Abstract
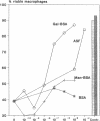
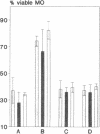
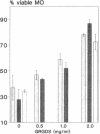
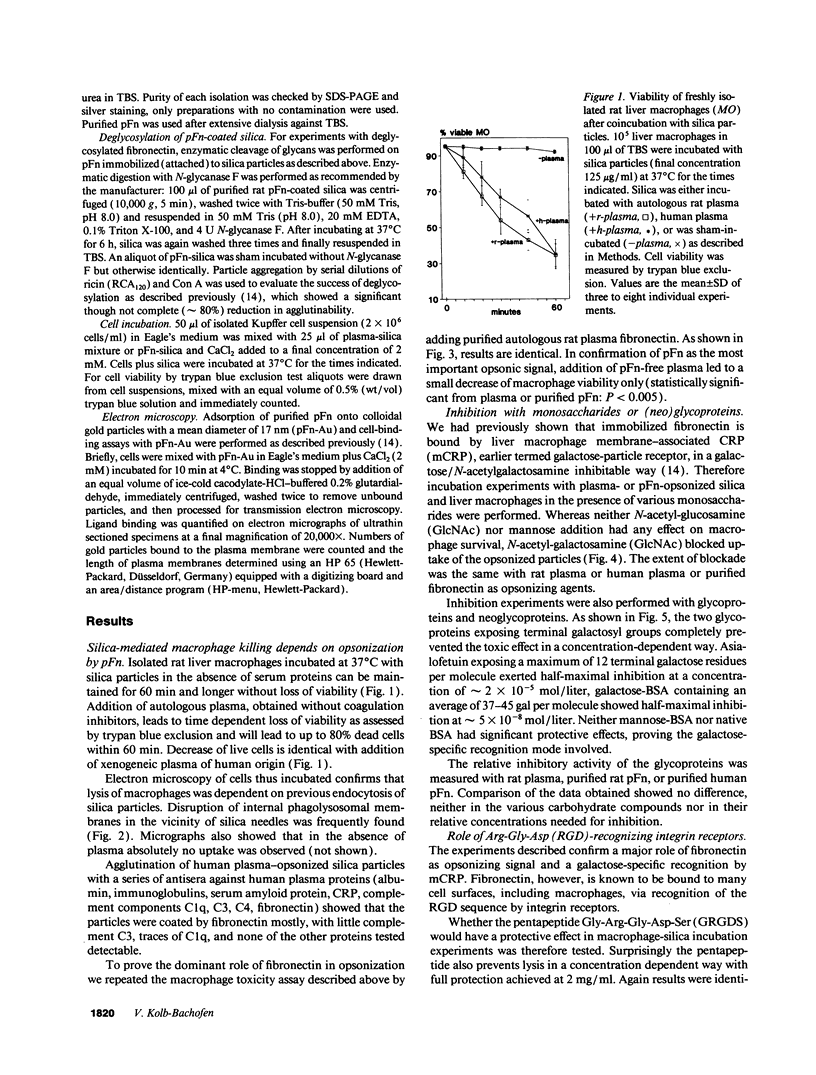
Silica particles (quartz dust) are toxic to macrophages after their uptake into these cells. These experiments describe the opsonization mechanism(s) and macrophage receptor(s) involved in silica uptake. Freshly isolated rat liver macrophages (Kupffer cells) were incubated at 37 degrees C with silica particles in the presence or absence of autologous or heterologous plasma or purified plasma fibronectin and cell viability was assessed at various times. Within 60 min of coincubation, > 80% of macrophages were lysed in the presence of plasma or purified fibronectin but not in their absence (viability > 90%). Lysis was slower with defibronectinized plasma (28% in 60 min). Macrophages could be protected from lysis by addition of the monosaccharide N-acetyl-D-galactosamine but not by N-acetyl-D-glucosamine. Galactosylated serum albumin but not mannosylated albumin or native albumin exerted full protection from lysis. The pentapeptide GRGDS also prevented macrophage lysis in synergy with N-acetyl-galactosamine. Enzymatic deglycosylation of fibronectin reduced lysis significantly. These findings indicate an important opsonizing activity for fibronectin and dual recognition via the lectin-like galactose-specific binding activity of membrane-associated C-reactive protein and by integrin receptor(s). Binding experiments (at 4 degrees C) revealed initial binding as primarily galactose-inhibitable, suggesting integrin-mediated binding as a later event necessary for effective uptake.
