Abstract
Activation of inflammasomes is an important aspect of innate immune responses to bacterial infection. Recent studies have linked Vibrio cholerae secreted toxins to inflammasome activation by using murine macrophages. To increase relevance to human infection, studies of inflammasome-dependent cytokine secretion were conducted with the human THP-1 monocytic cell line and corroborated in primary human peripheral blood mononuclear cells (PBMCs). Both El Tor and classical strains of V. cholerae activated ASC (apoptosis-associated speck-like protein-containing a CARD domain)-dependent release of interleukin-1β (IL-1β) when cultured with human THP-1 cells, but the pattern of induction was distinct, depending on the repertoire of toxins the strains produced. El Tor biotype strains induced release of IL-1β dependent on NOD-like receptor family pyrin domain-containing 3 (NLRP3) and ASC due to the secreted pore-forming toxin hemolysin. Unlike in studies with mouse macrophages, the MARTX toxin did not contribute to IL-1β release from human monocytic cells. Classical biotype strains, which do not produce either hemolysin or the MARTX toxin, activated low-level IL-1β release that was induced by cholera toxin (CT) and dependent on ASC but independent of NLRP3 and pyroptosis. El Tor strains likewise showed increased IL-1β production dependent on CT when the hemolysin gene was deleted. In contrast to studies with murine macrophages, this phenotype was dependent on a catalytically active CT A subunit capable of inducing production of cyclic AMP and not on the B subunit. These studies demonstrate that the induction of the inflammasome in human THP-1 monocytes and in PBMCs by V. cholerae varies with the biotype and is mediated by both NLRP3-dependent and -independent pathways.
INTRODUCTION
Vibrio cholerae is the causative agent of the diarrheal disease cholera. The O1 serogroup, which is most associated with disease, is divided into two biotypes, classical and El Tor. Classical strains were responsible for the six cholera pandemics that occurred during the 19th century and the first half of the 20th century and are noted for their severe clinical presentation. In 1961, the El Tor strains emerged as the cause of the ongoing seventh pandemic, completely displacing the classical strains from the environment and as a cause of cholera in humans (1). These strains colonize the intestine more effectively and spread between hosts more efficiently but cause fewer deaths and significantly more asymptomatic colonization (2).
The primary virulence factor for pandemic cholera strains is cholera toxin (CT), an ADP-ribosylating toxin that disables the Gαs subunit, resulting in increased cyclic AMP (cAMP) production and secretion of fluid from enterocytes due to the opening of chloride channels. The toxin is composed of the catalytically active A (CTA) subunit associated with five CTB subunits that bind to surface GM1 gangliosides, facilitating toxin endocytosis (3). In addition to enterotoxigenic activity, CT has also been demonstrated to have immunomodulatory effects (4). In mice infected with V. cholerae, CT is known to skew the immune response to promote bacterial survival by modulating the neutrophil response (5).
In addition to CT, strains of the El Tor biotype secrete accessory toxins, including the pore-forming toxin hemolysin, the actin cross-linking multifunctional autoprocessing repeats-in-toxin (MARTX) toxin, and the secreted metalloprotease hemagglutinin (HA)/protease (6, 7). These factors have been linked to evasion of neutrophil clearance during infection, facilitating colonization of the small intestine (8–10). In contrast, classical strains are not hemolytic because of a deletion within the hlyA gene (11–13) and do not induce actin cross-linking because of a large deletion that removes part of the rtxA gene (14). Given the difference in the toxin secretion profiles and the fact that CT, hemolysin, and the MARTX toxin all control innate immunity and thereby intestinal colonization, it is possible that the strains of different biotypes may not consistently stimulate the same pathways within the host innate immune system. In this study, we examined differences between the biotypes in the induction of inflammasome-dependent production of interleukin-1β (IL-1β).
