Abstract
The Criegee intermediates are carbonyl oxides that play critical roles in ozonolysis of alkenes in the atmosphere. So far, the mid-infrared spectrum of only the simplest Criegee intermediate CH2OO has been reported. Methyl substitution of CH2OO produces two conformers of CH3CHOO and consequently complicates the infrared spectrum. Here we report the transient infrared spectrum of syn- and anti-CH3CHOO, produced from CH3CHI + O2 in a flow reactor, using a step-scan Fourier-transform spectrometer. Guided and supported by high-level full-dimensional quantum calculations, rotational contours of the four observed bands are simulated successfully and provide definitive identification of both conformers. Furthermore, anti-CH3CHOO shows a reactivity greater than syn-CH3CHOO towards NO/NO2; at the later period of reaction, the spectrum can be simulated with only syn-CH3CHOO. Without NO/NO2, anti-CH3CHOO also decays much faster than syn-CH3CHOO. The direct infrared detection of syn- and anti-CH3CHOO should prove useful for field measurements and laboratory investigations of the Criegee mechanism.
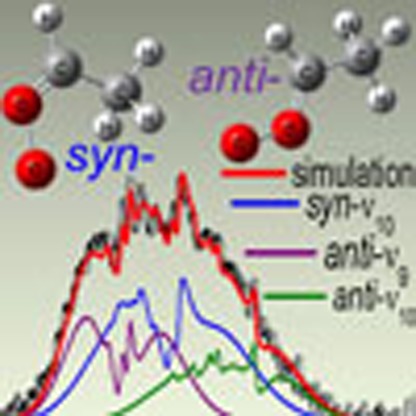 Criegee intermediates—formed by the breakdown of alkanes in the atmosphere—are difficult to identify in the gas phase. Here, the authors report the infrared spectrum of the Criegee intermediate CH3CHOO and identify both the syn- and anti-conformers.
Criegee intermediates—formed by the breakdown of alkanes in the atmosphere—are difficult to identify in the gas phase. Here, the authors report the infrared spectrum of the Criegee intermediate CH3CHOO and identify both the syn- and anti-conformers.
Recent advances in the direct detection of the Criegee intermediates have stimulated active research on their structures, spectroscopy, reactions and implications for atmospheric chemistry1. The Criegee intermediates are carbonyl oxides that are produced during the reactions of ozone (O3) with unsaturated hydrocarbons; they play a central role in controlling the atmospheric budget of OH, organic acids and secondary organic aerosols, especially under low-light conditions.
These Criegee intermediates have eluded detection in the gaseous phase until recently because of their great reactivity and small concentration. Previous understanding of the reactivity of Criegee intermediates, including reaction mechanisms and rate coefficients, was hence subject to large uncertainties, because only indirect experimental evidence was available. Welz et al.3 produced the simplest Criegee intermediate, formaldehyde oxide (CH2OO), in a flow cell and confirmed its identification with tunable vacuum-ultraviolet photoionization mass spectrometry2. Following the reaction scheme of CH2I+O2→I+CH2OO initially developed by Welz et al.3, CH2OO has now been detected with ultraviolet depletion4, ultraviolet absorption5,6, infrared absorption7 and microwave spectroscopy8,9. The kinetics of reactions of CH2OO with various atmospheric species have been directly investigated with some of these detection methods3,10,11,12,13,14,15.
In the atmosphere, O3 reacts with larger alkenes and produces more complex Criegee intermediates RR′COO; the nature and location of the substituents R/R′ are predicted to affect their reactivity16. For example, the OH yield increases from ∼10% for O3+C2H4 to ∼60% for O3 + trans-2-butene17,18. The methyl-substituted Criegee intermediate, acetaldehyde oxide (CH3CHOO), is an intermediate of reactions O3 + 2-alkenes (such as trans-2-butene) and serves as a prototype to understand various fundamental issues in larger Criegee intermediates. CH3CHOO exists in two conformers, syn-CH3CHOO and anti-CH3CHOO (Supplementary Fig. 1), with the former more stable than the latter by ∼15 kJ mol−1 (refs 19, 20). Because of a large barrier ∼160 kJ mol−1, the interconversion between syn-CH3CHOO and anti-CH3CHOO is unlikely. Taatjes et al.21 employed the reaction CH3CHI + O2 to produce CH3CHOO and detected both conformers with photoionization. By employing photoionization at energies 9.37 and 10.5 eV to detect predominantly anti-CH3CHOO and syn-CH3CHOO, respectively, these authors reported that anti-CH3CHOO is significantly more reactive towards H2O and SO2 than is syn-CH3CHOO.
Although photoionization can distinguish these two conformers of CH3CHOO via distinct ionization energies, a small contribution from the other conformer is unavoidable. The ultraviolet depletion22 and ultraviolet absorption experiments20 yielded a broad feature without structure and provided no information about the conformation of the CH3CHOO carrier. Recently Sheps et al.23 separated the ultraviolet spectra of two possible conformers based on their distinct reactivity towards H2O and SO2, but the two spectra were broad and overlapping with each other. Although microwave spectroscopy was successful in providing structural information24, to employ it for monitoring these species in laboratory kinetic experiments is difficult. Liu et al.25 employed infrared activation of cold CH3CHOO to produce OH, which was detected with laser-induced fluorescence, and assigned several absorption features in the region 5,600–6,100 cm−1 to be the CH-overtone and combination bands of syn-CH3CHOO; no bands of anti-CH3CHOO were identified. It is thus desirable to develop an alternative spectral method that can be applied conveniently and can distinguish clearly these two conformers of CH3CHOO.
We have demonstrated that coupling a step-scan Fourier-transform infrared (FTIR) spectrometer with a multipass absorption cell enables the recording of temporally resolved infrared absorption spectra of the simplest Criegee intermediate, CH2OO (ref. 7); five distinct absorption bands of CH2OO were clearly identified to provide a simple and direct detection method.
Here we report a further application of this technique to characterize the infrared absorption of methyl-substituted Criegee intermediate CH3CHOO in two conformations and to demonstrate the conformation-dependent reactivity towards its self-reaction and reaction with NO/NO2.
Results
Spectral analysis of CH3CHOO
Even though only one H atom of CH2OO was replaced with a methyl group to form CH3CHOO, the infrared spectrum of the latter is expected to be much more complicated, because both syn- and anti-conformers contribute to the infrared absorption. In addition, the methyl group introduces low-energy vibrational modes such as the CH3 torsion (internal rotation), which are populated with several vibrational quanta even at ambient temperatures; hot bands might consequently play important roles in the observed spectrum, as it was demonstrated in the spectrum of CH2BrOO (ref. 26). Furthermore, the internal rotation of the methyl moiety may introduce torsional splitting in vibrational bands. Consequently, spectral identification and simulation of observed bands of CH3CHOO are expected to be difficult. In this respect, sophisticated quantum-chemical calculations are essential to assist the spectral simulation and assignments.
