Abstract
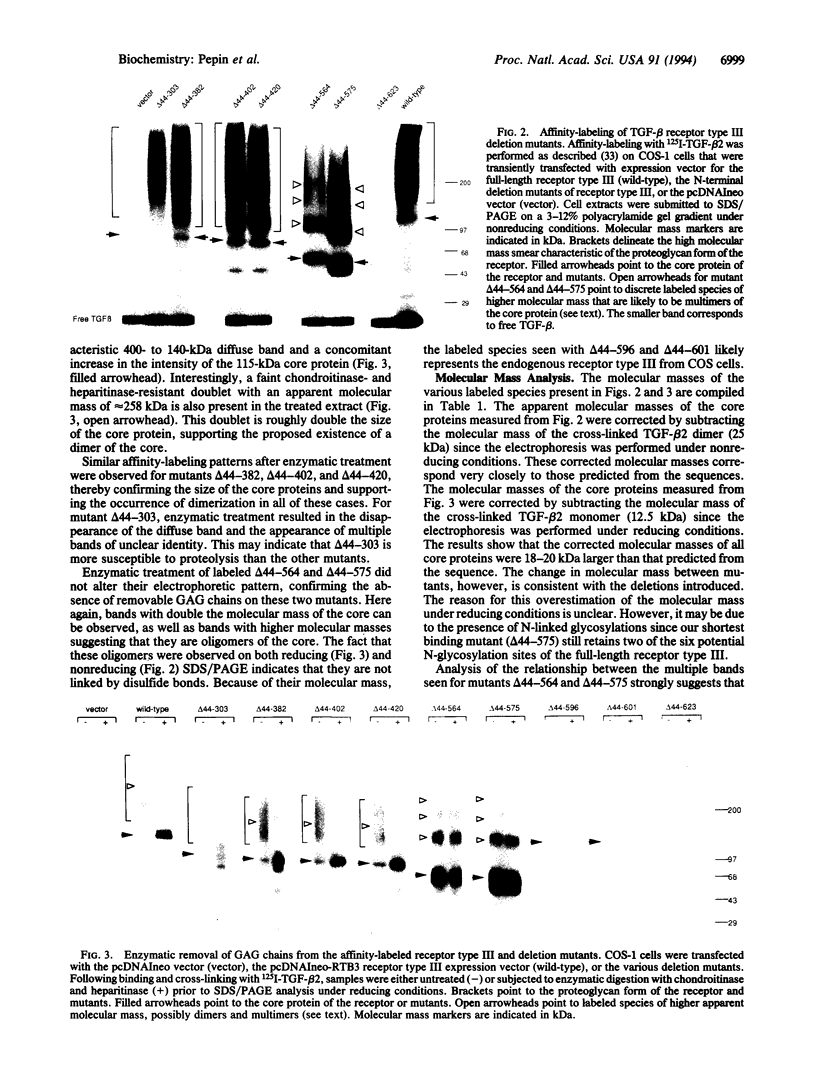
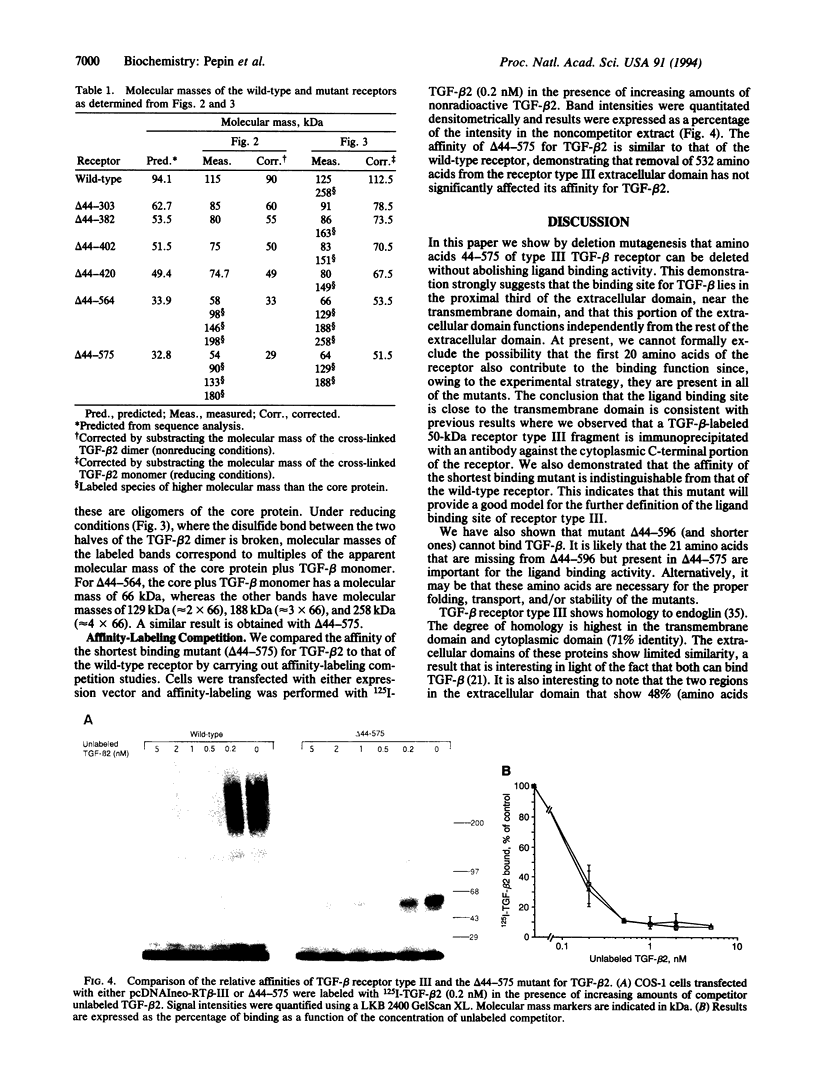
Transforming growth factor beta (TGF-beta) receptor type III is a membrane-anchored proteoglycan that binds TGF-beta via the core protein. We have determined, by deletion mutagenesis of the receptor type III, the minimal essential region of the extracellular domain that is capable of binding TGF-beta. Nine deletion mutants were produced, six of which are expressed on the cell surface and bind TGF-beta. We find that the shortest of these active mutants, which retains only 253 of the 785 amino acids of the extracellular domain, binds TGF-beta with the same affinity as the full-length receptor. These results indicate that the ligand binding domain lies proximal to the transmembrane domain and is functionally independent from the rest of the extracellular domain. We have determined from the mutants that one of the potential glycosaminoglycan attachment sites in the receptor type III is not utilized. Results from the nonglycosylated mutants confirm that the glycosaminoglycan chains are not required for the folding, targeting, and TGF-beta binding activity of the receptor. Moreover, we present evidence for dimerization and multimerization of the receptor.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bork P., Sander C. A large domain common to sperm receptors (Zp2 and Zp3) and TGF-beta type III receptor. FEBS Lett. 1992 Apr 6;300(3):237–240. doi: 10.1016/0014-5793(92)80853-9. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Massagué J. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989 Feb 5;264(4):2272–2278. [PubMed] [Google Scholar]
- Cheifetz S., Andres J. L., Massagué J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J Biol Chem. 1988 Nov 15;263(32):16984–16991. [PubMed] [Google Scholar]
- Cheifetz S., Bellón T., Calés C., Vera S., Bernabeu C., Massagué J., Letarte M. Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J Biol Chem. 1992 Sep 25;267(27):19027–19030. [PubMed] [Google Scholar]
- Cheifetz S., Hernandez H., Laiho M., ten Dijke P., Iwata K. K., Massagué J. Distinct transforming growth factor-beta (TGF-beta) receptor subsets as determinants of cellular responsiveness to three TGF-beta isoforms. J Biol Chem. 1990 Nov 25;265(33):20533–20538. [PubMed] [Google Scholar]
- Cheifetz S., Like B., Massagué J. Cellular distribution of type I and type II receptors for transforming growth factor-beta. J Biol Chem. 1986 Jul 25;261(21):9972–9978. [PubMed] [Google Scholar]
- Cheifetz S., Weatherbee J. A., Tsang M. L., Anderson J. K., Mole J. E., Lucas R., Massagué J. The transforming growth factor-beta system, a complex pattern of cross-reactive ligands and receptors. Cell. 1987 Feb 13;48(3):409–415. doi: 10.1016/0092-8674(87)90192-9. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinkers M. Isolation and characterization of mink lung epithelial cell mutants resistant to transforming growth factor beta. J Cell Physiol. 1987 Jan;130(1):1–5. doi: 10.1002/jcp.1041300102. [DOI] [PubMed] [Google Scholar]
