Abstract
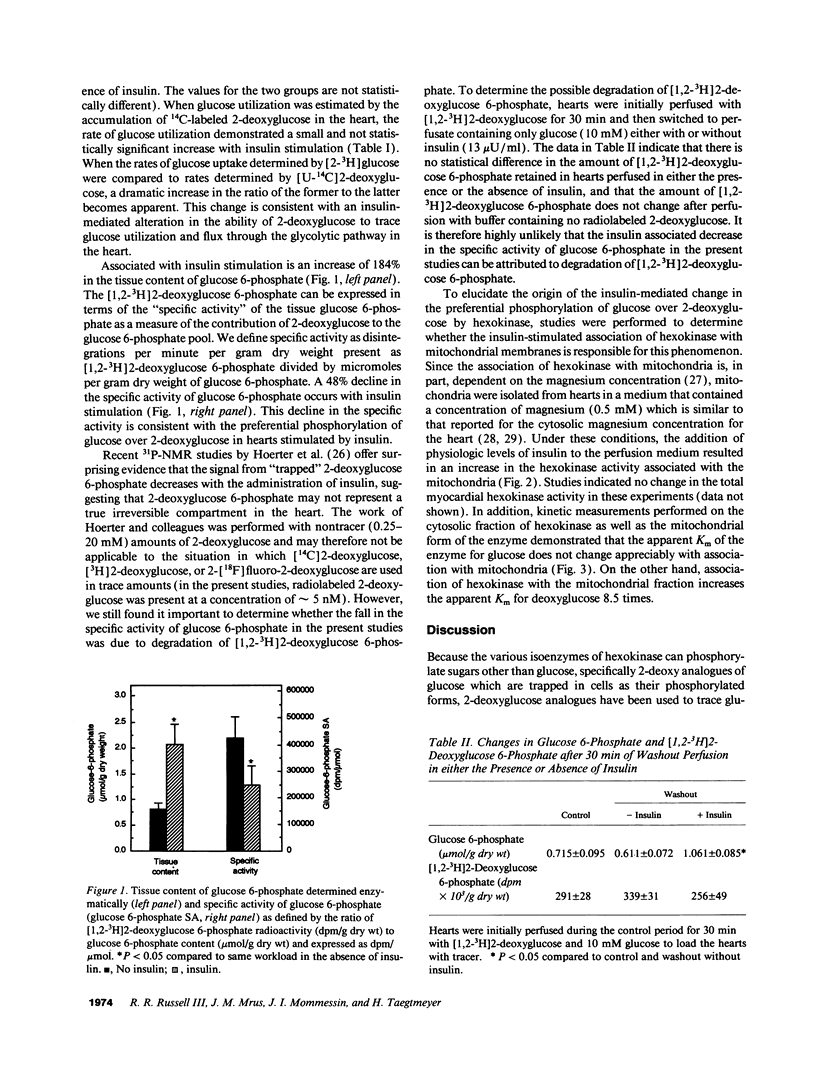
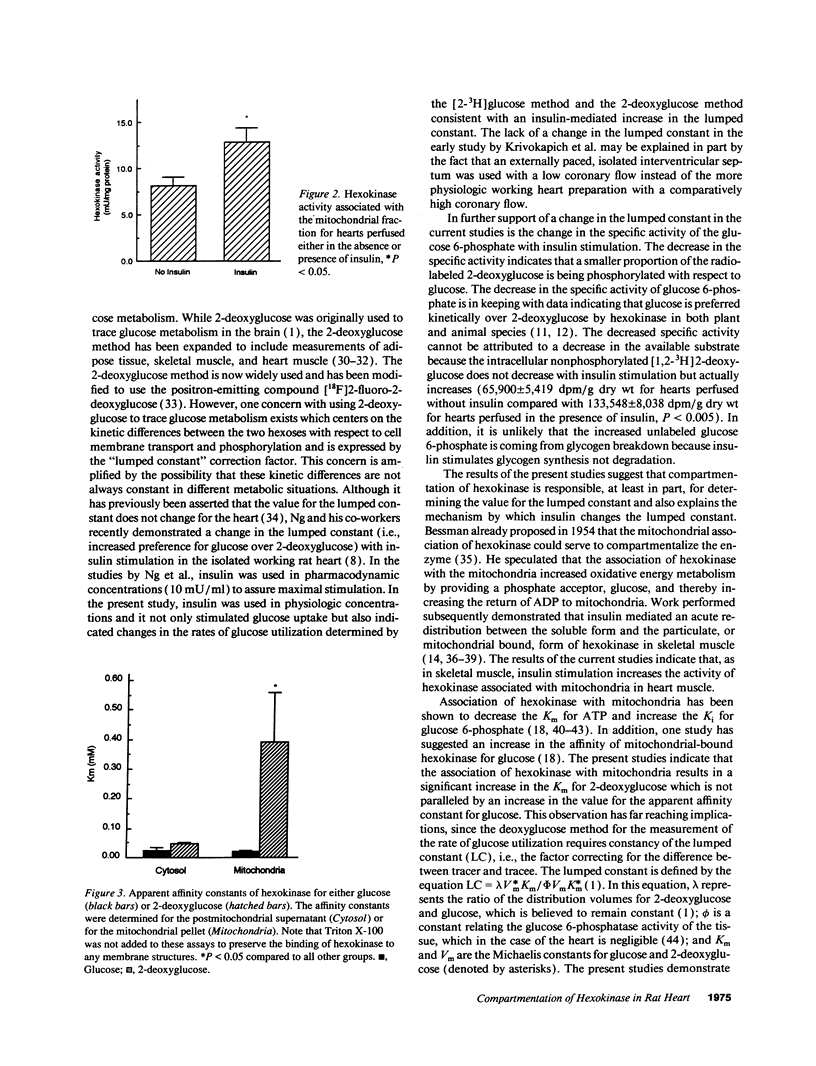
Radiolabeled analogues of 2-deoxyglucose are widely used to trace glucose metabolism in cell cultures, whole organs, and intact animals, although kinetic differences in transport and phosphorylation between these compounds and glucose exist. The present studies were undertaken to determine the effects of insulin stimulation on the phosphorylation of 2-deoxyglucose compared to glucose in the intact, saline-perfused working rat heart. Rates of glucose utilization determined from tritiated glucose differed from rates estimated from the accumulation of [14C]2-deoxyglucose in a nonconstant manner when comparing rates in the absence or presence of physiologic levels of insulin (13 microU/ml). The fraction of monophosphorylated hexoses that was accounted for by [14C]2-deoxyglucose 6-phosphate was dramatically decreased in hearts perfused in the presence of insulin. Additionally, hexokinase activity associated with the mitochondrial fraction of tissue extracts was increased in hearts stimulated by insulin. While this redistribution of hexokinase to the mitochondria did not affect the apparent affinity constant for glucose, hexokinase bound to mitochondria exhibited an 8.5-fold decrease in the affinity for 2-deoxyglucose when compared with hexokinase present in the cytosolic fraction. The findings are consistent with an insulin-mediated preferential uptake and phosphorylation of glucose compared to deoxyglucose. The results also imply that the redistribution of hexokinase and the differential effect of insulin on its affinity for tracer and tracee are responsible for changes in the "lumped constant" (i.e., the correction factor used to equate 2-deoxyglucose to glucose uptake). These changes must be taken into account when regional myocardial glucose metabolism is assessed by the 2-deoxyglucose method.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett J. E., Holman G. D., Munday K. A. Structural requirements for binding to the sugar-transport system of the human erythrocyte. Biochem J. 1973 Feb;131(2):211–221. doi: 10.1042/bj1310211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessell E. M., Foster A. B., Westwood J. H. The use of deoxyfluoro-D-glucopyranoses and related compounds in a study of yeast hexokinase specificity. Biochem J. 1972 Jun;128(2):199–204. doi: 10.1042/bj1280199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessman S. P., Geiger P. J. Compartmentation of hexokinase and creatine phosphokinase, cellular regulation, and insulin action. Curr Top Cell Regul. 1980;16:55–86. doi: 10.1016/b978-0-12-152816-4.50007-8. [DOI] [PubMed] [Google Scholar]
- Bessman S. P. Hexokinase acceptor theory of insulin action. New evidence. Isr J Med Sci. 1972 Mar;8(3):344–352. [PubMed] [Google Scholar]