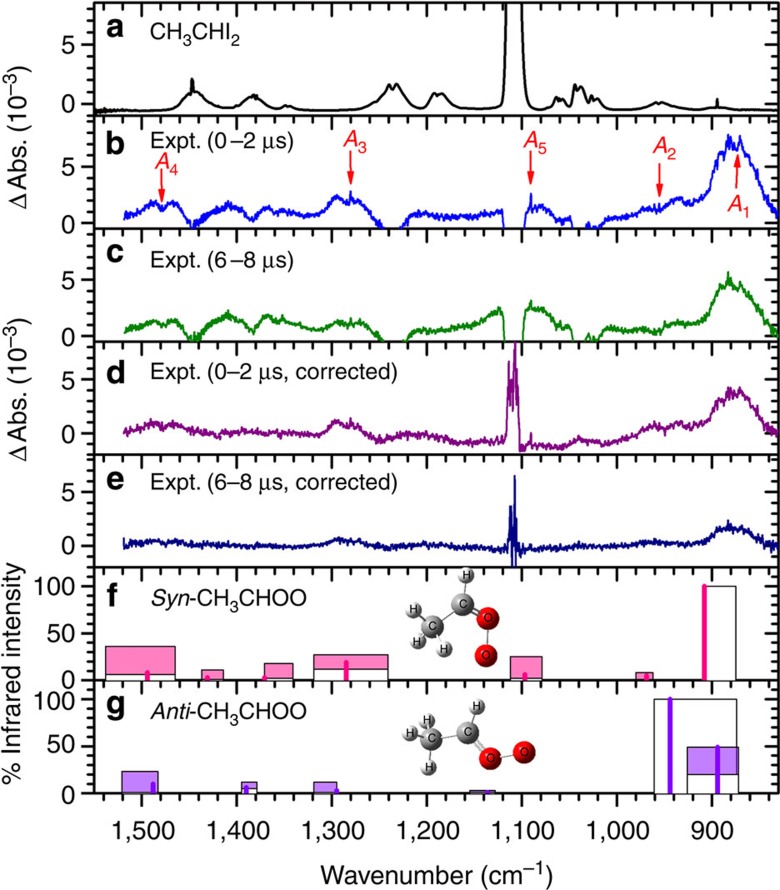
We employed a step-scan FTIR spectrometer coupled with a multi-reflection White cell to record time-resolved infrared spectra. The laser beam at 308 nm photodissociated a flowing mixture of 1,1-diiodoethane (CH3CHI2) and O2 to produce CH3CHI, which subsequently reacted with O2 to form CH3CHOO. The partial infrared absorption spectrum (830–1,550 cm−1) of a flowing mixture of CH3CHI2/N2/O2 (1/13/288, 84 Torr) at 328 K exhibits an intense absorption line of CH3CHI2 near 1,108 cm−1 and some much weaker ones near 894, 956, 1,024, 1,041, 1,061, 1,190, 1,236, 1,383 and 1,447 cm−1 (Fig. 1a). On irradiation with light at 308 nm, the absorption of CH3CHI2 decreased because of photolysis, whereas new bands marked A1–A5 appeared in the difference spectrum recorded 0–2 μs after laser irradiation (Fig. 1b). The A5 band was partially interfered with by the absorption of the precursor and the product acetaldehyde, but its sharp Q-branch is quite characteristic and can be readily recognized. These new lines decreased in intensity, as shown in Fig. 1c where 6–8 μs was recorded after ultraviolet irradiation and disappeared after ∼25 μs. To minimize interference from other products, we subtracted the spectrum recorded during 16.0–19.8 μs from these two spectra and stripped the contributions from the precursor and acetaldehyde, as shown in Fig. 1d,e.
Figure 1. Comparison of observed spectra with predicted stick spectra.
(a) Absorption spectrum of a flowing mixture of CH3CHI2/N2/O2 (1/13/288, 84 Torr) before photolysis. (b) Difference spectra recorded 0–2 μs and (c) 6–8 μs after irradiation of the sample at 308 nm. (d) Corrected spectra recorded 0–2 μs and (e) 6–8 μs after subtraction of the spectrum recorded at 16.0–19.8 μs and removal of the contributions of the precursor CH3CHI2 and stable product acetaldehyde. Resolution of all spectra is 0.5 cm−1. New features are marked with arrows and labelled as A1–A5. (f) Possible ranges of anharmonic vibrational wavenumbers and infrared intensities of syn-CH3CHOO and (g) anti-CH3CHOO predicted with various methods (Supplementary Tables 1 and 2) shown as filled boxes; those predicted with the MULTIMODE method are shown with thick lines.
As photolysis of CH3CHI2 produces mainly CH3CHI (ref. 27) and because this radical reacts readily with excessive O2 in the system28, possible carriers of the observed new bands include conformers of CH3CHOO and vinyl hydroperoxide (C2H3OOH), methyldioxirane and the stabilized adduct CH3CHIOO. The structures of these species are shown in Supplementary Fig. 1. For all species except CH3CHIOO, MP2, B3LYP and NEVPT2(8,8) methods were employed to predict their vibrational wavenumbers and infrared intensities. For CH3CHIOO, the NEVPT2(1,1) method was used. Furthermore, the most sophisticated predictions were performed for syn- and anti-CH3CHOO with high-level, full-dimensional quantum calculations, using the MULTIMODE method.29,30,31 The vibrational wavenumbers and infrared intensities of syn- and anti-CH3CHOO calculated with these theoretical methods are given in Supplementary Tables 1 and 2. Those of methyl dioxirane, trans- and cis-vinyl hydroperoxide and syn- and anti-CH3CHIOO are listed in Supplementary Tables 3–5, respectively. There is good consistency among these calculations, but there are significant variations in the calculated anharmonic vibrational wavenumbers and infrared intensities among these calculations. To indicate this, we show the ranges of values as filled rectangles, while the values predicted with the MULTIMODE method are depicted with thick lines in Fig. 1f,g, respectively, for syn- and anti-CH3CHOO. Possible ranges of vibrational anharmonic wavenumbers and intensities of CH3CHIOO, methyl dioxirane and vinyl hydroperoxide predicted with various methods are compared with the observed spectrum in Supplementary Fig. 2. A comparison of the observed new spectral features with those predicted theoretically excludes the possibility of assigning the observed spectral features A1–A5 to the carriers other than CH3CHOO.
As shown in Fig. 1f and Supplementary Table 1, the more stable syn-CH3CHOO conformer is predicted from the MULTIMODE calculations to have more intense absorptions near 908 (100), 969 (5), 1,097 (6), 1,285 (19) and 1,494 (8) cm−1; the numbers in parentheses give relative infrared intensity of each vibration. The observed new features near 871 (100), 956, 1,091 (10), 1,281 (40) and 1,477 (30) cm−1 agree satisfactorily with these predicted values. The predicted pattern of intense lines of anti-CH3CHOO near 894 (49), 944 (100), 1,295 (3) and 1,488 (10) cm−1 agrees less satisfactorily with the observed spectrum. We show below that the contribution from the anti-CH3CHOO conformer, although less significant, is important for explaining the overall shape of the spectrum and is of primary importance for explaining some of the fine-structure spectral features of the spectrum.
