Abstract
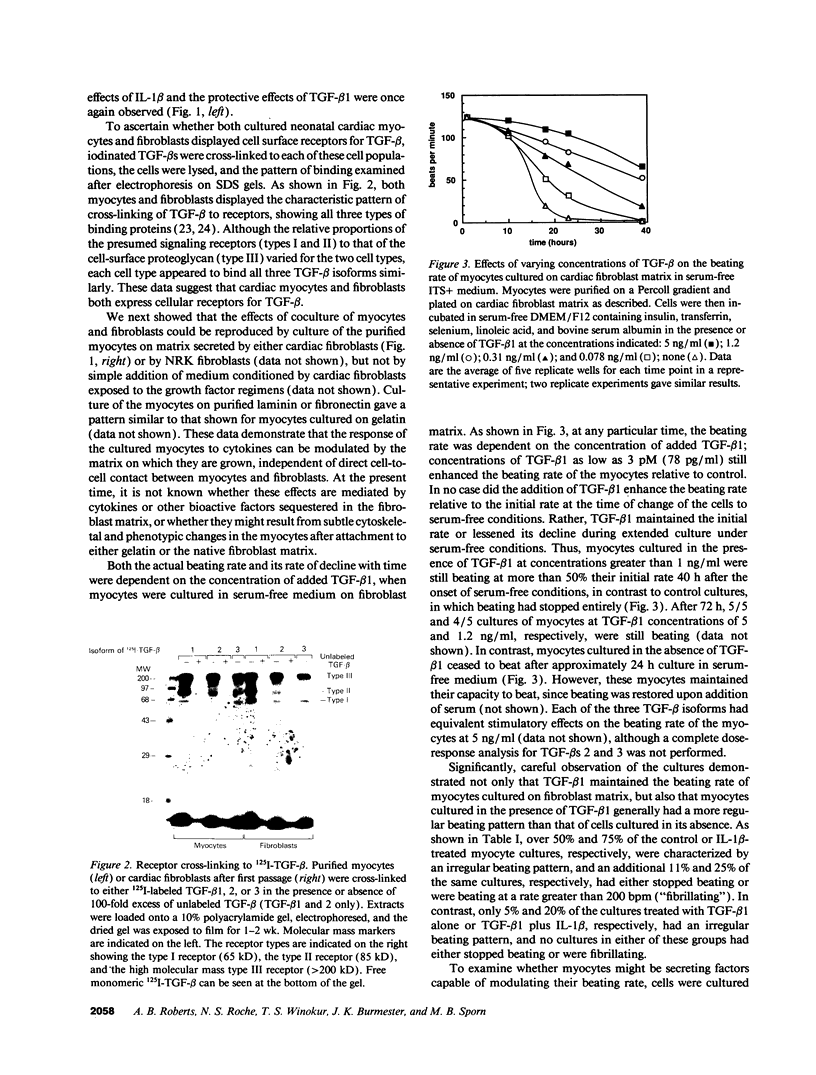
The three isoforms of transforming growth factor-beta (TGF-beta) have previously been implicated in embryonic development of the heart as well as in repair of myocardial damage after ischemia/reperfusion injury. TGF-beta 1 has also been localized intracellularly to both mitochondria and contractile filaments of cardiac myocytes, although its role in these structures has not been defined. We now report that exogenous TGF-beta stabilizes the beating rate of neonatal rat cardiac myocytes cultured on fibroblast matrix, and sustains their spontaneous rhythmic beating in serum-free medium. Moreover, using blocking antibodies to TGF-beta, we show that endogenous TGF-beta secreted by these myocytes acts in an autocrine fashion to maintain their beating rate. In contrast, IL-1 beta, an inflammatory mediator secreted by immune cells during myocardial injury, inhibits the beating of cardiac myocytes, and TGF-beta can overcome this inhibition. The antagonistic effects of TGF-beta and IL-1 were not observed when the myocytes were cultured on gelatin, as compared to native fibroblast matrix. The data indicate that TGF-beta is an important regulator of contractile function of the heart and have significant implications for understanding cardiac physiology in health and disease.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., Lehnert S. A., Faissner A., Duffie E. TGF beta in murine morphogenetic processes: the early embryo and cardiogenesis. Development. 1990 Apr;108(4):645–656. doi: 10.1242/dev.108.4.645. [DOI] [PubMed] [Google Scholar]
- Andres J. L., Stanley K., Cheifetz S., Massagué J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J Cell Biol. 1989 Dec;109(6 Pt 1):3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. B., Bittner V. Cardiac matrix alterations induced by adriamycin. Am J Pathol. 1988 Nov;133(2):298–305. [PMC free article] [PubMed] [Google Scholar]
- Caulfield J. B., Wolkowicz P. Inducible collagenolytic activity in isolated perfused rat hearts. Am J Pathol. 1988 May;131(2):199–205. [PMC free article] [PubMed] [Google Scholar]
- Collins F. Neurite outgrowth induced by the substrate associated material from nonneuronal cells. Dev Biol. 1980 Sep;79(1):247–252. doi: 10.1016/0012-1606(80)90089-5. [DOI] [PubMed] [Google Scholar]
- Danielpour D., Kim K. Y., Dart L. L., Watanabe S., Roberts A. B., Sporn M. B. Sandwich enzyme-linked immunosorbent assays (SELISAs) quantitate and distinguish two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) in complex biological fluids. Growth Factors. 1989;2(1):61–71. doi: 10.3109/08977198909069082. [DOI] [PubMed] [Google Scholar]
- Dubois C. M., Ruscetti F. W., Palaszynski E. W., Falk L. A., Oppenheim J. J., Keller J. R. Transforming growth factor beta is a potent inhibitor of interleukin 1 (IL-1) receptor expression: proposed mechanism of inhibition of IL-1 action. J Exp Med. 1990 Sep 1;172(3):737–744. doi: 10.1084/jem.172.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Figari I. S., Ranges G. E., Palladino M. A., Jr Transforming growth factor-beta 1 (TGF-beta 1) and recombinant human tumor necrosis factor-alpha reciprocally regulate the generation of lymphokine-activated killer cell activity. Comparison between natural porcine platelet-derived TGF-beta 1 and TGF-beta 2, and recombinant human TGF-beta 1. J Immunol. 1988 Apr 1;140(7):2312–2316. [PubMed] [Google Scholar]
- Gamble J. R., Vadas M. A. Endothelial adhesiveness for blood neutrophils is inhibited by transforming growth factor-beta. Science. 1988 Oct 7;242(4875):97–99. doi: 10.1126/science.3175638. [DOI] [PubMed] [Google Scholar]
