Abstract
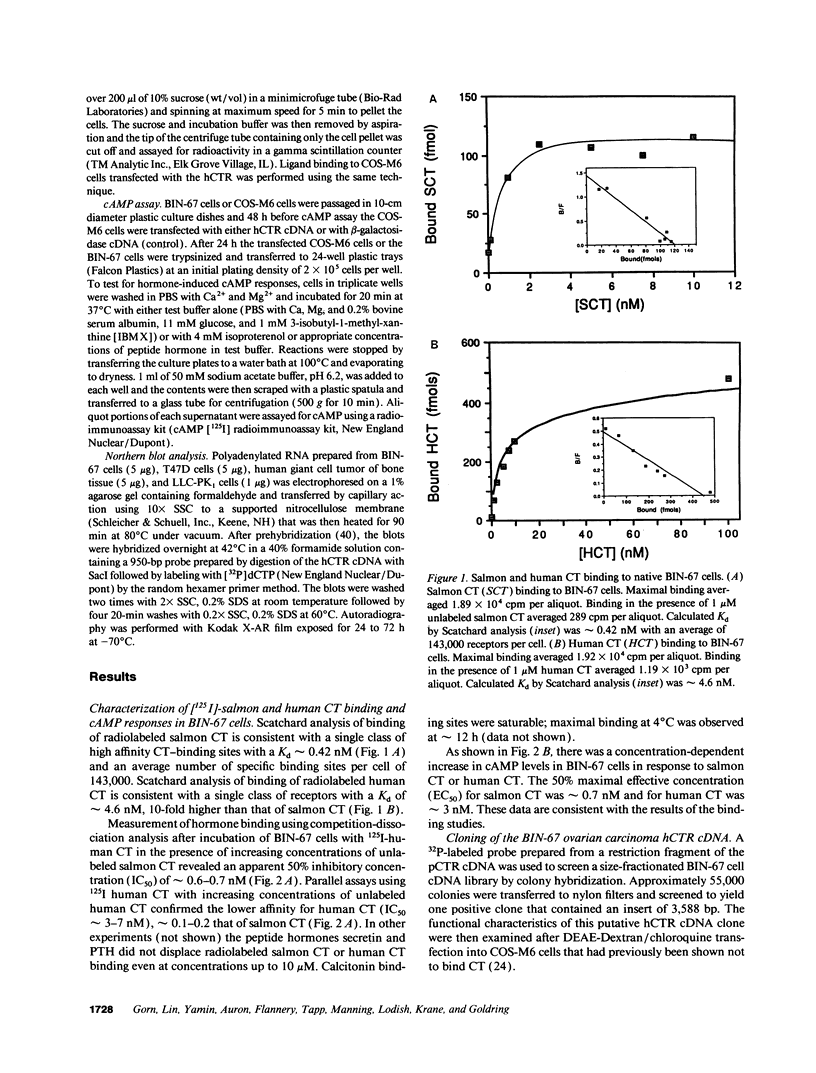
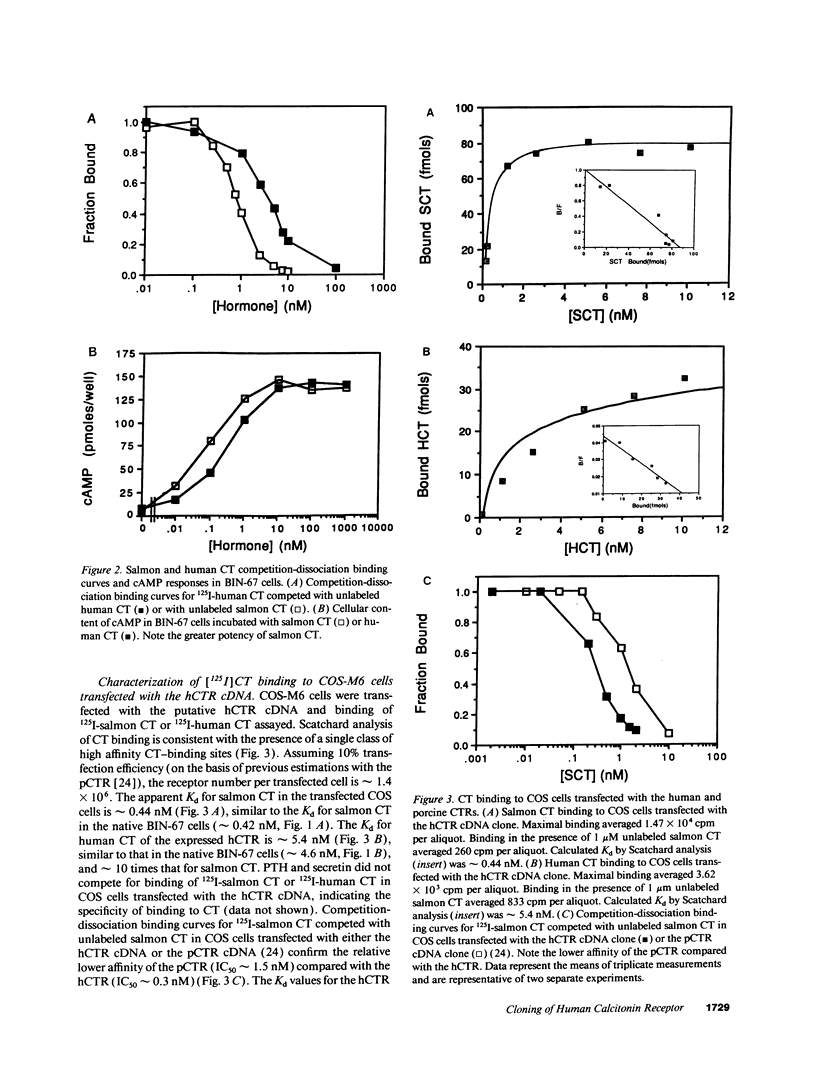
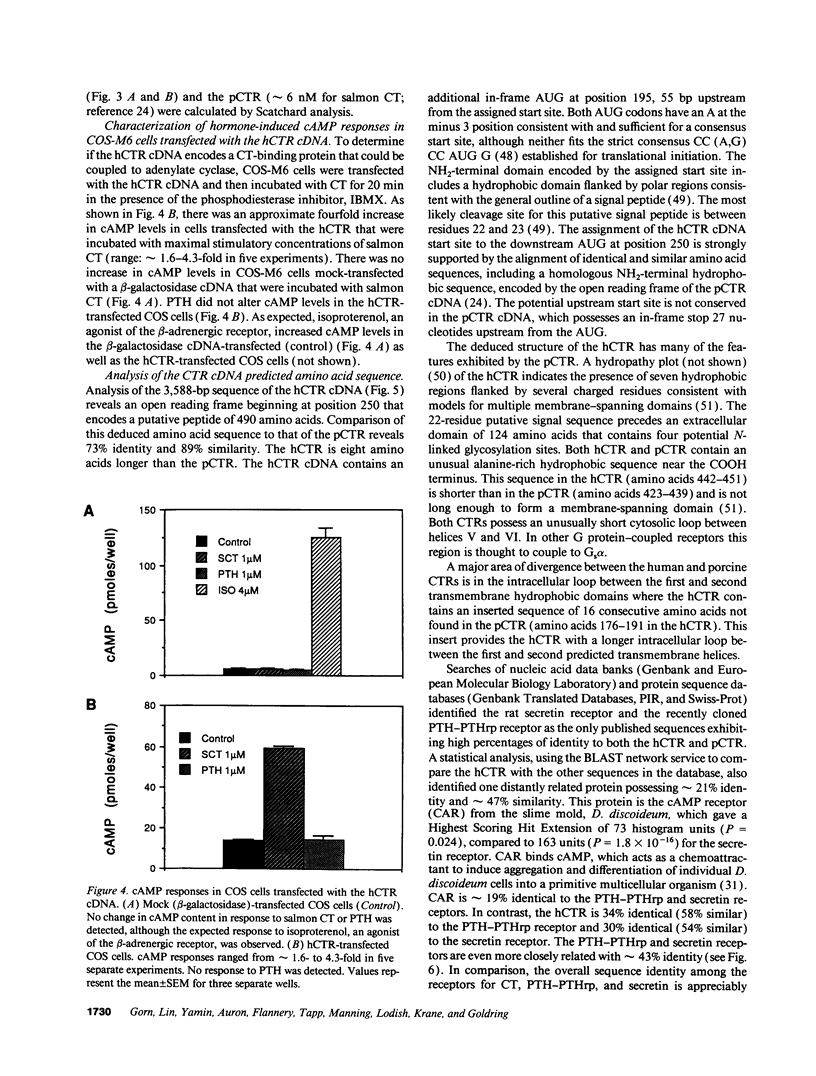
A human ovarian small cell carcinoma line (BIN-67) expresses abundant calcitonin (CT) receptors (CTR) (143,000 per cell) that are coupled, to adenylate cyclase. The dissociation constants (Kd) for the CTRs on these BIN-67 cells is approximately 0.42 nM for salmon CT and approximately 4.6 nM for human CT. To clone a human CTR (hCTR), a BIN-67 cDNA library was screened using a cDNA probe from a porcine renal CTR (pCTR) that we recently cloned. One positive clone of 3,588 bp was identified. Transfection of this cDNA into COS cells resulted in expression of receptors with high affinity for salmon CT (Kd = approximately 0.44 nM) and for human CT (Kd = approximately 5.4 nM). The expressed hCTR was coupled to adenylate cyclase. Northern analysis with the hCTR cDNA probe indicated a single transcript of approximately 4.2 kb. The cloned cDNA encodes a putative peptide of 490 amino acids with seven potential transmembrane domains. The amino acid sequence of the hCTR is 73% identical to the pCTR, although the hCTR contains an insert of 16 amino acids between transmembrane domain I and II. The structural differences may account for observed differences in binding affinity between the porcine renal and human ovarian CTRs. The CTRs are closely related to the receptors for parathyroid hormone-parathyroid hormone-related peptide and secretin; these receptors comprise a distinct family of G protein-coupled seven transmembrane domain receptors. Interestingly, the hCTR sequence is remotely related to the cAMP receptor of Dictyostelium discoideum (21% identical), but is not significantly related to other G protein-coupled receptor sequences now in the data bases.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Amara S. G., Arriza J. L., Leff S. E., Swanson L. W., Evans R. M., Rosenfeld M. G. Expression in brain of a messenger RNA encoding a novel neuropeptide homologous to calcitonin gene-related peptide. Science. 1985 Sep 13;229(4718):1094–1097. doi: 10.1126/science.2994212. [DOI] [PubMed] [Google Scholar]
- Attwood T. K., Eliopoulos E. E., Findlay J. B. Multiple sequence alignment of protein families showing low sequence homology: a methodological approach using database pattern-matching discriminators for G-protein-linked receptors. Gene. 1991 Feb 15;98(2):153–159. doi: 10.1016/0378-1119(91)90168-b. [DOI] [PubMed] [Google Scholar]