Inflammasomes are multiprotein complexes found in monocytic-lineage cells. They are classified in part by specific cytosolic pathogen recognition receptors (PRR) that, in response to danger signals, assemble to activate caspase-1. The PRR NOD-like receptor family pyrin domain-containing 3 (NLRP3) is known to respond to stimuli, including efflux of potassium (K+), that can be induced by pore-forming toxins. NLRP3 then can activate caspase-1 via the ASC (apoptosis-associated speck-like protein containing a CARD domain) adaptor protein. Active caspase-1 cleaves and activates proinflammatory cytokines IL-1β and IL-18, leading to their secretion, and induces pyroptosis. These cytokines, in turn, recruit monocytes, macrophages, and neutrophils to the site of infection and induce Th1 and Th17 adaptive immunity (15, 16). The V. cholerae accessory toxins hemolysin and the MARTX toxin have both been shown to activate the NLRP3 inflammasome in murine macrophages, resulting in the processing and secretion of IL-1β (17).
Noncanonical activation of IL-1β in mouse macrophages is primed by Toll-like receptor 4 (TLR4)-TRIF-IFNAR and activated by cytosolic lipopolysaccharide (LPS) binding caspase-11 in mice and caspase-4/5 in humans (18–23). Secreted CTB has been shown to act as a carrier of O111:B4 LPS into the cytosol, leading to activation of caspase-11 and resulting in IL-1β release (18, 22).
In this study, to increase relevance to human infection, we examined secreted factors of V. cholerae known to contribute to inflammasome activation in murine macrophages for similar effects in human THP-1 monocyte-like cells and primary human peripheral blood mononuclear cells (PBMCs). We found that hemolysin secreted by El Tor strains rapidly stimulates release of IL-1β dependent on NLRP3 and ASC. However, the MARTX toxin does not stimulate IL-1β release. We further found that the catalytically active CT holotoxin, but not CTB, induces ASC-dependent but NLRP3-independent release of IL-1β and that this occurs both for the nonhemolytic classical strains and for El Tor strains modified to remove the hemolysin gene. These findings with CT and the MARTX toxin are distinct from studies with mouse macrophages (18, 20, 22), suggesting that the MARTX toxin and CT produced by V. cholerae induce innate immune responses in human monocytes by a pathway distinct from those characterized in murine macrophages.
MATERIALS AND METHODS
Bacterial strains, growth media, and plasmids.
The bacterial strains used in this study are listed in Table 1. V. cholerae was grown in Luria-Bertani (LB) medium or agar supplemented with 100 μg/ml streptomycin or ampicillin, as appropriate.
TABLE 1.
V. cholerae strains used in this study
| Strain | Relevant description | Reference and/or source |
|---|---|---|
| N16961 | Wild type El Tor Inaba (Bangladesh) | 49 |
| P27459 | Wild type El Tor Inaba (Bangladesh) | 50 |
| E7946 | Wild type El Tor Ogawa (Bahrain) | 50 |
| 9459 | Wild type Classical (ATCC 9459) | Fisher Scientific, 51 |
| O395 | Wild type Classical (India) | 52 |
| P4 | P27459ΔctxAB | 53 |
| E4 | E7946ΔctxAB | J. Mekalanos |
| O395NT | O395ΔctxAB | 52 |
| O395N1 | O395ΔctxA | 52 |
| KFV103 | P4ΔhlyA | 24 |
| KFV105 | P4ΔrtxA | 24 |
| KFV70 | P4ΔhapA | 24 |
| KFV98 | KFV70ΔrtxA | 7 |
| KFV101 | KFV70ΔrtxAΔhlyA | 24 |
| VOV3 | P4ΔrtxAΔhlyA | 7 |
| VOV8 | P27459ΔhlyA | This study |
Strain VOV8 was generated for this study by using plasmids and methods described previously (24). For plasmid-based production of CT, the ctxAB operon was amplified from strain P27459 chromosomal DNA with oligonucleotides JQctxAB-F/NcoI (CCATGGTAAAGATAATATTTGTGTTTTTTATTTTCC) and JQctxAB-R/EcoRV (GATATCTTAATTTGCCATACTAATTGCGG) (the NcoI and EcoRI restriction sites are underlined). The 1.15-kb product was captured in the PBAD TOPO TA vector (Life Technologies), which allows expression under arabinose-inducible control of the araBAD promoter. The resulting plasmid, PBAD-ctxAB, was then modified by site-directed mutagenesis to change the ctxA codon at position E112 to lysine (codon shift from GAA to AAA). The resulting plasmid was transferred by electroporation at 1,800 V in a 0.1-cm cuvette into freshly cultured V. cholerae KFV101 washed three times in 2 mM CaCl2. After 1 h of outgrowth, bacteria with plasmids were selected on LB agar supplemented with streptomycin and ampicillin.
