogenic differentiation of bone marrow stromal stem cells. J Clin Invest. 2004;114:1704–1713. doi: 10.1172/JCI20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Holmbeck Kenn, Bianco Paolo, Caterina John, Yamada Susan, Kromer Mark, Kuznetsov Sergei A, Mankani Mahesh, Robey Pamela G, Robin Poole A, Pidoux Isabelle, Ward Jerrold M, Birkedal-Hansen Henning. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 176.Holmbeck Kenn, Bianco Paolo, Yamada Susan, Birkedal-Hansen Henning. MT1-MMP: a tethered collagenase. J Cell Physiol. 2004;200:11–9. doi: 10.1002/jcp.20065. [DOI] [PubMed] [Google Scholar]
- 177.Holmbeck Kenn, Bianco Paolo, Pidoux Isabelle, Inoue S, Clark Billinghurst R, Wu William, Chrysovergis Kali, Yamada Susan, Birkedal-Hansen Henning, Robin Poole A. The metalloproteinase MT1-MMP is required for normal development and maintenance of osteocyte processes in bone. J Cell Sci. 2005;118:147–156. doi: 10.1242/jcs.01581. [DOI] [PubMed] [Google Scholar]
- 178.Nabil G, Seidah NG, Mayer G, Zaid A, Rousselet E, Nassoury N, Poirier S, Essalmani R, Prat A. The activation and physiological functions of the proprotein convertases. Int J Biochem Cell Biol. 2008;40:1111–1125. doi: 10.1016/j.biocel.2008.01.030. [DOI] [PubMed] [Google Scholar]
- 179.Wang Xiaodong, Pai Jih-tung, Wiedenfeld Elizabeth A, Medina Julio C, Slaughter Clive A, Goldstein Joseph L, Brown Michael S. Purification of an interleukin-1 beta converting enzyme-related cysteine protease that cleaves sterol regulatory element-binding proteins between the leucine zipper and transmembrane domains. J Biol Chem. 1995;270:18044–18050. doi: 10.1074/jbc.270.30.18044. [DOI] [PubMed] [Google Scholar]
- 180.Guan Guimin, Dai Pei-Hua, Osborne Timothy F, Kim Jae B, Shechter Ishaiahu. Multiple sequence elements are involved in the transcriptional regulation of the human squalene synthase gene. J Biol Chem. 1997;272:10295–102302. doi: 10.1074/jbc.272.15.10295. [DOI] [PubMed] [Google Scholar]
- 181.Schröder M. Endoplasmic reticulum stress responses. Cell Mol Life Sci. 2008;65:862–894. doi: 10.1007/s00018-007-7383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Murakami Tomohiko, Kondo Shinichi, Ogata Maiko, Kanemoto Soshi, Saito Atsushi, Wanaka Atsushi, Imaizumi Kazunori. Cleavage of the membrane-bound transcription factor OASIS in response to endoplasmic reticulum stress. J Neurochem. 2006;96:1090–1100. doi: 10.1111/j.1471-4159.2005.03596.x. [DOI] [PubMed] [Google Scholar]
- 183.Murakami Tomohiko, Saito Atsushi, Hino Shin-ichiro, Kondo Shinichi, Kanemoto Soshi, Chihara Kazuyasu, Sekiya Hiroshi, Tsumagari Kenji, Ochiai Kimiko, Yoshinaga Kazuya, Saitoh Masahiro, Nishimura Riko, Yoneda Toshiyuki, Kou Ikuyo, Furuichi Tatsuya, Ikegawa Shiro, Ikawa Masahito, Okabe Masaru, Wanaka Akio, Imaizumi Kazunori. Signalling mediated by the endoplasmic reticulum stress transducer OASIS is involved in bone formation. Nature Cell Biol. 2009;11:1205–1211. doi: 10.1038/ncb1963. [DOI] [PubMed] [Google Scholar]
- 184.Chandhoke Taranpreet K, Huang Yu-Feng, Liu Fei, Gronowicz Gloria A, Adams Douglas J, Harrison John R, Kream Barbara E. Osteopenia in transgenic mice with osteoblast-targeted expression of the inducible cAMP early repressor. Bone. 2008;43:101–109. doi: 10.1016/j.bone.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Schlombs Kornelia, Wagner Thomas, Scheel Jochen. Site-1 protease is required for cartilage development in zebrafish. Proc Natl Acad Sci U S A. 2003;100:14024–14029. doi: 10.1073/pnas.2331794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Patra Debabrata, Xing Xiaoyun, Davies Sherri, Bryan Jennifer, Franz Carl, Hunziker Ernst B, Sandell Linda J. Site-1 protease is essential for endochondral bone formation in mice. J Cell Biol. 2007;179:687–700. doi: 10.1083/jcb.200708092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Yang Jian, Goldstein Joseph L, Hammer Robert E, Moon Young-Ah, Brown Michael S, Horton Jay D. Decreased lipid synthesis in livers of mice with disrupted Site-1 protease gene. Proc Natl Acad Sci U S A. 2010;98:13607–13612. doi: 10.1073/pnas.201524598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gorski Jeff P, Huffman Nichole T, Chittur Sridar, Midura Ronald J, Black Claudine, Oxford Julia, Seidah Nabil G. Inhibition of proprotein convertase SKI-1 blocks transcription of key extracellular matrix genes regulating osteoblastic mineralization. J Biol Chem. 2011;286 doi: 10.1074/jbc.M110.151647. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]