- Ebner R., Chen R. H., Shum L., Lawler S., Zioncheck T. F., Lee A., Lopez A. R., Derynck R. Cloning of a type I TGF-beta receptor and its effect on TGF-beta binding to the type II receptor. Science. 1993 May 28;260(5112):1344–1348. doi: 10.1126/science.8388127. [DOI] [PubMed] [Google Scholar]
- Fukushima D., Bützow R., Hildebrand A., Ruoslahti E. Localization of transforming growth factor beta binding site in betaglycan. Comparison with small extracellular matrix proteoglycans. J Biol Chem. 1993 Oct 25;268(30):22710–22715. [PubMed] [Google Scholar]
- Geiser A. G., Burmester J. K., Webbink R., Roberts A. B., Sporn M. B. Inhibition of growth by transforming growth factor-beta following fusion of two nonresponsive human carcinoma cell lines. Implication of the type II receptor in growth inhibitory responses. J Biol Chem. 1992 Feb 5;267(4):2588–2593. [PubMed] [Google Scholar]
- Gougos A., Letarte M. Primary structure of endoglin, an RGD-containing glycoprotein of human endothelial cells. J Biol Chem. 1990 May 25;265(15):8361–8364. [PubMed] [Google Scholar]
- Kimchi A., Wang X. F., Weinberg R. A., Cheifetz S., Massagué J. Absence of TGF-beta receptors and growth inhibitory responses in retinoblastoma cells. Science. 1988 Apr 8;240(4849):196–199. doi: 10.1126/science.2895499. [DOI] [PubMed] [Google Scholar]
- Laiho M., Weis F. M., Boyd F. T., Ignotz R. A., Massagué J. Responsiveness to transforming growth factor-beta (TGF-beta) restored by genetic complementation between cells defective in TGF-beta receptors I and II. J Biol Chem. 1991 May 15;266(14):9108–9112. [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990 Oct 25;265(30):18518–18524. [PubMed] [Google Scholar]
- Lin H. Y., Lodish H. F. Receptors for the TGF-beta superfamily: multiple polypeptides and serine/threonine kinases. Trends Cell Biol. 1993 Jan;3(1):14–19. doi: 10.1016/0962-8924(93)90195-7. [DOI] [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991 Nov 15;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Wrana J. L., Massagué J. Betaglycan presents ligand to the TGF beta signaling receptor. Cell. 1993 Jul 2;73(7):1435–1444. doi: 10.1016/0092-8674(93)90368-z. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Boyd F. T., Andres J. L. TGF-beta receptors and TGF-beta binding proteoglycans: recent progress in identifying their functional properties. Ann N Y Acad Sci. 1990;593:59–72. doi: 10.1111/j.1749-6632.1990.tb16100.x. [DOI] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E. J., Lee K., O'Connor-McCourt M. D. Characterization of transforming growth factor-beta (TGF-beta) receptors on BeWo choriocarcinoma cells including the identification of a novel 38-kDa TGF-beta binding glycoprotein. Mol Biol Cell. 1992 Nov;3(11):1295–1307. doi: 10.1091/mbc.3.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell E. J., O'Connor-McCourt M. D. A transforming growth factor beta (TGF-beta) receptor from human placenta exhibits a greater affinity for TGF-beta 2 than for TGF-beta 1. Biochemistry. 1991 Apr 30;30(17):4350–4356. doi: 10.1021/bi00231a034. [DOI] [PubMed] [Google Scholar]
