Abstract
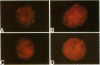
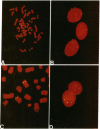
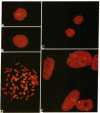
Among the small round cell tumors differential diagnosis is particularly difficult for their undifferentiated or primitive character. In this mixed group of tumors, only the primitive neuroectodermal tumors, which include Ewing's sarcoma (ES), show the unique and consistent feature of the (11;22)(q24;q12) translocation, which can therefore be considered a hallmark of these neoplasias. We analyzed four primitive neuroectodermal tumor cell lines, one osteosarcoma cell line, and 11 patients by fluorescent in situ hybridization with cosmid clones 23.2 and 5.8, bracketing the t(11;22) at 11q24. Metaphase spreads from tumor cell lines, and from biopsy specimens of three patients with ES were analyzed. In the remaining eight patients comprising five ES, two small cell osteosarcomas and one chronic osteomyelitis, only nuclei preparations were available for analysis. We detected the t(11;22) in interphase nuclei of the four primitive neuroectodermal tumor cell lines, of three patients in which the karyotype demonstrated the translocation and in five cases of ES in which cytogenetic analysis had not been possible. Two cases of small cell osteosarcoma and one chronic osteomyelitis were also analyzed and were both normal with respect to the t(11;22). By analyzing cell lines and small round cell tumor samples by fluorescent in situ hybridization, we established that interphase cytogenetics is a rapid alternative to chromosomal analysis for the detection of the t(11;22) and represents an invaluable tool for the differential diagnosis of small round cell tumors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anastasi J., Thangavelu M., Vardiman J. W., Hooberman A. L., Bian M. L., Larson R. A., Le Beau M. M. Interphase cytogenetic analysis detects minimal residual disease in a case of acute lymphoblastic leukemia and resolves the question of origin of relapse after allogeneic bone marrow transplantation. Blood. 1991 Mar 1;77(5):1087–1091. [PubMed] [Google Scholar]
- Bagnara G. P., Serra M., Giovannini M., Badiali M., Stella M., Montaldi A., Granchi D., Paolucci P., Rocchi P., Pession A. Establishment and characterization of a primitive neuroectodermal tumor of bone continuous cell line (LAP-35). Int J Cell Cloning. 1990 Nov;8(6):409–424. doi: 10.1002/stem.5530080644. [DOI] [PubMed] [Google Scholar]
- Biegel J. A., Womer R. B., Emanuel B. S. Complex karyotypes in a series of pediatric osteosarcomas. Cancer Genet Cytogenet. 1989 Mar;38(1):89–100. doi: 10.1016/0165-4608(89)90169-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fletcher J. A., Kozakewich H. P., Hoffer F. A., Lage J. M., Weidner N., Tepper R., Pinkus G. S., Morton C. C., Corson J. M. Diagnostic relevance of clonal cytogenetic aberrations in malignant soft-tissue tumors. N Engl J Med. 1991 Feb 14;324(7):436–442. doi: 10.1056/NEJM199102143240702. [DOI] [PubMed] [Google Scholar]
- Henderson D. W., Leppard P. J., Brennan J. S., Mukherjee T. M., Swift J. G. Primitive neuroepithelial tumours of soft tissues and of bone: further ultrastructural and immunocytochemical clarification of 'Ewing's sarcoma', including freeze-fracture analysis. J Submicrosc Cytol Pathol. 1989 Jan;21(1):35–57. [PubMed] [Google Scholar]
- Johnson G. D., Nogueira Araujo G. M. A simple method of reducing the fading of immunofluorescence during microscopy. J Immunol Methods. 1981;43(3):349–350. doi: 10.1016/0022-1759(81)90183-6. [DOI] [PubMed] [Google Scholar]
- Kolluri R. V., Manuelidis L., Cremer T., Sait S., Gezer S., Raza A. Detection of monosomy 7 in interphase cells of patients with myeloid disorders. Am J Hematol. 1990 Feb;33(2):117–122. doi: 10.1002/ajh.2830330208. [DOI] [PubMed] [Google Scholar]
- Lichter P., Tang C. J., Call K., Hermanson G., Evans G. A., Housman D., Ward D. C. High-resolution mapping of human chromosome 11 by in situ hybridization with cosmid clones. Science. 1990 Jan 5;247(4938):64–69. doi: 10.1126/science.2294592. [DOI] [PubMed] [Google Scholar]
- Limon J., Dal Cin P., Sandberg A. A. Application of long-term collagenase disaggregation for the cytogenetic analysis of human solid tumors. Cancer Genet Cytogenet. 1986 Dec;23(4):305–313. doi: 10.1016/0165-4608(86)90013-0. [DOI] [PubMed] [Google Scholar]