THP-1 cells.
Human monocytic leukemic THP-1 cells (C. Stehlik, lab collection) were cultured in RPMI 1640 (Life Technologies) supplemented with 10% fetal bovine serum (FBS). Stable THP-1shASC (ASCdef THP-1) cells were previously generated by lentiviral transduction with pLKO.1-based vectors (ASC sequence 5′-GCTCTTCAGTTTCACACCA-3′ and a nontargeting control sequence [Sigma]). Envelope and packaging plasmids were produced in HEK293T cells by puromycin selection (25, 26). THP-1 cells deficient in NLRP3 (NLRP3def THP-1) were purchased from InvivoGen and cultured in supplemented RPMI as described above, with 200 μg/ml hygromycin added at every other passage, in accordance with the manufacturer's instructions.
Monocyte isolation.
Human PBMCs were isolated by Ficoll-Hypaque centrifugation (Sigma) from healthy donor blood after informed consent was obtained under a protocol approved by the Northwestern University Institutional Review Board. Monocytes were isolated from PBMCs by countercurrent centrifugal elutriation in the presence of 10 μg/ml polymyxin B with a JE-6B rotor (Beckman Coulter) as described previously (27). Monocytes were washed in Hanks balanced salt solution and resuspended in RPMI medium supplemented with 20% FBS. Monocytes were routinely phenotyped to ensure >85% purity, as determined by flow cytometry for CD45 and CD14 (27).
Bacterial and LPS/CT treatment of THP-1 cells and PBMCs.
Liquid cultures of V. cholerae were grown from single colonies in LB medium with appropriate antibiotics overnight at 30°C with shaking. Overnight cultures were subcultured 1:1,000 in LB medium with antibiotics at 37°C with shaking until mid-log phase (A600 of ∼0.5). Bacterial pellets were washed twice and diluted in phosphate-buffered saline (PBS) to the appropriate number of CFU/ml by using A600 to determine culture density. A 50-μl volume of a V. cholerae suspension was added at a multiplicity of infection (MOI) of 25 to 5 × 105 to 1 × 106 THP-1 cells or PBMCs seeded in RPMI 1640 supplemented with 1% FBS without antibiotics in 12-well culture dishes. Plates were centrifuged at 500 × g for 5 min and then incubated at 37°C in 5% CO2 for 3 h, at which point supernatant fluids were collected and assayed. For 16-h experiments, after a 3-h bacterial treatment, the medium was replaced with fresh culture medium containing 100 μg/ml gentamicin and supernatant fluids were assayed at 13 h postinfection. For assays when agents were administered prior to the addition of bacteria, 5 μg of either CT or CTB, 50 μg of forskolin dissolved in dimethyl sulfoxide (DMSO), or the equivalent volume of DMSO as a vehicle control was added to cells for 1 h and then the cells were treated with bacteria as described above. All other treatments were conducted as indicated in the figure legends.
Immunoblotting.
THP-1 cells were washed in PBS and then resuspended, boiled, subjected to SDS-PAGE, transferred to Immobilon polyvinylidene difluoride membranes (Millipore), and probed with the appropriate antibodies; this was followed by chemiluminescence detection by ECL reagents (Thermo Scientific). Western blotting for actin cross-linking was performed with THP-1 cells seeded at 2 × 106/ml after 90 min of bacterial treatment with the strains indicated at an MOI of 25 with rabbit anti-actin monoclonal antibody (Sigma). Lysates were analyzed for caspase-1 after 5 min of infection at an MOI of 25 with a mouse anti-caspase 1 monoclonal antibody (Imgenex). For loading of a control, the blot was stripped and then reprobed with polyclonal rabbit anti-actin antibody (Sigma). Lysates were analyzed for pro-IL-1β after 3 h of infection at an MOI of 25 with a rabbit anti-IL-1β polyclonal antibody (Santa Cruz Biotechnology, Inc.). Western blotting for ASC was performed with cellular lysates from untreated cells with a custom-raised rabbit polyclonal antibody with a peptide covering amino acids 93 through 111 of ASC (25), with actin as a loading control.