- Geiser A. G., Burmester J. K., Webbink R., Roberts A. B., Sporn M. B. Inhibition of growth by transforming growth factor-beta following fusion of two nonresponsive human carcinoma cell lines. Implication of the type II receptor in growth inhibitory responses. J Biol Chem. 1992 Feb 5;267(4):2588–2593. [PubMed] [Google Scholar]
- Goldring M. B., Krane S. M. Modulation by recombinant interleukin 1 of synthesis of types I and III collagens and associated procollagen mRNA levels in cultured human cells. J Biol Chem. 1987 Dec 5;262(34):16724–16729. [PubMed] [Google Scholar]
- Gulick T., Chung M. K., Pieper S. J., Lange L. G., Schreiner G. F. Interleukin 1 and tumor necrosis factor inhibit cardiac myocyte beta-adrenergic responsiveness. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6753–6757. doi: 10.1073/pnas.86.17.6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey A. K., Hrubey P. S., Chandrasekhar S. Transforming growth factor-beta inhibition of interleukin-1 activity involves down-regulation of interleukin-1 receptors on chondrocytes. Exp Cell Res. 1991 Aug;195(2):376–385. doi: 10.1016/0014-4827(91)90387-a. [DOI] [PubMed] [Google Scholar]
- Heine U. I., Burmester J. K., Flanders K. C., Danielpour D., Munoz E. F., Roberts A. B., Sporn M. B. Localization of transforming growth factor-beta 1 in mitochondria of murine heart and liver. Cell Regul. 1991 Jun;2(6):467–477. doi: 10.1091/mbc.2.6.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J., Heinonen T. Interleukin-1 beta prevents the stimulatory effect of transforming growth factor-beta on collagen gene expression in human skin fibroblasts. Biochem J. 1990 Nov 1;271(3):827–830. doi: 10.1042/bj2710827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heino J., Ignotz R. A., Hemler M. E., Crouse C., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Concomitant regulation of integrins that share a common beta 1 subunit. J Biol Chem. 1989 Jan 5;264(1):380–388. [PubMed] [Google Scholar]
- Ignotz R. A., Heino J., Massagué J. Regulation of cell adhesion receptors by transforming growth factor-beta. Regulation of vitronectin receptor and LFA-1. J Biol Chem. 1989 Jan 5;264(1):389–392. [PubMed] [Google Scholar]
- Ignotz R. A., Massagué J. Cell adhesion protein receptors as targets for transforming growth factor-beta action. Cell. 1987 Oct 23;51(2):189–197. doi: 10.1016/0092-8674(87)90146-2. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Miller J. C., Hagler H. K., Chien K. R., Willerson J. T., Buja L. M. Association between inhibition of arachidonic acid release and prevention of calcium loading during ATP depletion in cultured rat cardiac myocytes. Am J Pathol. 1989 Sep;135(3):541–556. [PMC free article] [PubMed] [Google Scholar]
- Lefer A. M. Mechanisms of the protective effects of transforming growth factor-beta in reperfusion injury. Biochem Pharmacol. 1991 Sep 12;42(7):1323–1327. doi: 10.1016/0006-2952(91)90441-7. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P., Aoki N., Palladino M. A., Jr Mediation of cardioprotection by transforming growth factor-beta. Science. 1990 Jul 6;249(4964):61–64. doi: 10.1126/science.2164258. [DOI] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Tucker A. M. Phenotypic modulation of endothelial cells by transforming growth factor-beta depends upon the composition and organization of the extracellular matrix. J Cell Biol. 1988 Apr;106(4):1375–1384. doi: 10.1083/jcb.106.4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- Musso T., Espinoza-Delgado I., Pulkki K., Gusella G. L., Longo D. L., Varesio L. Transforming growth factor beta downregulates interleukin-1 (IL-1)-induced IL-6 production by human monocytes. Blood. 