Simulation of rotational contours
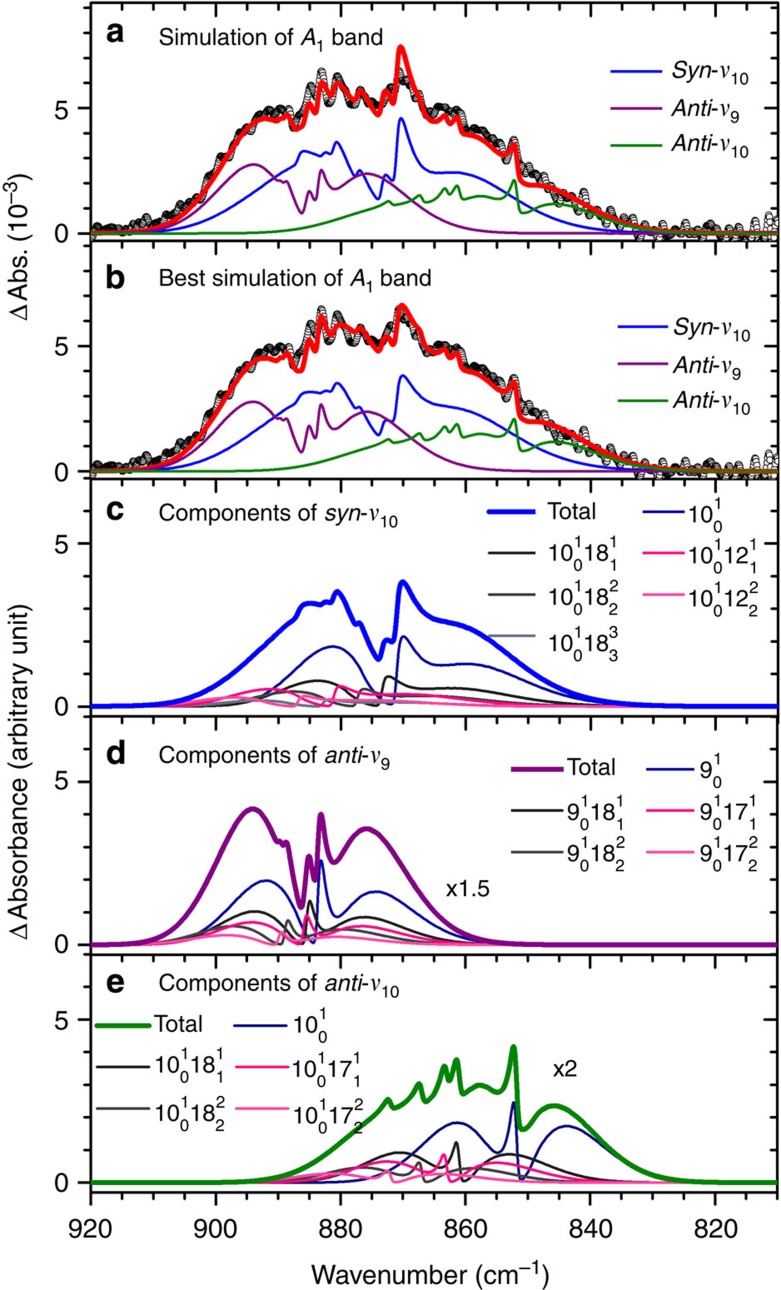
Further support for the presented assignment comes from analysis of the rotational contours of the observed bands. The rotational contour of the A1 band near 871 cm−1 recorded in the interval of 0–2 μs has several characteristic peaks; it cannot be simulated with a single band and was deconvoluted with guidance from calculations. The experimental data are presented with open circles and the resultant spectrum, simulated according to ratios of rotational constants of the excited and ground states predicted with the MULTIMODE method and the experimental rotational constants of the ground state, is shown as a thick solid line in Fig. 2a; the agreement is satisfactory. Figure 2b presents a comparison of experimental data with the spectrum simulated according to slightly modified ratios of rotational constants for an improved fit. For details of simulation using the PGopher programme32, see Supplementary Methods. This simulated feature consists of three bands: a dominant OO-stretching (ν10) band of syn-CH3CHOO at 871.2 cm−1 and two smaller bands of OO-stretching (ν9) and OO-stretching mixed with CH2 wagging (ν10) modes of anti-CH3CHOO at 883.7 and 851.8 cm−1, respectively, shown as thin lines in Fig. 2a,b; the fundamental Q-branches of these three modes correspond well with observed distinct peaks; hence, their vibrational wavenumbers can be determined accurately. It is found that several hot bands associated with the ν18 (208 cm−1) and ν12 (314 cm−1) low-energy vibrational modes of syn-CH3CHOO and with the ν18 (156 cm−1) and ν17 (255 cm−1) low-energy vibrational modes of anti-CH3CHOO contribute significantly to the observed spectral features. The individual contributions of fundamental and hot bands for these three vibrational modes are shown in Fig. 2c–e; the transitions of hot bands are expressed as 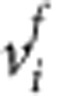 in which v is the vibrational mode number, and i and f are vibrational quantum numbers of the lower and upper states, respectively. Detailed positions and relative intensities are listed in Supplementary Table 6. This simulation implies that (1) the hot bands are all blue shifted from the fundamental; the unusual but necessary blue shifts of about 2 and 10 cm−1 for the hot bands involving ν18 and ν12 of syn-CH3CHOO, respectively, agree qualitatively with the theoretical predictions (Supplementary Table 6). The calculations also indicate that torsional splitting is small for all vibrational modes so that it has no consequence on our spectral simulation. (2) At 328 K, assuming a Boltzmann distribution and that the infrared intensities of hot bands are the same as that of the fundamental band (in qualitative accord with calculations), observed relative intensities of these hot bands imply energies of the first excited states of ν18 and ν12 modes of syn-CH3CHOO and those of ν18 and ν17 of anti-CH3CHOO to be ∼193, 282, 149 and 239 cm−1, respectively, which is consistent with theoretical predictions (Table 1 and Supplementary Tables 1 and 2). (3) The population fraction of anti-CH3CHOO is 0.30 and 0.38 at 328 K, respectively, if infrared intensities predicted with the B3LYP and MULTIMODE methods are used. This fraction is consistent with a value of ∼0.30 derived from ultraviolet experiments23, but greater than values ∼0.20 from microwave experiments24 and ∼0.10 from photoionization experiments21.
in which v is the vibrational mode number, and i and f are vibrational quantum numbers of the lower and upper states, respectively. Detailed positions and relative intensities are listed in Supplementary Table 6. This simulation implies that (1) the hot bands are all blue shifted from the fundamental; the unusual but necessary blue shifts of about 2 and 10 cm−1 for the hot bands involving ν18 and ν12 of syn-CH3CHOO, respectively, agree qualitatively with the theoretical predictions (Supplementary Table 6). The calculations also indicate that torsional splitting is small for all vibrational modes so that it has no consequence on our spectral simulation. (2) At 328 K, assuming a Boltzmann distribution and that the infrared intensities of hot bands are the same as that of the fundamental band (in qualitative accord with calculations), observed relative intensities of these hot bands imply energies of the first excited states of ν18 and ν12 modes of syn-CH3CHOO and those of ν18 and ν17 of anti-CH3CHOO to be ∼193, 282, 149 and 239 cm−1, respectively, which is consistent with theoretical predictions (Table 1 and Supplementary Tables 1 and 2). (3) The population fraction of anti-CH3CHOO is 0.30 and 0.38 at 328 K, respectively, if infrared intensities predicted with the B3LYP and MULTIMODE methods are used. This fraction is consistent with a value of ∼0.30 derived from ultraviolet experiments23, but greater than values ∼0.20 from microwave experiments24 and ∼0.10 from photoionization experiments21.