Full text
PDF
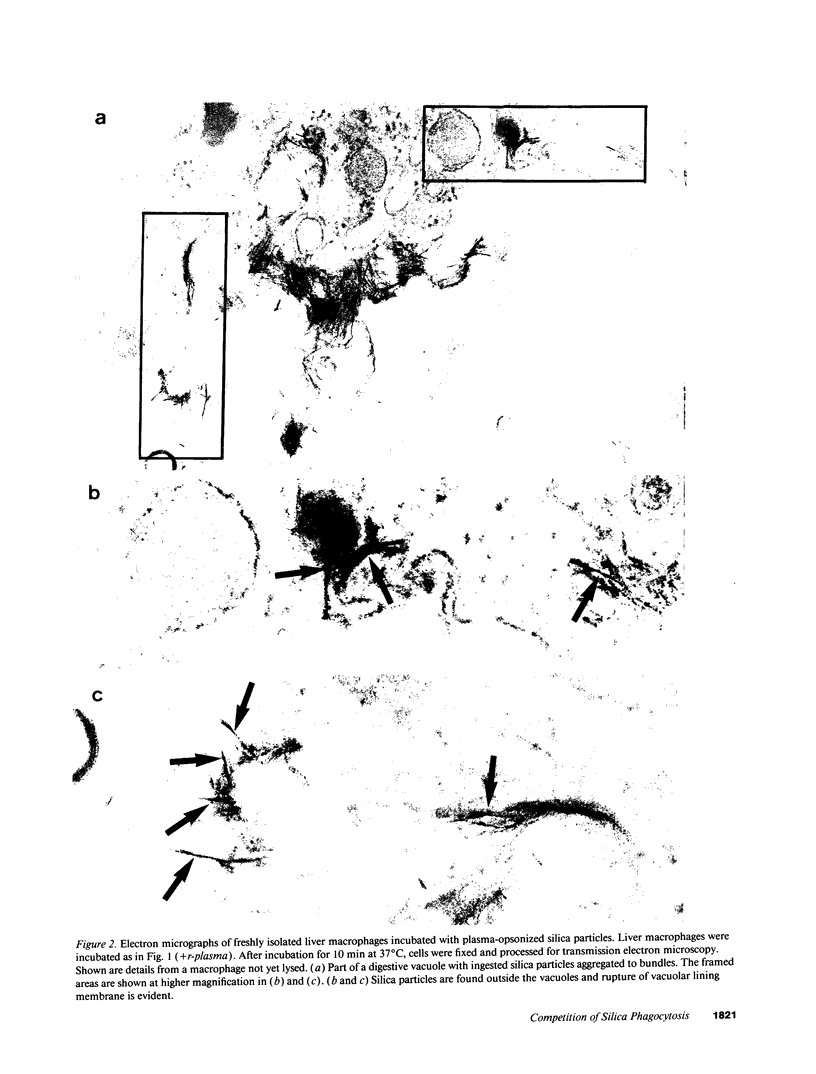
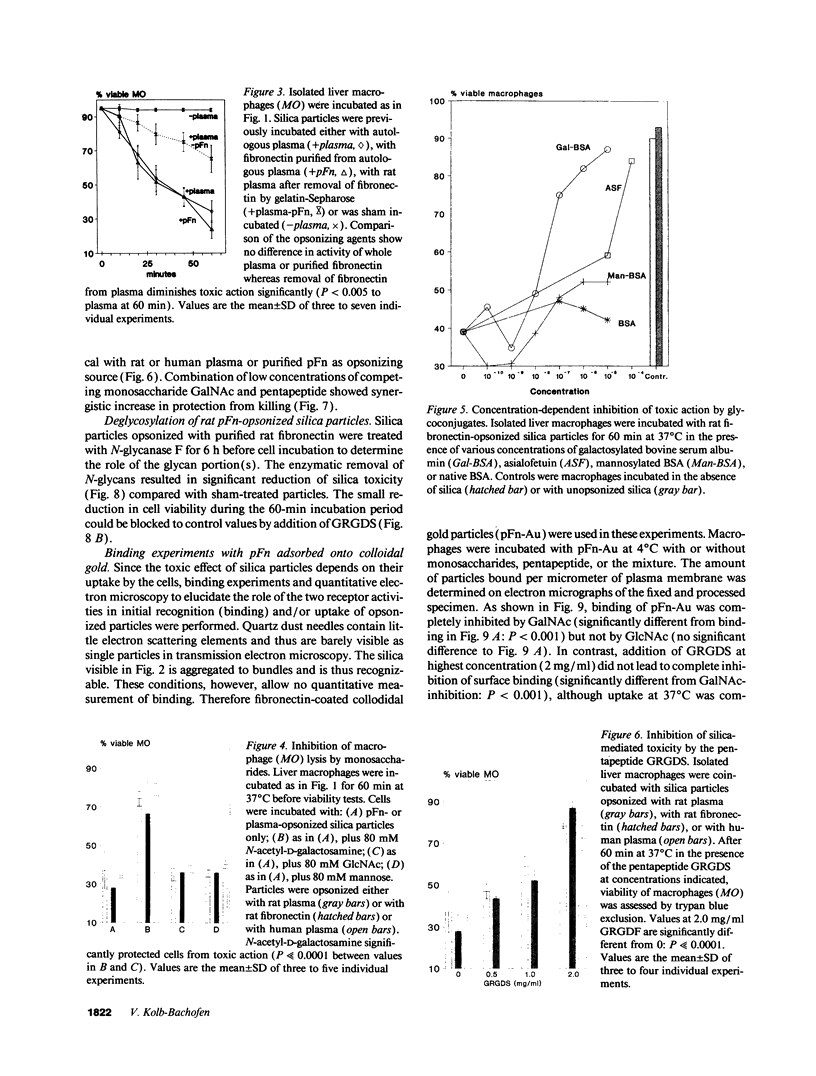

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Harington J. S., Birbeck M. An examination of the cytotoxic effects of silica on macrophages. J Exp Med. 1966 Aug 1;124(2):141–154. doi: 10.1084/jem.124.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosnan C. F., Bornstein M. B., Bloom B. R. The effects of macrophage depletion on the clinical and pathologic expression of experimental allergic encephalomyelitis. J Immunol. 1981 Feb;126(2):614–620. [PubMed] [Google Scholar]
- Cardarelli P. M., Blumenstock F. A., McKeown-Longo P. J., Saba T. M., Mazurkiewicz J. E., Dias J. A. High-affinity binding of fibronectin to cultured Kupffer cells. J Leukoc Biol. 1990 Nov;48(5):426–437. doi: 10.1002/jlb.48.5.426. [DOI] [PubMed] [Google Scholar]
- Cardarelli P. M., Yamagata S., Scholz W., Moscinski M. A., Morgan E. L. Fibronectin augments anti-CD3-mediated IL-2 receptor (CD25) expression on human peripheral blood lymphocytes. Cell Immunol. 1991 Jun;135(1):105–117. doi: 10.1016/0008-8749(91)90258-d. [DOI] [PubMed] [Google Scholar]
- Chaouat G., Howard J. G. Influence of reticuloendothelial blockade on the induction of tolerance and immunity by polysaccharides. Immunology. 1976 Feb;30(2):221–227. [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. F., Clyman R. I., Enenstein J., Waleh N., Pytela R., Kramer R. H. The integrin complex alpha v beta 3 participates in the adhesion of microvascular endothelial cells to fibronectin. Exp Cell Res. 1991 May;194(1):69–77. doi: 10.1016/0014-4827(91)90131-d. [DOI] [PubMed] [Google Scholar]
- D'Souza S. E., Ginsberg M. H., Plow E. F. Arginyl-glycyl-aspartic acid (RGD): a cell adhesion motif. Trends Biochem Sci. 1991 Jul;16(7):246–250. doi: 10.1016/0968-0004(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Davis G. S. Pathogenesis of silicosis: current concepts and hypotheses. Lung. 1986;164(3):139–154. doi: 10.1007/BF02713638. [DOI] [PubMed] [Google Scholar]
- Du Clos T. W. C-reactive protein reacts with the U1 small nuclear ribonucleoprotein. J Immunol. 1989 Oct 15;143(8):2553–2559. [PubMed] [Google Scholar]