- Brain S. D., MacIntyre I., Williams T. J. A second form of human calcitonin gene-related peptide which is a potent vasodilator. Eur J Pharmacol. 1986 May 27;124(3):349–352. doi: 10.1016/0014-2999(86)90238-4. [DOI] [PubMed] [Google Scholar]
- COPP D. H., CAMERON E. C., CHENEY B. A., DAVIDSON A. G., HENZE K. G. Evidence for calcitonin--a new hormone from the parathyroid that lowers blood calcium. Endocrinology. 1962 May;70:638–649. doi: 10.1210/endo-70-5-638. [DOI] [PubMed] [Google Scholar]
- Chakraborty M., Chatterjee D., Kellokumpu S., Rasmussen H., Baron R. Cell cycle-dependent coupling of the calcitonin receptor to different G proteins. Science. 1991 Mar 1;251(4997):1078–1082. doi: 10.1126/science.1847755. [DOI] [PubMed] [Google Scholar]
- Chausmer A. B., Stevens M. D., Severn C. Autoradiographic evidence for a calcitonin receptor on testicular Leydig cells. Science. 1982 May 14;216(4547):735–736. doi: 10.1126/science.6281881. [DOI] [PubMed] [Google Scholar]
- Chausmer A. B., Stevens M. D., Zears R. Influence of parathyroid hormone and calcitonin on tissue zinc homeostasis in the rat. Metabolism. 1980 Jul;29(7):617–623. doi: 10.1016/0026-0495(80)90105-5. [DOI] [PubMed] [Google Scholar]
- Chausmer A., Stuart C., Stevens M. Identification of testicular cell plasma membrane receptors for calcitonin. J Lab Clin Med. 1980 Nov;96(5):933–938. [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickersin G. R., Kline I. W., Scully R. E. Small cell carcinoma of the ovary with hypercalcemia: a report of eleven cases. Cancer. 1982 Jan 1;49(1):188–197. doi: 10.1002/1097-0142(19820101)49:1<188::aid-cncr2820490137>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Dohlman H. G., Caron M. G., DeBlasi A., Frielle T., Lefkowitz R. J. Role of extracellular disulfide-bonded cysteines in the ligand binding function of the beta 2-adrenergic receptor. Biochemistry. 1990 Mar 6;29(9):2335–2342. doi: 10.1021/bi00461a018. [DOI] [PubMed] [Google Scholar]
- Findlay D. M., Michelangeli V. P., Moseley J. M., Martin T. J. Calcitonin binding and degradation by two cultured human breast cancer cell lines (MCF 7 and T 47D). Biochem J. 1981 May 15;196(2):513–520. doi: 10.1042/bj1960513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Findlay D. M., deLuise M., Michelangeli V. P., Ellison M., Martin T. J. Properties of a calcitonin receptor and adenylate cyclase in BEN cells, a human cancer cell line. Cancer Res. 1980 Apr;40(4):1311–1317. [PubMed] [Google Scholar]