Figure 2. Spectral simulation of band A1.
(a) Comparison of experimental data (open circles, recorded 0–2 μs) with spectrum simulated according to theoretical predictions (thick red solid line) and (b) the best simulated spectrum (thick red solid line) with slightly modified parameters; contributions of ν10 of syn-CH3CHOO, and ν9 and ν10 of anti-CH3CHOO are shown with thin lines. Resolution is 0.5 cm−1. (c) Contributions of fundamental and hot bands of ν10 of syn-CH3CHOO, (d) ν9 of anti-CH3CHOO and (e) ν10 of anti-CH3CHOO. Detailed positions and relative intensities are listed in Supplementary Table 6. Spectral width of simulation is 0.64 cm−1. The transitions of hot bands are expressed with 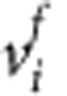 in which v is the vibrational mode number, and i and f are vibrational quantum numbers of the lower and upper states, respectively.
in which v is the vibrational mode number, and i and f are vibrational quantum numbers of the lower and upper states, respectively.
Table 1. Comparison of experimentally observed wavenumbers (cm−1) and intensities with the vibrational wavenumbers and infrared intensities of representative vibrational modes of CH3CHOO predicted with the MULTIMODE method.
| Symbol | Syn-CH3CHOO | Anti-CH3CHOO | Description* | ||||
|---|---|---|---|---|---|---|---|
| Mode | Experiment | Calculation | Mode | Experiment | Calculation | ||
| A' | ν4 | 1,476.8 (30)† | 1,494 (8)† | ν4 | 1,479.0 (14)† | 1,488 (10)† | CO str./HCO bend |
| A' | ν7 | 1,280.8 (40) | 1,285 (19) | ν7 | 1,279.4 (17) | 1,295 (3) | HCO bend/CO str. |
| A' | ν8 | 1,090.6 (10) | 1,097 (6) | ν8 | 1,136 (1) | CH2 wag/CCH bend | |
| A' | ν9 | ∼956.0 (−) | 969 (5) | ν10 | 851.8 (73) | 894 (49) | CCH bend/CH2 wag |
| A' | ν10 | 871.2 (100) | 908 (100) | ν9 | 883.7 (100) | 944 (100) | OO str. |
| A' | ν12 | 314 (3)‡ | ν12 | 330 (7) | CCO/COO iph bend | ||
| A” | ν17 | 449 (0) | ν17 | 255 (0)§ | op deformation | ||
| A” | ν18 | 208 (1)‡ | ν18 | 156 (0)§ | CH3 torsion | ||
Ip, in-plane; iph, in-phase; op, out-of-plane; str., stretch.
*Approximate mode description. For anti-CH3CHOO, the HCO bending mode is replaced with CH ip bending mode for ν7, ν8 is mainly CCH bend, the CCH bend is replaced with OO stretch for ν10, and the iph bend is replaced with oph bend for ν12.
†Relative infrared intensities normalized to the most intense line. The maximal intensities of syn- and anti-CH3CHOO are predicted to be 44.4 and 43.6 km mol−1, respectively, with the MULTIMODE method (18-mode coupling); values of 13-mode coupling for syn- and 14-mode coupling for anti-CH3CHOO are 50.3 and 61.1 km mol−1, respectively.
‡Harmonic vibrational wavenumbers, as the anharmonic treatment of this mode is problematic using the methods employed here. Anharmonic vibrational wavenumbers are ν12=290 cm−1 and ν18=221 cm−1 predicted with the VCI/NEVPT2(8,8)/aug-cc-pVDZ method.
§Harmonic vibrational wavenumbers, as the anharmonic treatment of this mode is problematic using the methods employed here. Anharmonic vibrational wavenumbers are ν17=378 cm−1 and ν18=241 cm−1 predicted with the VCI/NEVPT2(8,8)/aug-cc-pVDZ method.
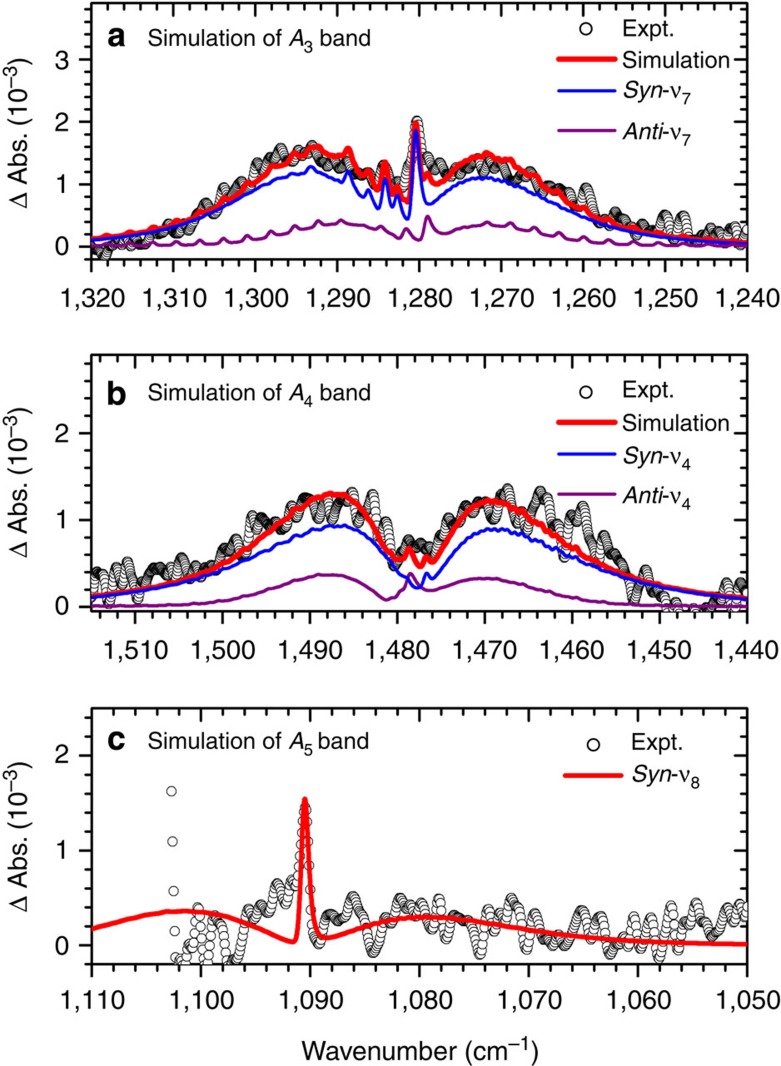
The weaker bands A3 and A4 can be simulated likewise with contributions of syn- and anti-CH3CHOO, as presented in Fig. 3. Band A3 has a prominent Q-branch at 1,280.8 cm−1, which is assigned to the HCO bending coupled with the CO-stretching (ν7) mode of syn-CH3CHOO. A second feature with a weaker Q-branch at 1,279.4 cm−1 is assigned to the corresponding ν7 mode of anti-CH3CHOO (Fig. 3a). Band A4 has prominent P- and R-branches; this band is assigned to the CO-stretching mode coupled with the HCO bending (ν4) mode of syn-CH3CHOO. The observed weak Q-branch arises from a small contribution of the ν4 mode of anti-CH3CHOO. The contributions of fundamental and hot bands to these features are presented in Supplementary Figs 3 and 4. The a- and b-type spectra of related vibrational modes of syn- and anti-CH3CHOO are presented in Supplementary Figs 5 and 6, respectively. As band A2 is rather weak and subject to interference from absorption of C2H4, we could only estimate its position to be ∼956 cm−1 from the Q-branch and assign it to the ν9 mode of syn-CH3CHOO. Band A5 suffers from partial interference due to the precursor, but the prominent Q-branch indicates that this band is due to syn-CH3CHOO and not anti-CH3CHOO. The observed position at 1,090.6 cm−1 is much closer to the predicted anharmonic vibrational wavenumber of 1,097 cm−1 for syn-CH3CHOO than the value 1,136 cm−1 for anti-CH3CHOO.