IL-1β enzyme-linked immunosorbent assay (ELISA).
Following treatment, supernatant fluids were collected and any recovered THP-1 cells or PBMCs were pelleted at 500 × g; this was followed by a second centrifugation at 13,700 × g to remove bacteria. Supernatant fluids were stored at −20°C until analyzed. All samples were analyzed by quantitative sandwich enzyme colorimetric immunoassays according to the manufacturer's instructions (R&D Systems), and the plates were read on a Molecular Devices SpectraMax M5 microplate reader at 450 nm and IL-1β was quantified in picograms per milliliter on the basis of a standard curve.
CT ELISA.
A total of 5 × 107 CFU of mid-log-phase PBS-washed cultures prepared as described above were added to 1 ml of RPMI 1640 with 1% FBS and grown statically at 37°C for 3 h. For strains requiring arabinose induction, bacteria were grown in RPMI medium without glucose and supplemented with 1% arabinose to induce ctxAB gene expression. Supernatant fluids were cleared of bacteria by centrifugation at 16,000 × g for 1 min and assayed with Nunc-Immuno 96-well microtiter plates precoated with bovine monosialoganglioside GM1 (Sigma) as previously described (28). Supernatant fluids were incubated in the microtiter plates, probed with rabbit anti-CT serum (Sigma), alkaline phosphatase-conjugated goat-anti-rabbit secondary antibody (Sigma), and p-nitrophenylphosphate (SIGMAFAST p-nitrophenylphosphate tablets; Sigma). Color was allowed to develop for 6 min, and the plate was read with a Molecular Devices SpectraMax M5 microplate reader at 450 nm. CT was quantified in nanograms per milliliter on the basis of a CT standard curve prepared from toxin serially diluted in water.
Hemolysis assays.
Hemolysis was determined by streaking on blood agar plates prepared from tryptose agar base (Difco) agar mixed with 5% sheep blood cells (Lampire Biological Products). For liquid assays, sheep blood cells at a concentration of 5% in PBS were washed three times at 2,000 × g for 10 min. Fifty microliters of LB culture supernatant fluids from V. cholerae grown until mid-log phase at 37°C was collected and incubated in a 96-well plate in triplicate with 50 μl of 5% sheep red blood cells in a 96-well microtiter dish. Plates were incubated at 37°C for 4 h. The plate was spun at 1,000 × g for 5 min to pellet the intact red blood cells. Percent hemolysis was quantified by dividing the sample A540 (minus the A540 for lysis obtained with LB medium alone) by the A540 upon 100% lysis by 1% Triton X-100.
cAMP production in cells cocultured with V. cholerae.
Bacterial treatment was done as described above, except that 107 THP-1 cells were seeded into 12-well tissue culture dishes. Two hours prior to bacterial addition, cells were washed three times with PBS and the medium was changed to phenol red-free RPMI without antibiotics containing 1% FBS and 1% arabinose for induction of the araBAD promoter. After the addition of bacteria, plates were centrifuged at 500 × g for 5 min. The plates were then incubated at 37°C in 5% CO2 for 3 h. Cells were collected, washed once in PBS, and then lysed with 500 μl of PBS with 1% Triton X-100 at 37°C for 10 min, and 100 μl of lysate was analyzed with the Enzo Life Sciences cAMP Complete ELISA according to the manufacturer's protocol, except for the use of the alternative lysis buffer indicated above to lyse the cells. To generate a positive control for the assay, 10 μM forskolin was added to an extra well 30 min prior to the end of the incubation period and assayed for maximum cAMP production in parallel with the other samples. Plates were read on a Molecular Devices SpectraMax M5 plate reader, and concentrations (in picograms per milliliter) were determined on the basis of a cAMP standard curve. Measured cAMP levels were normalized to the amount of protein present in the sample determined with Precision Red Advanced Protein Assay Reagent (Cytoskeleton, Inc.) in accordance with the manufacturer's recommendations.
Statistical analysis.
All statistical analyses were performed with GraphPad Prism 4 or 6 for Macintosh software (GraphPad Software Inc., San Diego, CA). ELISA data were analyzed by two-tailed Student t test. P values of <0.05 were considered statistically significant.