- Fiore C. E., Castorina F., Malatino L. S., Tamburino C. Antalgic activity of calcitonin: effectiveness of the epidural and subarachnoid routes in man. Int J Clin Pharmacol Res. 1983;3(4):257–260. [PubMed] [Google Scholar]
- Fischer J. A., Tobler P. H., Kaufmann M., Born W., Henke H., Cooper P. E., Sagar S. M., Martin J. B. Calcitonin: regional distribution of the hormone and its binding sites in the human brain and pituitary. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7801–7805. doi: 10.1073/pnas.78.12.7801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouchereau-Peron M., Moukhtar M. S., Benson A. A., Milhaud G. Characterization of specific receptors for calcitonin in porcine lung. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3973–3975. doi: 10.1073/pnas.78.6.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J., Raisz L. G. Thyrocalcitonin: inhibitor of bone resorption in tissue culture. Science. 1965 Dec 10;150(3702):1465–1467. doi: 10.1126/science.150.3702.1465. [DOI] [PubMed] [Google Scholar]
- Gattei V., Bernabei P. A., Pinto A., Bezzini R., Ringressi A., Formigli L., Tanini A., Attadia V., Brandi M. L. Phorbol ester induced osteoclast-like differentiation of a novel human leukemic cell line (FLG 29.1). J Cell Biol. 1992 Jan;116(2):437–447. doi: 10.1083/jcb.116.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goebell H., Ammann R., Herfarth C., Horn J., Hotz J., Knoblauch M., Schmid M., Jaeger M., Akovbiantz A., Linder E. A double-blind trial of synthetic salmon calcitonin in the treatment of acute pancreatitis. Scand J Gastroenterol. 1979;14(7):881–889. doi: 10.3109/00365527909181420. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Dayer J. M., Ausiello D. A., Krane S. M. A cell strain cultured from porcine kidney increases cyclic AMP content upon exposure to calcitonin or vasopressin. Biochem Biophys Res Commun. 1978 Jul 28;83(2):434–440. doi: 10.1016/0006-291x(78)91009-4. [DOI] [PubMed] [Google Scholar]
- Goldring S. R., Roelke M. S., Petrison K. K., Bhan A. K. Human giant cell tumors of bone identification and characterization of cell types. J Clin Invest. 1987 Feb;79(2):483–491. doi: 10.1172/JCI112838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goltzman D., Mitchell J. Interaction of calcitonin and calcitonin gene-related peptide at receptor sites in target tissues. Science. 1985 Mar 15;227(4692):1343–1345. doi: 10.1126/science.2983422. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Sheiness D. K., Bishop J. M. Transcripts from the cellular homologs of retroviral oncogenes: distribution among chicken tissues. Mol Cell Biol. 1982 Jun;2(6):617–624. doi: 10.1128/mcb.2.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubler U., Hoffman B. J. A simple and very efficient method for generating cDNA libraries. Gene. 1983 Nov;25(2-3):263–269. doi: 10.1016/0378-1119(83)90230-5. [DOI] [PubMed] [Google Scholar]
- Haas H. G., Dambacher M. A., Guncaga J., Lauffenbruger T. Renal effects of calcitonin and parathyroid extract in man. Studies in hypoparathyroidism. J Clin Invest. 1971 Dec;50(12):2689–2702. doi: 10.1172/JCI106770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotz J., Goebell H. Long-term effects of calcitonin on gastric secretion in normals, peptic ulcer and high risk patients. Z Gastroenterol Verh. 1976;(10):71–77. [PubMed] [Google Scholar]
- Ishihara T., Nakamura S., Kaziro Y., Takahashi T., Takahashi K., Nagata S. Molecular cloning and expression of a cDNA encoding the secretin receptor. EMBO J. 1991 Jul;10(7):1635–1641. doi: 10.1002/j.1460-2075.1991.tb07686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jüppner H., Abou-Samra A. B., Freeman M., Kong X. F., Schipani E., Richards J., Kolakowski L. F., Jr, Hock J., Potts J. T., Jr, Kronenberg H. M. A G protein-linked receptor for parathyroid hormone and parathyroid hormone-related peptide. Science. 1991 Nov 15;254(5034):1024–1026. doi: 10.1126/science.1658941. [DOI] [PubMed] [Google Scholar]
- Klein P. S., Sun T. J., Saxe C. L., 3rd, Kimmel A. R., Johnson R. L., Devreotes P. N. A chemoattractant receptor controls development in Dictyostelium discoideum. Science. 1988 Sep 16;241(4872):1467–1472. doi: 10.1126/science.3047871. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Harris T. L., Flannery M. S., Aruffo A., Kaji E. H., Gorn A., Kolakowski L. F., Jr, Lodish H. F., Goldring S. R. Expression cloning of an adenylate cyclase-coupled calcitonin receptor. Science. 1991 Nov 15;254(5034):1022–1024. doi: 10.1126/science.1658940. [DOI] [PubMed] [Google Scholar]
- Marx S. J., Aurbach G. D., Gavin J. R., 3rd, Buell D. W. Calcitonin receptors on cultured human lymphocytes. J Biol Chem. 1974 Nov 10;249(21):6812–6816. [PubMed] [Google Scholar]
- Moll R., Levy R., Czernobilsky B., Hohlweg-Majert P., Dallenbach-Hellweg G., Franke W. W. Cytokeratins of normal epithelia and some neoplasms of the female genital tract. Lab Invest. 1983 Nov;49(5):599–610. [PubMed] [Google Scholar]