- Nederlof P. M., van der Flier S., Raap A. K., Tanke H. J., van der Ploeg M., Kornips F., Geraedts J. P. Detection of chromosome aberrations in interphase tumor nuclei by nonradioactive in situ hybridization. Cancer Genet Cytogenet. 1989 Oct 1;42(1):87–98. doi: 10.1016/0165-4608(89)90011-3. [DOI] [PubMed] [Google Scholar]
- Nesbit M. E., Jr Advances and management of solid tumors in children. Cancer. 1990 Feb 1;65(3 Suppl):696–702. doi: 10.1002/1097-0142(19900201)65:3+<696::aid-cncr2820651313>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- Noguera R., Navarro S., Triche T. J. Translocation (11;22) in small cell osteosarcoma. Cancer Genet Cytogenet. 1990 Mar;45(1):121–124. doi: 10.1016/0165-4608(90)90074-k. [DOI] [PubMed] [Google Scholar]
- Reynolds C. P., Smith R. G., Frenkel E. P. The diagnostic dilemma of the "small round cell neoplasm": catecholamine fluorescence and tissue culture morphology as markers for neuroblastoma. Cancer. 1981 Nov 1;48(9):2088–2094. doi: 10.1002/1097-0142(19811101)48:9<2088::aid-cncr2820480929>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A. Chromosome abnormalities in human cancer and leukemia. Mutat Res. 1991 Apr;247(2):231–240. doi: 10.1016/0027-5107(91)90019-k. [DOI] [PubMed] [Google Scholar]
- Sandberg A. A., Turc-Carel C., Gemmill R. M. Chromosomes in solid tumors and beyond. Cancer Res. 1988 Mar 1;48(5):1049–1059. [PubMed] [Google Scholar]
- Seeger R. C., Reynolds C. P. Treatment of high-risk solid tumors of childhood with intensive therapy and autologous bone marrow transplantation. Pediatr Clin North Am. 1991 Apr;38(2):393–424. doi: 10.1016/s0031-3955(16)38084-1. [DOI] [PubMed] [Google Scholar]
- Selleri L., Hermanson G. G., Eubanks J. H., Lewis K. A., Evans G. A. Molecular localization of the t(11;22)(q24;q12) translocation of Ewing sarcoma by chromosomal in situ suppression hybridization. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):887–891. doi: 10.1073/pnas.88.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teyssier J. R. The chromosomal analysis of human solid tumors. A triple challenge. Cancer Genet Cytogenet. 1989 Jan;37(1):103–125. doi: 10.1016/0165-4608(89)90080-0. [DOI] [PubMed] [Google Scholar]
- Thiele C. J., McKeon C., Triche T. J., Ross R. A., Reynolds C. P., Israel M. A. Differential protooncogene expression characterizes histopathologically indistinguishable tumors of the peripheral nervous system. J Clin Invest. 1987 Sep;80(3):804–811. doi: 10.1172/JCI113137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkachuk D. C., Westbrook C. A., Andreeff M., Donlon T. A., Cleary M. L., Suryanarayan K., Homge M., Redner A., Gray J., Pinkel D. Detection of bcr-abl fusion in chronic myelogeneous leukemia by in situ hybridization. Science. 1990 Oct 26;250(4980):559–562. doi: 10.1126/science.2237408. [DOI] [PubMed] [Google Scholar]
- Trask B. J. Fluorescence in situ hybridization: applications in cytogenetics and gene mapping. Trends Genet. 1991 May;7(5):149–154. doi: 10.1016/0168-9525(91)90378-4. [DOI] [PubMed] [Google Scholar]
- Triche T. J., Askin F. B. Neuroblastoma and the differential diagnosis of small-, round-, blue-cell tumors. Hum Pathol. 1983 Jul;14(7):569–595. doi: 10.1016/s0046-8177(83)80202-0. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C., Aurias A., Mugneret F., Lizard S., Sidaner I., Volk C., Thiery J. P., Olschwang S., Philip I., Berger M. P. Chromosomes in Ewing's sarcoma. I. An evaluation of 85 cases of remarkable consistency of t(11;22)(q24;q12). Cancer Genet Cytogenet. 1988 Jun;32(2):229–238. doi: 10.1016/0165-4608(88)90285-3. [DOI] [PubMed] [Google Scholar]
- Turc-Carel C., Philip I., Berger M. P., Philip T., Lenoir G. M. Chromosome study of Ewing's sarcoma (ES) cell lines. Consistency of a reciprocal translocation t(11;22)(q24;q12). Cancer Genet Cytogenet. 1984 May;12(1):1–19. doi: 10.1016/0165-4608(84)90002-5. [DOI] [PubMed] [Google Scholar]
- Whang-Peng J., Triche T. J., Knutsen T., Miser J., Kao-Shan S., Tsai S., Israel M. A. Cytogenetic characterization of selected small round cell tumors of childhood. Cancer Genet Cytogenet. 1986 Apr 1;21(3):185–208. doi: 10.1016/0165-4608(86)90001-4. [DOI] [PubMed] [Google Scholar]
- Wilson T. Trends in confocal microscopy. Trends Neurosci. 1989 Dec;12(12):486–493. doi: 10.1016/0166-2236(89)90104-5. [DOI] [PubMed] [Google Scholar]