Figure 3. Spectral simulation of bands A3 to A5.
(a) Comparison of experimental data (open circles, recorded 0–4 μs) with simulated spectrum (thick red solid line) for band A3; contributions of ν7 bands of syn-CH3CHOO and anti-CH3CHOO are shown with thin lines. (b) Comparison for band A4; contributions of ν4 bands of syn-CH3CHOO and anti-CH3CHOO are shown with thin lines. (c) Comparison of band A5 (recorded 0–2 μs); only the ν8 band of syn-CH3CHOO contributes. Spectral width of simulation is 0.64 cm−1.
As shown in Table 1, the agreement between observed vibrational wavenumbers of syn- and anti-CH3CHOO, and those calculated with the MULTIMODE method is quite satisfactory with differences <17 cm−1, except for the OO-stretching mode, which has differences of 37 cm−1 for syn-CH3CHOO and 60 cm−1 for anti-CH3CHOO. A similar shift between experiment and MULTIMODE calculations was reported previously for CH2OO for an analogous band33. Conceivably, the origin of the deviation is, as in CH2OO, a slight deficiency in the level of electronic structure theory which is more sensitive to the OO-stretching mode.
Our observations also conform to an expectation that similar to CH2OO, CH3CHOO has a significant zwitterionic character with a strengthened C−O bond and a weakened O−O bond. The observed wavenumber of the OO-stretching mode of syn-CH3CHOO near 871 cm−1 is smaller than the corresponding value 908 cm−1 of CH2OO, consistent with theoretical predictions showing that the length of the O–O bond increases from 1.349 Å in CH2OO to 1.380 Å in syn-CH3CHOO.
Conformation-dependent reactivity
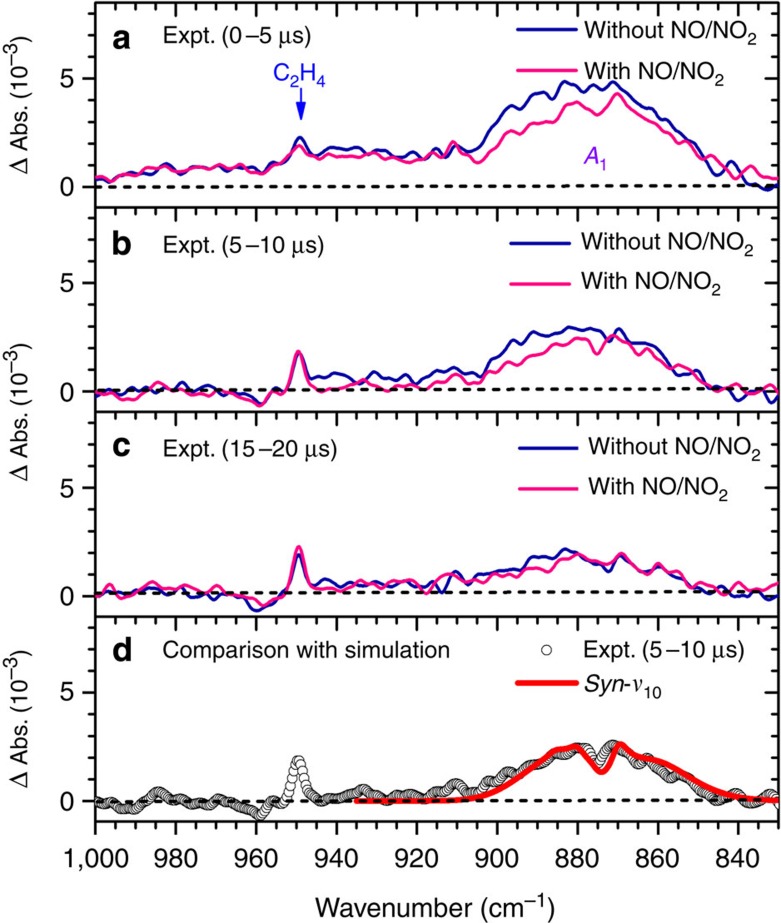
Further support for this assignment of the A1 band comes from a comparison of low-resolution spectra recorded in experiments with and without NO/NO2. Taatjes et al.21 reported that anti-CH3CHOO is more reactive towards H2O, SO2 and NO2 than syn-CH3CHOO, with the effect much more significant for the former two. A direct test with H2O in our system is difficult because of the intense infrared absorption of H2O and the hygroscopic KBr windows we employed. A test with SO2 is also infeasible, because the windows were contaminated by the products easily. Instead, we added NO/NO2 to explore the differences. As shown in Fig. 4a, the difference between bands A1 in the range 870–910 cm−1 recorded in experiments with and without adding NO/NO2 is significant during the interval 0–5 μs and the difference in the range 830–870 cm−1 is smaller but non-negligible. The differences in these two regions are consistent with the contributions of ν9 and ν10 bands of anti-CH3CHOO shown in Fig. 2a. This difference in spectra recorded in experiments with and without NO/NO2 diminished at a later period and the spectrum recorded 5–10 μs after photolysis in the experiment with NO/NO2 (Fig. 4b) agrees with that simulated for syn-CH3CHOO only, as shown in Fig. 4d; this provides additional support that our simulation of these two conformers is reliable and most anti-CH3CHOO was consumed within 10 μs.