- Borrebaek B. Mitochondrial-bound hexokinase of the rat epididymal adipose tissue and its possible relation to the action of insulin. Biochem Med. 1970 Jun;3(6):485–497. doi: 10.1016/0006-2944(70)90041-4. [DOI] [PubMed] [Google Scholar]
- Borrebaek B., Spydevold O. The effects of insulin and glucose on mitochondrial-bound hexokinase activity of rat epididymal adipose tissue. Diabetologia. 1969 Feb;5(1):42–43. doi: 10.1007/BF01212218. [DOI] [PubMed] [Google Scholar]
- Crane P. D., Pardridge W. M., Braun L. D., Oldendorf W. H. Kinetics of transport and phosphorylation of 2-fluoro-2-deoxy-D-glucose in rat brain. J Neurochem. 1983 Jan;40(1):160–167. doi: 10.1111/j.1471-4159.1983.tb12666.x. [DOI] [PubMed] [Google Scholar]
- Felgner P. L., Wilson J. E. Effect of neutral salts on the interaction of rat brain hexokinase with the outer mitochondrial membrane. Arch Biochem Biophys. 1977 Jul;182(1):282–294. doi: 10.1016/0003-9861(77)90309-5. [DOI] [PubMed] [Google Scholar]
- Ferré P., Leturque A., Burnol A. F., Penicaud L., Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J. 1985 May 15;228(1):103–110. doi: 10.1042/bj2280103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry C. H. Measurement and control of intracellular magnesium ion concentration in guinea pig and ferret ventricular myocardium. Magnesium. 1986;5(5-6):306–316. [PubMed] [Google Scholar]
- Gots R. E., Bessman S. P. An ultrasensitive radioassay for hexokinase. Anal Biochem. 1973 Mar;52(1):272–279. doi: 10.1016/0003-2697(73)90349-7. [DOI] [PubMed] [Google Scholar]
- Hernandez A., Crane R. K. Association of heart hexokinase with subcellular structure. Arch Biochem Biophys. 1966 Jan;113(1):223–229. doi: 10.1016/0003-9861(66)90176-7. [DOI] [PubMed] [Google Scholar]
- Hoerter J., Dormont D., Girault M., Guéron M., Syrota A. Insulin increases the rate of degradation of 2-deoxy-glucose-6-phosphate in the perfused rat heart: a 31P NMR study. J Mol Cell Cardiol. 1991 Oct;23(10):1101–1115. doi: 10.1016/0022-2828(91)90200-6. [DOI] [PubMed] [Google Scholar]
- Hom F. G., Goodner C. J., Berrie M. A. A [3H]2-deoxyglucose method for comparing rates of glucose metabolism and insulin responses among rat tissues in vivo. Validation of the model and the absence of an insulin effect on brain. Diabetes. 1984 Feb;33(2):141–152. doi: 10.2337/diab.33.2.141. [DOI] [PubMed] [Google Scholar]
- Hom F. G., Goodner C. J. Insulin dose-response characteristics among individual muscle and adipose tissues measured in the rat in vivo with 3[H]2-deoxyglucose. Diabetes. 1984 Feb;33(2):153–159. doi: 10.2337/diab.33.2.153. [DOI] [PubMed] [Google Scholar]
