Abstract
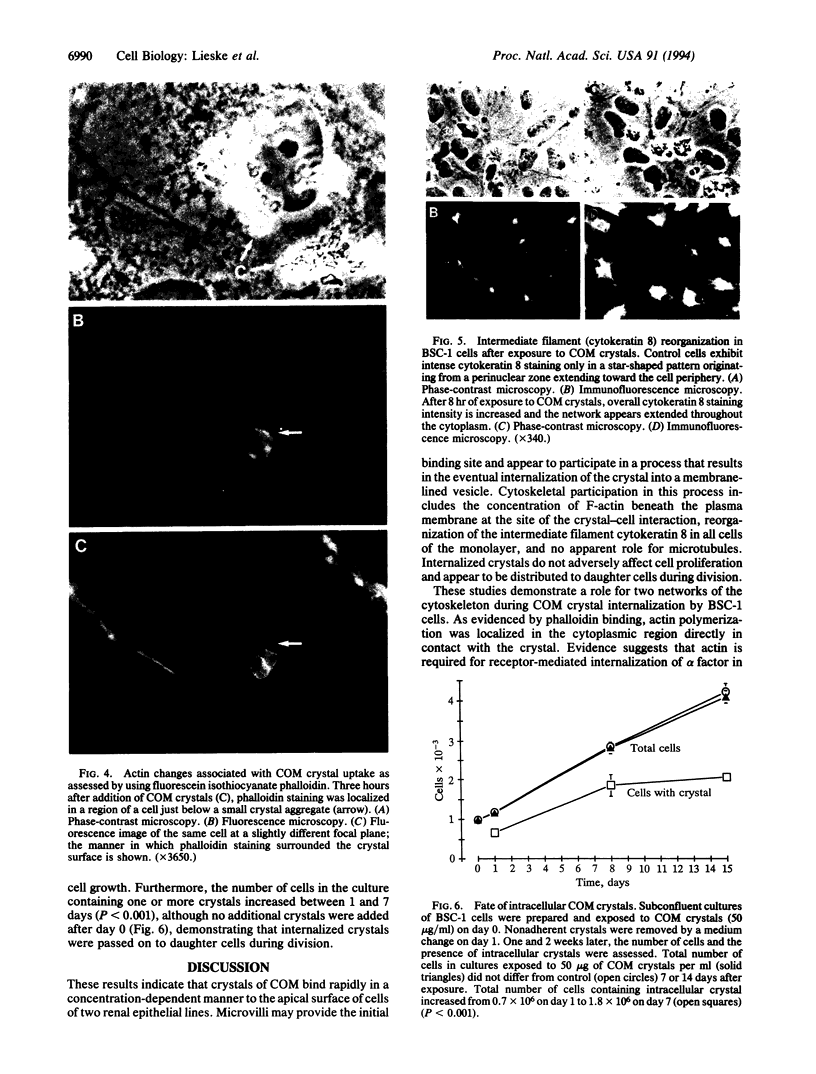
Renal tubular fluid is supersaturated with calcium and oxalate ions, which can nucleate to form crystals of calcium oxalate monohydrate (COM), the most abundant constituent of kidney stones. However, the mechanisms by which nascent crystals are retained in the nephron and then grow into kidney stones are unclear. An interaction of COM crystals with the surface of renal epithelial cells could be a critical initiating event in nephrolithiasis. To investigate this possibility we used cultures of monkey kidney epithelial cells (BSC-1 line) as a model system and found that [14C]COM crystals bound to the cell surface within seconds. Scanning electron microscopy revealed that crystals bind first to apical microvilli, which subsequently migrate over the crystalline surface. When visualized by transmission electron microscopy, intracellular crystals were located within vesicles. Cytoskeletal responses to crystal uptake were sought by immunofluorescence microscopy, which revealed concentration of F-actin at sites of crystal contact as well as a generalized reorganization of the intermediate filament network containing cytokeratin 8. Uptake of COM crystals did not adversely affect renal epithelial cell growth, and internalized crystals were apparently distributed to daughter cells during division. Rapid adherence of COM crystals to the apical surface of tubular epithelial cells could promote crystal retention in the kidney. Elucidation of factors that regulate this process may provide insight into the pathogenesis of nephrolithiasis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axline S. G., Reaven E. P. Inhibition of phagocytosis and plasma membrane mobility of the cultivated macrophage by cytochalasin B. Role of subplasmalemmal microfilaments. J Cell Biol. 1974 Sep;62(3):647–659. doi: 10.1083/jcb.62.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlayson B., Reid F. The expectation of free and fixed particles in urinary stone disease. Invest Urol. 1978 May;15(6):442–448. [PubMed] [Google Scholar]
- Gottlieb T. A., Ivanov I. E., Adesnik M., Sabatini D. D. Actin microfilaments play a critical role in endocytosis at the apical but not the basolateral surface of polarized epithelial cells. J Cell Biol. 1993 Feb;120(3):695–710. doi: 10.1083/jcb.120.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanein D., Sabanay H., Addadi L., Geiger B. Selective interactions of cells with crystal surfaces. Implications for the mechanism of cell adhesion. J Cell Sci. 1993 Feb;104(Pt 2):275–288. doi: 10.1242/jcs.104.2.275. [DOI] [PubMed] [Google Scholar]