Figure 4. Comparison of spectra recorded with and without added NO/NO2.
(a) A flowing mixture of CH3CHI2/N2/O2 (1/21/129, 90 Torr) with and without added NO/NO2 (∼3.0/0.18 Torr) recorded 0–5 μs, (b) 5–10 μs and (c) 15–20 μs after irradiation at 248 nm. (d) Comparison of experimental data (open circles) with simulated spectrum (thick red solid line) of ν10 of syn-CH3CHOO. Spectral width of simulation is 2.6 cm−1.
Further indication of the greater reactivity of anti-CH3CHOO than that of syn-CH3CHOO comes from the observation of a more rapid decrease of peaks at 851.8 and 883.7 cm−1 (assigned to anti-CH3CHOO) relative to that at 871.2 cm−1 (assigned to syn-CH3CHOO) in the earlier stage of reaction. Without NO/NO2, nearly all anti-CH3CHOO diminished and only syn-CH3CHOO survived after 10 μs; syn-CH3CHOO became nearly diminished after 25 μs. The decay of syn-CH3CHOO is likely to be mainly due to its self-reaction, similar to that observed for CH2OO (refs 7, 14, 15). In Supplementary Fig. 7, we plotted the reciprocal absorbance (I−1) integrated from 850 to 900 cm−1 for band A1 as a function of reaction period; the integrated absorbance I is proportional to concentration. The slope at the initial stage of reaction is twice that at a later stage of reaction. A qualitative analysis indicates that the overall removal of anti-CH3CHOO could be up to 30 times faster than that of syn-CH3CHOO; this probably reflects the difference in reactivity of these two conformers associated with some reactions with themselves or with other species. We are unable to derive absolute concentration of CH3CHOO, because the infrared probe volume does not follow the photolysis volume in our experiments14; hence, accurate rate coefficient cannot be determined. In any case, it is clear that in the CH3CHI + O2 system the reactivity of anti-CH3CHOO is much greater than that of syn-CH3CHOO, so that most anti-CH3CHOO was consumed within 10 μs under our experimental conditions. Future theoretical investigations on this distinct conformation-dependent reactivity will provide some insight on this important observation.
Discussion
We recorded the transient infrared spectrum of both syn- and anti-CH3CHOO, produced from CH3CHI + O2 in a flow reactor, using a step-scan Fourier-transform spectrometer. The rotational contours, vibrational wavenumbers and relative infrared intensities of these observed bands provide definitive identification of both conformers; the rotational contours are critical in distinguishing these conformers. The assignments and spectral simulation are guided and supported by high-level full-dimensional quantum calculations. We found that anti-CH3CHOO is more reactive towards NO/NO2 than syn-CH3CHOO. Without NO/NO2, anti-CH3CHOO also decays much more rapidly than syn-CH3CHOO. This feature shall enable one to prepare nearly pure syn-CH3CHOO either by adding NO/NO2 or by waiting till a later reaction period. This distinct conformation-dependent reactivity is of fundamental interest and is also important in modelling reactions involving Criegee intermediates both in laboratory and in the atmosphere.
Our infrared identification provides an alternative way to probe CH3CHOO directly in the atmosphere or in laboratory experiments. Although nearly all observed bands of anti-CH3CHOO overlapped with syn-CH3CHOO, the Q-branch of ν8 near 1,090.6 cm−1 is contributed solely by syn-CH3CHOO, and that of ν7 near 1,280.8 cm−1 is also dominated by syn-CH3CHOO; they can be unambiguously detected with a narrow-width infrared laser. This convenient detection scheme will be useful in providing information on the exact mechanism and detailed kinetics of reactions involving CH3CHOO.
Methods
Experimental
The White cell of base path length 15 cm and effective absorbing path length 3.6 m was coupled with a step-scan FTIR spectrometer (Bruker, Vertex 80v) and served as a flow reactor. The flow reactor has volume ∼1,370 cm3 and accommodates two rectangular quartz windows (3 × 12 cm2) on opposite sides of the cell, to allow the photolysis beam to propagate through the cell. The photolysis light at 308 nm was generated from a XeCl excimer laser (Coherent, CompexPro 102F, 11 Hz, ∼170 mJ per pulse, beam size 2.9 × 1.2 cm2). In earlier experiments, light at 248 nm from a KrF excimer laser (Coherent, CompexPro 102F, 11 Hz, ∼200 mJ per pulse, beam size 1.5 × 1.0 cm2) was used. The photolysis beam passed the White cell and was reflected five times with two external mirrors, to enhance the photolysis. With appropriate optical filters to allow passage of light in a narrow spectral region, we performed undersampling to decrease the size of the interferogram, hence the duration of data acquisition. The infrared probing light was detected with a HgCdTe detector from which dc- and ac-coupled signals were recorded at each scan step on irradiation with the photolysis laser. These signals were sent either directly to the external 14-bit digitizer with resolution of 4 or 10 ns. For the ac signal, 5,000 data points at 4 (10) ns intervals were acquired to cover a period of 19.8 (48.0) μs after photolysis. These signals were typically averaged over 15 laser shots at each scan step. For spectra in the range 753–1,504 cm−1 at resolution 0.5 cm−1, 3,046 scan steps were completed in ∼1.5 h. For spectra in the range 1,089−1,633 cm−1 at resolution 0.5 cm−1, 2,206 scan steps were completed in ∼1 h. The spectral width (full width half maximum) at this resolution after apodization is 0.64 cm−1. Fourteen spectra recorded under similar conditions were averaged to yield a spectrum with a satisfactory ratio of signal to noise. For experiments with added NO/NO2, the resolution is 2.0 cm−1 (full width half maximum 2.6 cm−1) and one spectrum was recorded.
A small stream of O2 from a main stream at flow rate of FO2≅23 STP cm3 s−1 (STP denotes standard temperature 273 K and pressure 1 atm) served to purge the windows. A small stream of N2 (∼3.5 Torr in the reactor) was bubbled through liquid CH3CHI2 before entering the reactor. The total pressure was maintained in the range PT=80–85 Torr, with a partial pressure of PCH3CHI2≅0.28 Torr determined from its infrared absorption spectra. The average efficiency of photolysis of CH3CHI2 is estimated to be ∼8% according to an absorption cross-section of ∼3.1 × 10−18 cm2 per molecule (ref. 27) and a laser fluence of ∼7 × 1016 photons per cm2 at 308 nm. To obtain a desirable pressure of CH3CHI2, the samples and the flow reactor were heated to 328 K with heated water circulated from a thermostated bath through the jacket of the reactor. CH3CHI2 (96–98%), O2 (99.99%) and N2 (99.9995%) were used as received.