- Moseley J. M., Findlay D. M., Gorman J. J., Michelangeli V. P., Martin T. J. The calcitonin receptor on T 47D breast cancer cells. Evidence for glycosylation. Biochem J. 1983 Jun 15;212(3):609–616. doi: 10.1042/bj2120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moseley J. M., Findlay D. M., Martin T. J., Gorman J. J. Covalent cross-linking of a photoactive derivative of calcitonin to human breast cancer cell receptors. J Biol Chem. 1982 May 25;257(10):5846–5851. [PubMed] [Google Scholar]
- Moxham C. P., Malbon C. C. Fat cell beta 1-adrenergic receptor: structural evidence for existence of disulfide bridges essential for ligand binding. Biochemistry. 1985 Oct 22;24(22):6072–6077. doi: 10.1021/bi00343a007. [DOI] [PubMed] [Google Scholar]
- Murad F., Brewer H. B., Jr, Vaughan M. Effect of thyrocalcitonin on adenosine 3':5'-cyclic phosphate formation by rat kidney and bone. Proc Natl Acad Sci U S A. 1970 Feb;65(2):446–453. doi: 10.1073/pnas.65.2.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhla A. M., Bardin C. W., Salomon Y., Mather J. P., Jänne O. A. The actions of calcitonin on the TM3 Leydig cell line and on rat Leydig cell-enriched cultures. J Androl. 1989 Jul-Aug;10(4):311–320. doi: 10.1002/j.1939-4640.1989.tb00110.x. [DOI] [PubMed] [Google Scholar]
- Nicholson G. C., D'Santos C. S., Evans T., Moseley J. M., Kemp B. E., Michelangeli V. P., Martin T. J. Human placental calcitonin receptors. Biochem J. 1988 Mar 15;250(3):877–882. doi: 10.1042/bj2500877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petermann J. B., Born W., Chang J. Y., Fischer J. A. Identification in the human central nervous system, pituitary, and thyroid of a novel calcitonin gene-related peptide, and partial amino acid sequence in the spinal cord. J Biol Chem. 1987 Jan 15;262(2):542–545. [PubMed] [Google Scholar]
- Seed B., Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci U S A. 1987 May;84(10):3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silvestroni L., Menditto A., Frajese G., Gnessi L. Identification of calcitonin receptors in human spermatozoa. J Clin Endocrinol Metab. 1987 Oct;65(4):742–746. doi: 10.1210/jcem-65-4-742. [DOI] [PubMed] [Google Scholar]
- Stevenson J. C., Abeyasekera G., Hillyard C. J., Phang K. G., MacIntyre I., Campbell S., Townsend P. T., Young O., Whitehead M. I. Calcitonin and the calcium-regulating hormones in postmenopausal women: effect of oestrogens. Lancet. 1981 Mar 28;1(8222):693–695. doi: 10.1016/s0140-6736(81)91973-5. [DOI] [PubMed] [Google Scholar]