- Kartha S., Atkin B., Martin T. E., Toback F. G. Cytokeratin reorganization induced by adenosine diphosphate in kidney epithelial cells. Exp Cell Res. 1992 Jun;200(2):219–226. doi: 10.1016/0014-4827(92)90167-7. [DOI] [PubMed] [Google Scholar]
- Kartha S., Toback F. G. Adenine nucleotides stimulate migration in wounded cultures of kidney epithelial cells. J Clin Invest. 1992 Jul;90(1):288–292. doi: 10.1172/JCI115851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kübler E., Riezman H. Actin and fimbrin are required for the internalization step of endocytosis in yeast. EMBO J. 1993 Jul;12(7):2855–2862. doi: 10.1002/j.1460-2075.1993.tb05947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieske J. C., Spargo B. H., Toback F. G. Endocytosis of calcium oxalate crystals and proliferation of renal tubular epithelial cells in a patient with type 1 primary hyperoxaluria. J Urol. 1992 Nov;148(5):1517–1519. doi: 10.1016/s0022-5347(17)36954-9. [DOI] [PubMed] [Google Scholar]
- Lieske J. C., Toback F. G. Regulation of renal epithelial cell endocytosis of calcium oxalate monohydrate crystals. Am J Physiol. 1993 May;264(5 Pt 2):F800–F807. doi: 10.1152/ajprenal.1993.264.5.F800. [DOI] [PubMed] [Google Scholar]
- Lieske J. C., Walsh-Reitz M. M., Toback F. G. Calcium oxalate monohydrate crystals are endocytosed by renal epithelial cells and induce proliferation. Am J Physiol. 1992 Apr;262(4 Pt 2):F622–F630. doi: 10.1152/ajprenal.1992.262.4.F622. [DOI] [PubMed] [Google Scholar]
- Molitoris B. A., Nelson W. J. Alterations in the establishment and maintenance of epithelial cell polarity as a basis for disease processes. J Clin Invest. 1990 Jan;85(1):3–9. doi: 10.1172/JCI114427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riese R. J., Mandel N. S., Wiessner J. H., Mandel G. S., Becker C. G., Kleinman J. G. Cell polarity and calcium oxalate crystal adherence to cultured collecting duct cells. Am J Physiol. 1992 Feb;262(2 Pt 2):F177–F184. doi: 10.1152/ajprenal.1992.262.2.F177. [DOI] [PubMed] [Google Scholar]
- Riese R. J., Riese J. W., Kleinman J. G., Wiessner J. H., Mandel G. S., Mandel N. S. Specificity in calcium oxalate adherence to papillary epithelial cells in cultures. Am J Physiol. 1988 Nov;255(5 Pt 2):F1025–F1032. doi: 10.1152/ajprenal.1988.255.5.F1025. [DOI] [PubMed] [Google Scholar]
- Speert D. P., Gordon S. Phagocytosis of unopsonized Pseudomonas aeruginosa by murine macrophages is a two-step process requiring glucose. J Clin Invest. 1992 Sep;90(3):1085–1092. doi: 10.1172/JCI115924. [DOI] [PMC free article] [PubMed] [Google Scholar]