1990 Dec 15;76(12):2466–2469. [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J., Pignat W., Leighton J., Märki F., Vosbeck K., Alkan S. Transforming growth factor beta 2 differentially modulates interleukin-1 beta- and tumour-necrosis-factor-alpha-stimulated phospholipase A2 and prostaglandin E2 synthesis in rat renal mesangial cells. Biochem J. 1990 Aug 15;270(1):269–271. doi: 10.1042/bj2700269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. D., Dagle J. M., Walder J. A., Weeks D. L., Runyan R. B. Epithelial-mesenchymal transformation of embryonic cardiac endothelial cells is inhibited by a modified antisense oligodeoxynucleotide to transforming growth factor beta 3. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1516–1520. doi: 10.1073/pnas.88.4.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potts J. D., Runyan R. B. Epithelial-mesenchymal cell transformation in the embryonic heart can be mediated, in part, by transforming growth factor beta. Dev Biol. 1989 Aug;134(2):392–401. doi: 10.1016/0012-1606(89)90111-5. [DOI] [PubMed] [Google Scholar]
- Qian S. W., Burmester J. K., Merwin J. R., Madri J. A., Sporn M. B., Roberts A. B. Identification of a structural domain that distinguishes the actions of the type 1 and 2 isoforms of transforming growth factor beta on endothelial cells. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6290–6294. doi: 10.1073/pnas.89.14.6290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Anzano M. A., Lamb L. C., Smith J. M., Sporn M. B. New class of transforming growth factors potentiated by epidermal growth factor: isolation from non-neoplastic tissues. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5339–5343. doi: 10.1073/pnas.78.9.5339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A. B., Heine U. I., Flanders K. C., Sporn M. B. Transforming growth factor-beta. Major role in regulation of extracellular matrix. Ann N Y Acad Sci. 1990;580:225–232. doi: 10.1111/j.1749-6632.1990.tb17931.x. [DOI] [PubMed] [Google Scholar]
- Schneider M. D., Parker T. G. Cardiac growth factors. Prog Growth Factor Res. 1991;3(1):1–26. doi: 10.1016/0955-2235(91)90010-2. [DOI] [PubMed] [Google Scholar]
- Segarini P. R. TGF-beta receptors. Ciba Found Symp. 1991;157:29–50. [PubMed] [Google Scholar]
- Thompson N. L., Bazoberry F., Speir E. H., Casscells W., Ferrans V. J., Flanders K. C., Kondaiah P., Geiser A. G., Sporn M. B. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988;1(1):91–99. doi: 10.3109/08977198809000251. [DOI] [PubMed] [Google Scholar]
- Thompson N. L., Flanders K. C., Smith J. M., Ellingsworth L. R., Roberts A. B., Sporn M. B. Expression of transforming growth factor-beta 1 in specific cells and tissues of adult and neonatal mice. J Cell Biol. 1989 Feb;108(2):661–669. doi: 10.1083/jcb.108.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner M., Chantry D., Katsikis P., Berger A., Brennan F. M., Feldmann M. Induction of the interleukin 1 receptor antagonist protein by transforming growth factor-beta. Eur J Immunol. 1991 Jul;21(7):1635–1639. doi: 10.1002/eji.1830210708. [DOI] [PubMed] [Google Scholar]
- Vincent R., Nadeau D. Adjustment of the osmolality of Percoll for the isopycnic separation of cells and cell organelles. Anal Biochem. 1984 Sep;141(2):322–328. doi: 10.1016/0003-2697(84)90049-6. [DOI] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., Wong H. L., Dougherty S. F., Ellingsworth L. R. Antagonistic and agonistic effects of transforming growth factor-beta and IL-1 in rheumatoid synovium. J Immunol. 1990 Oct 15;145(8):2514–2519. [PubMed] [Google Scholar]
- Weber K. T. Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol. 1989 Jun;13(7):1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y., Mann D. M., Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990 Jul 19;346(6281):281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yayon A., Klagsbrun M., Esko J. D., Leder P., Ornitz D. M. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991 Feb 22;64(4):841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]