- Jelicks L. A., Gupta R. K. Intracellular free magnesium and high energy phosphates in the perfused normotensive and spontaneously hypertensive rat heart. A 31P NMR study. Am J Hypertens. 1991 Feb;4(2 Pt 1):131–136. doi: 10.1093/ajh/4.2.131. [DOI] [PubMed] [Google Scholar]
- Katz J., Dunn A. Glucose-2-t as a tracer for glucose metabolism. Biochemistry. 1967 Jan;6(1):1–5. doi: 10.1021/bi00853a001. [DOI] [PubMed] [Google Scholar]
- Katzen H. M., Soderman D. D., Wiley C. E. Multiple forms of hexokinase. Activities associated with subcellular particulate and soluble fractions of normal and streptozotocin diabetic rat tissues. J Biol Chem. 1970 Aug 25;245(16):4081–4096. [PubMed] [Google Scholar]
- Kosow D. P., Rose I. A. Ascites tumor mitochondrial hexokinase II. Effect of binding on kinetic properties. J Biol Chem. 1968 Jul 10;243(13):3623–3630. [PubMed] [Google Scholar]
- Krivokapich J., Huang S. C., Selin C. E., Phelps M. E. Fluorodeoxyglucose rate constants, lumped constant, and glucose metabolic rate in rabbit heart. Am J Physiol. 1987 Apr;252(4 Pt 2):H777–H787. doi: 10.1152/ajpheart.1987.252.4.H777. [DOI] [PubMed] [Google Scholar]
- Kyriazi H. T., Basford R. E. An examination of the in vivo distribution of brain hexokinase between the cytosol and the outer mitochondrial membrane. Arch Biochem Biophys. 1986 Jul;248(1):253–271. doi: 10.1016/0003-9861(86)90423-6. [DOI] [PubMed] [Google Scholar]
- Lackner R., Challiss R. A., West D., Newsholme E. A. A problem in the radiochemical assay of glucose-6-phosphatase in muscle. Biochem J. 1984 Mar 1;218(2):649–651. doi: 10.1042/bj2180649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. C., Nash W. W., Shine K. I., Phelps M. E., Ricchiuti N. Glucose metabolism during ischemia due to excessive oxygen demand or altered coronary flow in the isolated arterially perfused rabbit septum. Circ Res. 1981 Sep;49(3):640–648. doi: 10.1161/01.res.49.3.640. [DOI] [PubMed] [Google Scholar]
- Mayer S. E., Mayfield A. C., Haas J. A. Heart muscle hexokinase: subcellular distribution and inhibition by glucose 6-phosphate. Mol Pharmacol. 1966 Sep;2(5):393–405. [PubMed] [Google Scholar]
- Ng C. K., Holden J. E., DeGrado T. R., Raffel D. M., Kornguth M. L., Gatley S. J. Sensitivity of myocardial fluorodeoxyglucose lumped constant to glucose and insulin. Am J Physiol. 1991 Feb;260(2 Pt 2):H593–H603. doi: 10.1152/ajpheart.1991.260.2.H593. [DOI] [PubMed] [Google Scholar]
- Nguyê V. T., Mossberg K. A., Tewson T. J., Wong W. H., Rowe R. W., Coleman G. M., Taegtmeyer H. Temporal analysis of myocardial glucose metabolism by 2-[18F]fluoro-2-deoxy-D-glucose. Am J Physiol. 1990 Oct;259(4 Pt 2):H1022–H1031. doi: 10.1152/ajpheart.1990.259.4.H1022. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G., Fenstermacher J. D. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983 Mar;3(1):1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- Patlak C. S., Blasberg R. G. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. Generalizations. J Cereb Blood Flow Metab. 1985 Dec;5(4):584–590. doi: 10.1038/jcbfm.1985.87. [DOI] [PubMed] [Google Scholar]