- Thornton J. M., Flores T. P., Jones D. T., Swindells M. B. Protein structure. Prediction of progress at last. Nature. 1991 Nov 14;354(6349):105–106. doi: 10.1038/354105a0. [DOI] [PubMed] [Google Scholar]
- Toverud S. U., Cooper C. W., Munson P. L. Calcium metabolism during lactation: elevated blood levels of calcitonin. Endocrinology. 1978 Aug;103(2):472–479. doi: 10.1210/endo-103-2-472. [DOI] [PubMed] [Google Scholar]
- Upchurch K. S., Parker L. M., Scully R. E., Krane S. M. Differential cyclic AMP responses to calcitonin among human ovarian carcinoma cell lines: a calcitonin-responsive line derived from a rare tumor type. J Bone Miner Res. 1986 Jun;1(3):299–304. doi: 10.1002/jbmr.5650010309. [DOI] [PubMed] [Google Scholar]
- Warshawsky H., Goltzman D., Rouleau M. F., Bergeron J. J. Direct in vivo demonstration by radioautography of specific binding sites for calcitonin in skeletal and renal tissues of the rat. J Cell Biol. 1980 Jun;85(3):682–694. doi: 10.1083/jcb.85.3.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead M., Lane G., Young O., Campbell S., Abeyasekera G., Hillyard C. J., MacIntyre I., Phang K. G., Stevenson J. C. Interrelations of calcium-regulating hormones during normal pregnancy. Br Med J (Clin Res Ed) 1981 Jul 4;283(6283):10–12. doi: 10.1136/bmj.283.6283.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlwend A., Malmström K., Henke H., Murer H., Vassalli J. D., Fischer J. A. Calcitonin and calcitonin gene-related peptide interact with the same receptor in cultured LLC-PK1 kidney cells. Biochem Biophys Res Commun. 1985 Sep 16;131(2):537–542. doi: 10.1016/0006-291x(85)91269-0. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Datta H. K., Moonga B. S., MacIntyre I. Evidence that the action of calcitonin on rat osteoclasts is mediated by two G proteins acting via separate post-receptor pathways. J Endocrinol. 1990 Sep;126(3):473–481. doi: 10.1677/joe.0.1260473. [DOI] [PubMed] [Google Scholar]
- Zhu G. C., Dudley D. T., Saltiel A. R. Amylin increases cyclic AMP formation in L6 myocytes through calcitonin gene-related peptide receptors. Biochem Biophys Res Commun. 1991 Jun 14;177(2):771–776. doi: 10.1016/0006-291x(91)91855-7. [DOI] [PubMed] [Google Scholar]
- von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986 Jun 11;14(11):4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]