- Engvall E., Ruoslahti E. Binding of soluble form of fibroblast surface protein, fibronectin, to collagen. Int J Cancer. 1977 Jul 15;20(1):1–5. doi: 10.1002/ijc.2910200102. [DOI] [PubMed] [Google Scholar]
- Heppleston A. G., Styles J. A. Activity of a macrophage factor in collagen formation by silica. Nature. 1967 Apr 29;214(5087):521–522. doi: 10.1038/214521a0. [DOI] [PubMed] [Google Scholar]
- Kempka G., Roos P. H., Kolb-Bachofen V. A membrane-associated form of C-reactive protein is the galactose-specific particle receptor on rat liver macrophages. J Immunol. 1990 Feb 1;144(3):1004–1009. [PubMed] [Google Scholar]
- Kolb-Bachofen V., Abel F. Participation of D-galactose-specific receptors of liver macrophages in recognition of fibronectin-opsonized particles. Carbohydr Res. 1991 Jun 25;213:201–213. doi: 10.1016/s0008-6215(00)90609-8. [DOI] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Levy M. H., Wheelock E. F. Effects of intravenous silica on immune and non-immune functions of the murine host. J Immunol. 1975 Jul;115(1):41–48. [PubMed] [Google Scholar]
- Li J. J., Sanders R. L., McAdam K. P., Hales C. A., Thompson B. T., Gelfand J. A., Burke J. F. Impact of C-reactive protein (CRP) on surfactant function. J Trauma. 1989 Dec;29(12):1690–1697. doi: 10.1097/00005373-198912000-00019. [DOI] [PubMed] [Google Scholar]
- McKeown M., Knowles G., McCulloch C. A. Role of the cellular attachment domain of fibronectin in the phagocytosis of beads by human gingival fibroblasts in vitro. Cell Tissue Res. 1990 Dec;262(3):523–530. doi: 10.1007/BF00305249. [DOI] [PubMed] [Google Scholar]
- Mould A. P., Komoriya A., Yamada K. M., Humphries M. J. The CS5 peptide is a second site in the IIICS region of fibronectin recognized by the integrin alpha 4 beta 1. Inhibition of alpha 4 beta 1 function by RGD peptide homologues. J Biol Chem. 1991 Feb 25;266(6):3579–3585. [PubMed] [Google Scholar]
- Nagy B., Katona E., Erdei J., Karmazsin L., Fachet J. Fibronectin in bronchoalveolar lavage fluid and plasma of dogs with acute inflammation of the lungs. APMIS. 1991 Apr;99(4):387–390. doi: 10.1111/j.1699-0463.1991.tb05166.x. [DOI] [PubMed] [Google Scholar]
- O'Rourke E. J., Halstead S. B., Allison A. C., Platts-Mills T. A. Specific lethality of silica for human peripheral blood mononuclear phagocytes, in vitro. J Immunol Methods. 1978;19(2-3):137–151. doi: 10.1016/0022-1759(78)90174-6. [DOI] [PubMed] [Google Scholar]