Computational
The harmonic and anharmonic vibrational wavenumbers have been computed using quadratic force field obtained with the MP2 (ref. 34) and the NEVPT2 methods35 implemented in MOLPRO quantum chemistry package36, using a complete active space defined by four highest occupied orbitals and four lowest unoccupied orbitals; no symmetry has been used in these calculations to avoid problems during numerical determination of force-field coefficients. The anharmonic wavenumbers37 have only an approximate character, as the effects of three- and four-mode couplings have been neglected due to great computational complexity.
For CH3CHOO, we also employed the MULTIMODE method. See Supplementary Methods for more details. The MULTIMODE calculations employ full-dimensional potential energy surfaces (PESs) for syn-CH3CHOO and anti-CH3CHOO conformers. Most geometries are generated from classical dynamical trajectories with density functional theory (B3LYP). More points are chosen randomly around the equilibrium geometry and the torsion saddle point. To further increase the accuracy of the PESs, additional points are chosen along the grids of normal mode along the fully relaxed torsion path for both syn- and anti-CH3CHOO. Finally, we get about 39,000 ab initio energies, which are computed with the CCSD(T)-F12b method38,39 and aug-cc-pVDZ basis for carbon and oxygen atoms, and cc-pVDZ basis for hydrogen atoms.
The PESs are fitted with permutationally invariant method, which has been described in detail somewhere else30,40. The Morse variable is applied in the PES function, in which yij=exp(–rij/α) and α is fixed at 2 bohr. The maximum polynomial order is 5 for both PES and the number of coefficients is 5,801. The fitting root mean squares of the PESs are 0.076 and 0.068 cm−1 for syn- and anti-CH3CHOO, respectively. The comparisons of energies and harmonic frequencies between the PES and CCSD(T)-F12b calculation for syn- and anti-conformer are given in Supplementary Tables 9 and 10. As seen, great agreement is achieved between the PES and direct ab initio calculation. In addition, the dipole moment surfaces, which are computed with MP2 methods, are fitted as well for the infrared intensity calculation.
The infrared intensities are calculated using the dipole transition matrix and each element is determined by integrating the ground and vibrationally excited-state wave functions and the dipole moment components. The calculated energies and intensities of fundamental states for syn- and anti-CH3CHOO are listed in Supplementary Tables 1 and 2. Several fundamental states, especially the CH-stretching modes, are difficult to assign due to their strong coupling with other modes.
Rotation constants of the ground vibrational state and fundamental vibrational states are determined rigorously from calculated energy difference of J=1 and 0. The rotation constants of syn-CH3CHOO ground vibrational state agree with those determined experimentally24. The computed rotation constants for syn- and anti-CH3CHOO are shown in Supplementary Tables 7 and 8, respectively. These small energy differences are sensitive to the treatment of the torsion degree of freedom, and so several sets of calculations, including ones that eliminated that degree of freedom, were performed. The rotation constants that appear to be most consistent with the simulation of experimental bands are from the smaller set of calculation with 13 modes coupling for syn-CH3CHOO (14 modes for anti) and these are given in the tables.
Author contributions
H.Y.L. and Y.H.H. performed the experiments and H.Y.L. analysed the data. X.W. performed the MULTIMODE calculations. Y.N. performed calculations with other methods. J.B. and H.A.W. conceived and designed the calculations, and contributed to writing sections of the paper. Y.P.L. conceived and designed the experiments and wrote a major part of the paper.
Additional information
How to cite this article: Lin, H.-Y. et al. Infrared identification of the Criegee intermediates syn- and anti-CH3CHOO, and their distinct conformation-dependent reactivity. Nat. Commun. 6:7012 doi: 10.1038/ncomms8012 (2015).
Supplementary Material
Supplementary Figures 1-7, Supplementary Tables 1-10, Supplementary Methods and Supplementary References
Acknowledgments
Y.P.L. and H.A.W. thank the Ministry of Science and Technology (grants number MOST103-2745-M-009-001-ASP and MOST102-2113-M-009-015-MY3); Ministry of Education, Taiwan (‘Aim for the Top University Plan' of National Chiao Tung University) for support; and the National Center for High-Performance Computing for computer time. X.W. thanks NASA for financial support (NNX12AF42G from the NASA Astrophysics Research and Analysis programme) and J.M.B. thanks the US Department of Energy (DE-FG02-97ER14782).
References
- Taatjes C. A., Shallcross D. E. & Percival C. J. Research frontiers in the chemistry of Criegee intermediates and tropospheric ozonolysis. Phys. Chem. Chem. Phys. 16, 1704–1718 (2014). [DOI] [PubMed] [Google Scholar]
- Taatjes C. A. et al. Direct observation of the gas-phase Criegee intermediate (CH2OO). J. Am. Chem. Soc. 130, 11883–11885 (2008). [DOI] [PubMed] [Google Scholar]
- Welz O. et al. Direct kinetic measurements of Criegee intermediate (CH2OO) formed by reaction of CH2I with O2. Science 335, 204–207 (2012). [DOI] [PubMed] [Google Scholar]
- Beames J. M., Liu F., Lu L. & Lester M. I. Ultraviolet spectrum and photochemistry of the simplest Criegee intermediate CH2OO. J. Am. Chem. Soc. 134, 20045–20048 (2012). [DOI] [PubMed] [Google Scholar]
- Sheps L. Absolute ultraviolet absorption spectrum of a Criegee intermediate CH2OO. J. Phys. Chem. Lett. 4, 4201–4205 (2013). [DOI] [PubMed] [Google Scholar]
- Ting W.-L., Chen Y.-H., Chao W., Smith M. C. & Lin J. J.-M. The UV absorption spectrum of the simplest Criegee intermediate CH2OO. Phys. Chem. Chem. Phys. 16, 10438–10443 (2014). [DOI] [PubMed] [Google Scholar]
- Su Y.-T., Huang Y.-H., Witek H. A. & Lee Y.-P. Infrared absorption spectrum of the simplest Criegee intermediate CH2OO. Science 340, 174–176 (2013). [DOI] [PubMed] [Google Scholar]
- Nakajima M. & Endo Y. Communication: determination of the molecular structure of the simplest Criegee intermediate CH2OO. J. Chem. Phys. 139, 101103 (2013). [DOI] [PubMed] [Google Scholar]