RESULTS
V. cholerae El Tor, but not classical, strains induce rapid ASC-dependent secretion of IL-1β.
To initiate this study, undifferentiated THP-1 monocytic cells were incubated with various wild-type V. cholerae strains. Cells were not primed with LPS prior to the addition of bacteria since V. cholerae can activate NF-κB without priming and this activation does not depend on secreted toxins (see Fig. S1 in the supplemental material).
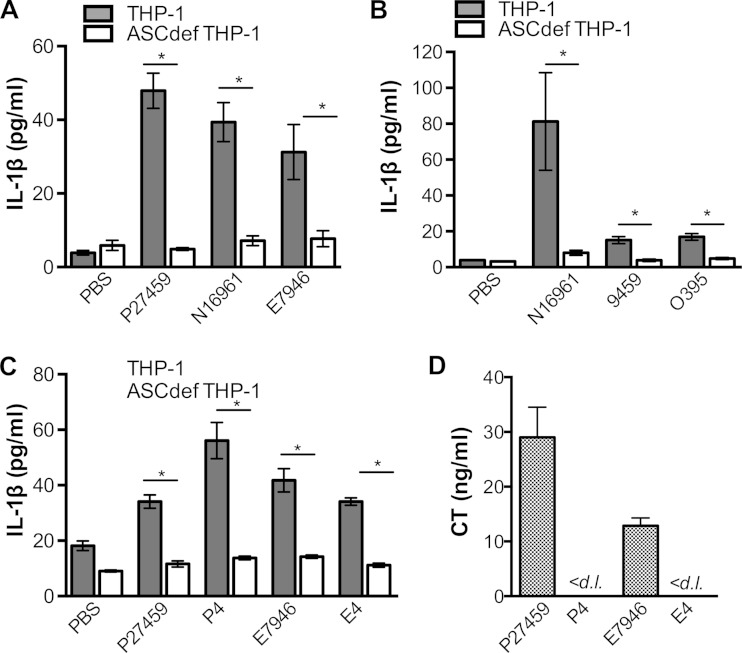
When incubated with undifferentiated THP-1 monocytes for 3 h at an MOI of 25, all of the wild-type V. cholerae El Tor biotype strains tested showed measurably greater IL-1β secretion than cells mock inoculated with PBS (Fig. 1A). The levels detected are lower than those in other studies, as they are due solely to stimulation by V. cholerae and, because of the absence of priming, are not also induced by exogenously added Escherichia coli LPS. This stimulation of IL-1β secretion occurred irrespective of whether the El Tor strains produced the Inaba O-antigen serotype (N16961 and P27459) or the Ogawa O-antigen serotype (E7946) (Fig. 1A). Parallel assays with small interfering RNA ASC knockdown cells (confirmed by Western blotting not to produce the adaptor protein ASC; see Fig. S2 in the supplemental material) showed that the increase in IL-1β release was dependent upon ASC, linking IL-1β secretion levels to the activation of inflammasomes (Fig. 1A).
FIG 1.
Rapid, ASC-dependent, CT-independent secretion of IL-1β from THP-1 cells is induced by El Tor, but not classical, V. cholerae strains. THP-1 (gray bars) or ASCdef THP-1 (white bars) cells were treated at an MOI of 25 with wild-type V. cholerae El Tor strain P27459, N16961, or E7946; wild-type classical strain 9459 or O395; or ΔctxAB mutant P4 (derived from P27459) or E4 (derived from E7946). (A to C) Supernatant fluid from cells treated with bacteria for 3 h were collected and assayed for IL-1β by ELISA. (D) CT secretion into RPMI culture medium was measured by ELISA after 3 h of growth. Data are presented as the mean ± the standard deviation of triplicate samples. *, P < 0.05; <d. l., below the detection limit.
In comparison, with two different isolates, classical strains 9459 and O395 induced much less IL-1β secretion from THP-1 monocytes than did El Tor strain N16961 under our test conditions (Fig. 1B). The difference in the abilities of these strains to induce IL-1β production was not due to differences in bacterial growth rates, as all of the strains grew equally well in culture medium (see Fig. S3A in the supplemental material). This difference was also not due to unexpected lysis from pyroptosis due to treatment with classical strains, as the cells were not lysed in this time frame (see Fig. S3B). Thus, there is a distinct quantitative difference between El Tor and classical strains in the induction of ASC-dependent IL-1β secretion from THP-1 cells, independent of their growth parameters.