- Phelps M. E., Hoffman E. J., Selin C., Huang S. C., Robinson G., MacDonald N., Schelbert H., Kuhl D. E. Investigation of [18F]2-fluoro-2-deoxyglucose for the measure of myocardial glucose metabolism. J Nucl Med. 1978 Dec;19(12):1311–1319. [PubMed] [Google Scholar]
- Phelps M. E., Huang S. C., Hoffman E. J., Selin C., Sokoloff L., Kuhl D. E. Tomographic measurement of local cerebral glucose metabolic rate in humans with (F-18)2-fluoro-2-deoxy-D-glucose: validation of method. Ann Neurol. 1979 Nov;6(5):371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- Russell R. R., 3rd, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol. 1991 Dec;261(6 Pt 2):H1756–H1762. doi: 10.1152/ajpheart.1991.261.6.H1756. [DOI] [PubMed] [Google Scholar]
- SOLS A., CRANE R. K. Substrate specificity of brain hexokinase. J Biol Chem. 1954 Oct;210(2):581–595. [PubMed] [Google Scholar]
- Salotra P. T., Singh V. N. Regulation of glucose metabolism in rat lung: subcellular distribution, isozyme pattern, and kinetic properties of hexokinase. Arch Biochem Biophys. 1982 Jul;216(2):758–764. doi: 10.1016/0003-9861(82)90267-3. [DOI] [PubMed] [Google Scholar]
- Schneider C. A., Nguyê V. T., Taegtmeyer H. Feeding and fasting determine postischemic glucose utilization in isolated working rat hearts. Am J Physiol. 1991 Feb;260(2 Pt 2):H542–H548. doi: 10.1152/ajpheart.1991.260.2.H542. [DOI] [PubMed] [Google Scholar]
- Sokoloff L., Reivich M., Kennedy C., Des Rosiers M. H., Patlak C. S., Pettigrew K. D., Sakurada O., Shinohara M. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J Neurochem. 1977 May;28(5):897–916. doi: 10.1111/j.1471-4159.1977.tb10649.x. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H., Hems R., Krebs H. A. Utilization of energy-providing substrates in the isolated working rat heart. Biochem J. 1980 Mar 15;186(3):701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viitanen P. V., Geiger P. J., Erickson-Viitanen S., Bessman S. P. Evidence for functional hexokinase compartmentation in rat skeletal muscle mitochondria. J Biol Chem. 1984 Aug 10;259(15):9679–9686. [PubMed] [Google Scholar]
- Walters E., McLean P. The effect of anti-insulin serum and alloxan-diabetes on the distribution and multiple forms of hexokinase in lactating rat mammary gland. Biochem J. 1968 Oct;109(5):737–741. doi: 10.1042/bj1090737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson J. E. Brain hexokinase. A proposed relation between soluble-particulate distribution and activity in vivo. J Biol Chem. 1968 Jul 10;243(13):3640–3647. [PubMed] [Google Scholar]