- Olden K., Pratt R. M., Yamada K. M. Role of carbohydrates in protein secretion and turnover: effects of tunicamycin on the major cell surface glycoprotein of chick embryo fibroblasts. Cell. 1978 Mar;13(3):461–473. doi: 10.1016/0092-8674(78)90320-3. [DOI] [PubMed] [Google Scholar]
- Oschilewski M., Schwab E., Kiesel U., Opitz U., Stünkel K., Kolb-Bachofen V., Kolb H. Administration of silica or monoclonal antibody to Thy-1 prevents low-dose streptozotocin-induced diabetes in mice. Immunol Lett. 1986 Jun;12(5-6):289–294. doi: 10.1016/0165-2478(86)90032-5. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Saba T. M. Fibronectin: relevance to phagocytic host response to injury. Circ Shock. 1989 Dec;29(4):257–278. [PubMed] [Google Scholar]
- Saba T. M. Physiology and physiopathology of the reticuloendothelial system. Arch Intern Med. 1970 Dec;126(6):1031–1052. [PubMed] [Google Scholar]
- Salonen E. M., Vartio T., Hedman K., Vaheri A. Binding of fibronectin by the acute phase reactant C-reactive protein. J Biol Chem. 1984 Feb 10;259(3):1496–1501. [PubMed] [Google Scholar]
- Schlepper-Schäfer J., Kolb-Bachofen V. Red cell aging results in a change of cell surface carbohydrate epitopes allowing for recognition by galactose-specific receptors of rat liver macrophages. Blood Cells. 1988;14(1):259–274. [PubMed] [Google Scholar]
- Swanson S. J., McPeek M. M., Mortensen R. F. Characteristics of the binding of human C-reactive protein (CRP) to laminin. J Cell Biochem. 1989 May;40(1):121–132. doi: 10.1002/jcb.240400112. [DOI] [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylänne J. RGD peptides may only temporarily inhibit cell adhesion to fibronectin. FEBS Lett. 1990 Jul 2;267(1):43–45. doi: 10.1016/0014-5793(90)80283-o. [DOI] [PubMed] [Google Scholar]
- Ziskind M., Jones R. N., Weill H. Silicosis. Am Rev Respir Dis. 1976 May;113(5):643–665. doi: 10.1164/arrd.1976.113.5.643. [DOI] [PubMed] [Google Scholar]
- Zisman B., Wheelock E. F., Allison A. C. Role of macrophages and antibody in resistance of mice against yellow fever virus. J Immunol. 1971 Jul;107(1):236–243. [PubMed] [Google Scholar]