- McCarthy M. C. et al. The simplest Criegee intermediate (H2C=O-O):Isotopic spectroscopy, equilibrium structure, and possible formation from atmospheric lightning. J. Phys. Chem. Lett. 4, 4133–4139 (2013). [Google Scholar]
- Taatjes C. A. et al. Direct measurement of Criegee intermediate (CH2OO) reactions with acetone, acetaldehyde, and hexafluoroacetone. Phys. Chem. Chem. Phys. 14, 10391–10400 (2012). [DOI] [PubMed] [Google Scholar]
- Stone D., Blitz M., Daubney L., Howes N. U. M. & Seakins P. Kinetics of CH2OO reactions with SO2, NO2, NO, H2O and CH3CHO as a function of pressure. Phys. Chem. Chem. Phys. 16, 1139–1149 (2014). [DOI] [PubMed] [Google Scholar]
- Su Y.-T. et al. Extremely rapid self-reaction of the simplest Criegee intermediate CH2OO and its implications in atmospheric chemistry. Nat. Chem. 6, 477–483 (2014). [DOI] [PubMed] [Google Scholar]
- Liu Y., Bayes K. D. & Sander S. P. Measuring rate constants for reactions of the simplest Criegee intermediate (CH2OO) by monitoring the OH radical. J. Phys. Chem. A. 118, 741–747 (2014). [DOI] [PubMed] [Google Scholar]
- Buras Z. J., Elsamra R. M. I. & Green W. H. Direct determination of the simplest Criegee intermediate (CH2OO) self reaction rate. J. Phys. Chem. Lett. 5, 2224–2228 (2014). [DOI] [PubMed] [Google Scholar]
- Ting W.-L. et al. Detailed mechanism of the CH2I + O2 reaction: yield and self-reaction of the simplest Criegee intermediate CH2OO. J. Chem. Phys. 141, 104308 (2014). [DOI] [PubMed] [Google Scholar]
- Anglada J. M., González J. & Torrent-Sucarrat M. Effects of the substituents on the reactivity of carbonyl oxides. A theoretical study on the reaction of substituted carbonyl oxides with water. Phys. Chem. Chem. Phys. 13, 13034–13045 (2011). [DOI] [PubMed] [Google Scholar]
- Fenske J. D. et al. The pressure dependence of the OH radical yield from ozone−alkene reactions. J. Phys. Chem. A. 104, 7821–7833 (2000). [Google Scholar]
- Hasson A. S. et al. Reaction of Criegee intermediates with water vapor- an additional source of OH radicals in alkene ozonolysis? J. Phys. Chem. A. 107, 6176–6182 (2003). [Google Scholar]
- Kuwata K. T., Hermes M. R., Carlson M. J. & Zogg C. K. Computational studies of the isomerization and hydration reactions of acetaldehyde oxide and methyl vinyl carbonyl oxide. J. Phys. Chem. A. 114, 9192–9204 (2010). [DOI] [PubMed] [Google Scholar]
- Smith M. C. et al. UV absorption spectrum of the C2 Criegee intermediate CH3CHOO. J. Chem. Phys. 141, 074302 (2014). [DOI] [PubMed] [Google Scholar]
- Taatjes C. A. et al. Direct measurements of conformer-dependent reactivity of the Criegee intermediate CH3CHOO. Science 340, 177–180 (2013). [DOI] [PubMed] [Google Scholar]
- Beames J. M., Liu F., Lu L. & Lester M. I. UV spectroscopic characterization of an alkyl substituted Criegee intermediate CH3CHOO. J. Chem. Phys. 138, 244307 (2013). [DOI] [PubMed] [Google Scholar]
- Sheps L., Scully A. M. & Au K. UV absorption probing of the conformer-dependent reactivity of a Criegee intermediate CH3CHOO. Phys. Chem. Chem. Phys. 16, 26701–26706 (2014). [DOI] [PubMed] [Google Scholar]
- Nakajima M. & Endo Y. Communication: spectroscopic characterization of an alkyl substituted Criegee intermediate syn-CH3CHOO through pure rotational transitions. J. Chem. Phys. 140, 011101 (2014). [DOI] [PubMed] [Google Scholar]
- Liu F., Beames J. M., Petit A. S., McCoy A. B. & Lester M. I. Infrared-driven unimolecular reaction of CH3CHOO Criegee intermediates to OH radical products. Science 345, 1596–1598 (2014). [DOI] [PubMed] [Google Scholar]
- Huang Y.-H. & Lee Y.-P. Infrared absorption of gaseous CH2BrOO detected with a step-scan Fourier-transform absorption spectrometer. J. Chem. Phys. 141, 164302 (2014). [DOI] [PubMed] [Google Scholar]
- Schmitt G. & Comes F. J. Photolysis of CH2I2 and 1,1-C2H4I2 at 300 nm. J. Photochem. 14, 107–123 (1980). [Google Scholar]
- Enami S. et al. Formation of the iodine monoxide radical from gas-phase reactions of iodoalkyl radicals with molecular oxygen. Chem. Phys. Lett. 445, 152–156 (2007). [Google Scholar]
- Bowman J. M., Carter S. & Huang X. MULTIMODE: a code to calculate rovibrational energies of polyatomic molecules. Int. Rev. Phys. Chem. 22, 533–549 (2003). [Google Scholar]
- Braams B. J. & Bowman J. M. Permutationally invariant potential energy surfaces in high dimensionality. Int. Rev. Phys. Chem. 28, 577–606 (2009). [DOI] [PubMed] [Google Scholar]
- Bowman J. M., Wang X. & Homayoon Z. Ab initio computational spectroscopy and vibrational dynamics of polyatomic molecules: Applications to syn and anti-CH3CHOO and NO3. J. Mol. Spectrosc. 311, 2–11 (2015). [Google Scholar]
- Western C. M. PGOPHER, a Program for Simulating Rotational Structure University of Bristol UK (2010) version 7.1.108 http://pgopher.chm.bris.ac.uk. [Google Scholar]
- Li J. et al. High-level, first-principles, full-dimensional quantum calculation of the ro-vibrational spectrum of the simplest Criegee intermediate (CH2OO). J. Phys. Chem. Lett. 5, 2364–2369 (2014). [DOI] [PubMed] [Google Scholar]
- Møller C. & Plesset M. S. Note on an approximation treatment for many-electron systems. Phys. Rev. 46, 618–622 (1934). [Google Scholar]
- Angeli C., Cimiraglia R., Evangelisti S., Leininger T. & Malrieu J.-P. Introduction of n-electron valence states for multireference perturbation theory. J. Chem. Phys. 114, 10252 (2001). [Google Scholar]
- Werner H.-J., Knowles P. J., Knizia G., Manby F. R. & Schütz M. Molpro: a general-purpose quantum chemistry program package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2, 242–253 (2012). [Google Scholar]
- Neff M. & Rauhut G. Toward large scale vibrational configuration interaction calculations. J. Chem. Phys. 131, 124129 (2009). [DOI] [PubMed] [Google Scholar]
- Adler T. B., Knizia G. & Werner H.-J. A simple and efficient CCSD(T)-F12 approximation. J. Chem. Phys. 127, 221106 (2007). [DOI] [PubMed] [Google Scholar]
- Knizia G., Adler T. B. & Werner H.-J. Simplified CCSD(T)-F12 methods: theory and benchmarks. J. Chem. Phys. 130, 054104 (2009). [DOI] [PubMed] [Google Scholar]
- Bowman J. M., Czakó G. & Fu B. High-dimensional ab initio potential energy surfaces for reaction dynamics calculations. Phys. Chem. Chem. Phys. 13, 8094–8111 (2011). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-7, Supplementary Tables 1-10, Supplementary Methods and Supplementary References