Rapid secretion of IL-1β from THP-1 monocytic cells is independent of CT.
Previous studies have shown that purified CTB, when added to murine macrophages, can serve as a carrier of exogenously added E. coli LPS into the cytosol, resulting in caspase-11-dependent noncanonical activation (22). Noncanonical activation of IL-1β is primed by TLR4-TRIF-IFNAR and cytosolic LPS to activate alternative caspase-11 in mice and is marked by potent cell lysis (18–20). Caspase-4, a human functional ortholog of murine caspase-11, was shown to recognize and bind intracellular LPS, leading to oligomerization and activation of caspase-4, similar to caspase-11 in murine bone marrow-derived macrophages. Indeed, caspase-4 could complement a caspase-11 knockout phenotype, indicating that the noncanonical pathway is conserved in mice and humans (23).
However, similar activation of IL-1β secretion does not occur when CT and CTB are added to undifferentiated THP-1 cells in combination with ultrapurified E. coli O111:B4 LPS (see Fig. S4 in the supplemental material). Indeed, the rapid induction of IL-1β secretion by El Tor V. cholerae strains was not due to CT, as complete deletion of both the ctxA and ctxB genes that encode the CTA and CTB subunits, respectively, did not reduce the levels of ASC-dependent IL-1β secretion (Fig. 1C). In addition, the levels of IL-1β secretion did not correlate with naturally varied levels of CT secretion by two different El Tor isolates (Fig. 1D). These data demonstrate that CT is not linked to the rapid release of IL-1β from THP-1 cells.
Rapid secretion of IL-1β from THP-1 cells is due to hemolysin, but not the MARTX toxin or HA/protease.
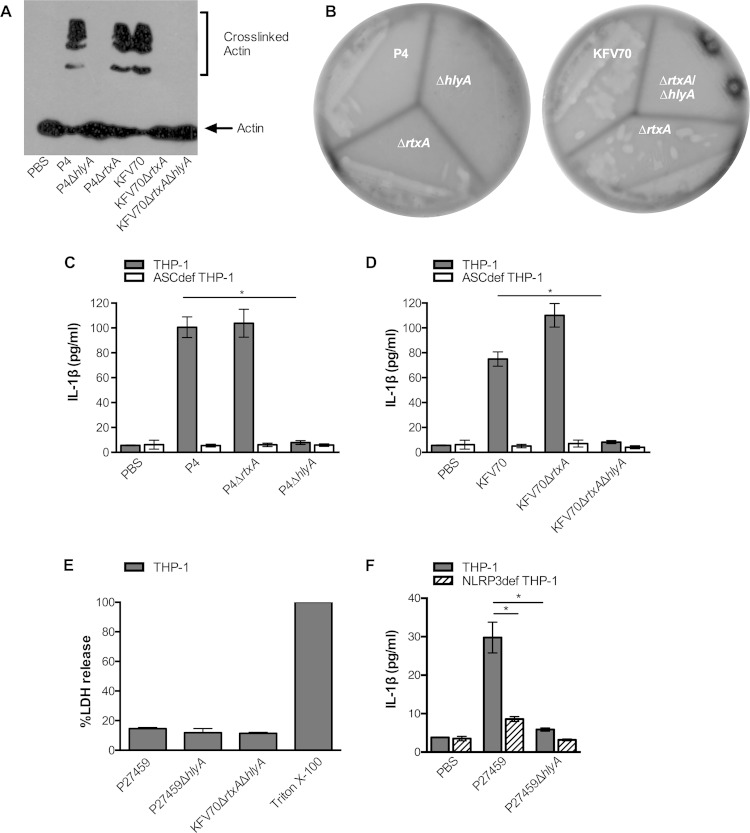
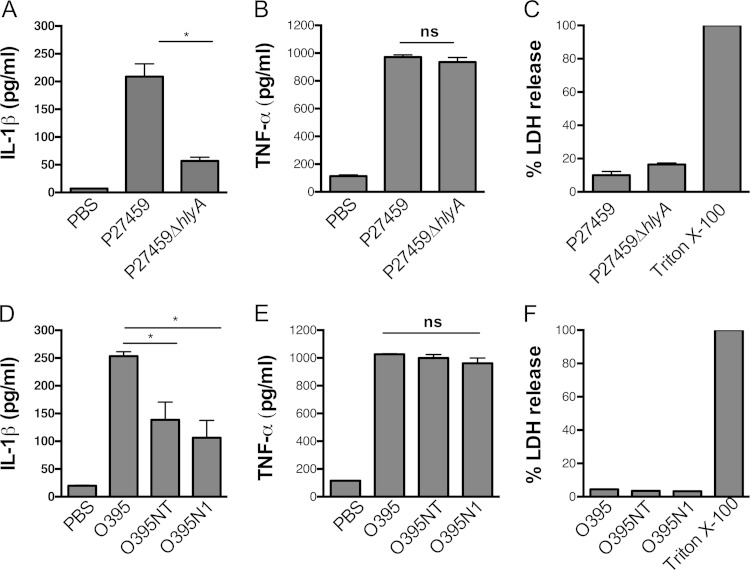
To assess if the accessory toxins account for inflammasome activation in THP-1 cells, as previously shown in murine macrophages (17), V. cholerae P4 (El Tor P27459ΔctxAB) was confirmed to produce the accessory toxins by its abilities to induce covalent cross-linking of actin in THP-1 cells dependent on the MARTX toxin encoded by the rtxA gene (Fig. 2A) and to secrete hemolysin dependent upon the hemolysin gene hlyA (Fig. 2B; see Fig. S5 in the supplemental material).
FIG 2.