- Morén A., Ichijo H., Miyazono K. Molecular cloning and characterization of the human and porcine transforming growth factor-beta type III receptors. Biochem Biophys Res Commun. 1992 Nov 30;189(1):356–362. doi: 10.1016/0006-291x(92)91566-9. [DOI] [PubMed] [Google Scholar]
- Moustakas A., Lin H. Y., Henis Y. I., Plamondon J., O'Connor-McCourt M. D., Lodish H. F. The transforming growth factor beta receptors types I, II, and III form hetero-oligomeric complexes in the presence of ligand. J Biol Chem. 1993 Oct 25;268(30):22215–22218. [PubMed] [Google Scholar]
- Ohta M., Greenberger J. S., Anklesaria P., Bassols A., Massagué J. Two forms of transforming growth factor-beta distinguished by multipotential haematopoietic progenitor cells. Nature. 1987 Oct 8;329(6139):539–541. doi: 10.1038/329539a0. [DOI] [PubMed] [Google Scholar]
- Philip A., O'Connor-McCourt M. D. Interaction of transforming growth factor-beta 1 with alpha 2-macroglobulin. Role in transforming growth factor-beta 1 clearance. J Biol Chem. 1991 Nov 25;266(33):22290–22296. [PubMed] [Google Scholar]
- Segarini P. R., Rosen D. M., Seyedin S. M. Binding of transforming growth factor-beta to cell surface proteins varies with cell type. Mol Endocrinol. 1989 Feb;3(2):261–272. doi: 10.1210/mend-3-2-261. [DOI] [PubMed] [Google Scholar]
- Segarini P. R., Seyedin S. M. The high molecular weight receptor to transforming growth factor-beta contains glycosaminoglycan chains. J Biol Chem. 1988 Jun 15;263(17):8366–8370. [PubMed] [Google Scholar]
- Siepl C., Malipiero U. V., Fontana A. Transforming growth factor-beta (TGF-beta)-resistant helper T lymphocyte clones show a concomitant loss of all three types of TGF-beta receptors. J Immunol. 1991 May 1;146(9):3063–3067. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchida K., Lewis K. A., Mathews L. S., Vale W. W. Molecular characterization of rat transforming growth factor-beta type II receptor. Biochem Biophys Res Commun. 1993 Mar 31;191(3):790–795. doi: 10.1006/bbrc.1993.1286. [DOI] [PubMed] [Google Scholar]
- Wang X. F., Lin H. Y., Ng-Eaton E., Downward J., Lodish H. F., Weinberg R. A. Expression cloning and characterization of the TGF-beta type III receptor. Cell. 1991 Nov 15;67(4):797–805. doi: 10.1016/0092-8674(91)90074-9. [DOI] [PubMed] [Google Scholar]
- Wrana J. L., Attisano L., Cárcamo J., Zentella A., Doody J., Laiho M., Wang X. F., Massagué J. TGF beta signals through a heteromeric protein kinase receptor complex. Cell. 1992 Dec 11;71(6):1003–1014. doi: 10.1016/0092-8674(92)90395-s. [DOI] [PubMed] [Google Scholar]