Rapid secretion of IL-1β from THP-1 cells induced by V. cholerae El Tor is due to secreted hemolysin and dependent on NLRP3. (A) Western blotting for actin indicating the presence or absence of MARTX toxin-dependent actin laddering in THP-1 cells after treatment with isogenic V. cholerae strains derived from the El Tor wild-type strain P27459, including the ΔctxAB mutant P4 and the ΔctxABΔhapA mutant KFV70 with additional deletions in rtxA or hlyA as indicated. (B) The strains indicated were streaked onto blood agar showing zones of clearing around strains that secrete hemolysin. IL-1β secretion (C, D, and F) or percent lysis as measured by LDH release (E) from THP-1 (gray bars), ASCdef THP-1 (white bars), or NLRP3def THP-1 cells (striped bars) treated with the V. cholerae strains indicated at an MOI of 25 for 3 h. Data are presented as the mean ± the standard deviation of triplicate samples. *, P < 0.05.
When incubated with THP-1 cells, ΔctxAB mutant strain P4 induced IL-1β secretion dependent upon ASC, and additional deletion of the hlyA gene completely abolished ASC-dependent IL-1β secretion (Fig. 2C). This activity was not redundant with the MARTX toxin, since the deletion of rtxA from P4 did not affect the levels of IL-1β secretion (Fig. 2C). Indeed, the P4ΔhlyA mutant strain did not induce IL-1β secretion despite its ability to produce a functional MARTX toxin, as shown by the ability of the strain to efficiently cross-link actin (Fig. 2A).
We also considered if HA/protease could affect IL-1β secretion. This secreted protease is known to decrease the effects of the MARTX toxin and hemolysin during infection (7) and to degrade the MARTX toxin in stationary-phase cultures (29) but has a minimal effect on the MARTX toxin when added to THP-1 cells or hemolysin in plates or liquid culture (Fig. 2A and B; see Fig. S5 in the supplemental material). The absence of the hapA gene also did not alter the ability of El Tor V. cholerae to induce ASC-dependent IL-1β secretion, demonstrating that HA/protease does not contribute to inflammasome activation. In addition, it did not change the conclusion that hemolysin, but not the MARTX toxin, is essential for IL-1β secretion (Fig. 2D).
It is possible that the increased levels of IL-1β detected in supernatant fluids is due to hemolysin-dependent cell lysis that would release pro-IL-1β from cells that would also be detected by ELISA. However, minimal release of lactate dehydrogenase (LDH) was observed upon bacterial treatment and the LDH levels observed were equal in all of the samples tested independent of hlyA (Fig. 2E). Further, these data show that hemolysin induced IL-1β secretion in THP-1 cells is not accompanied by pyroptosis.
The pore-forming hemolysin is known to induce the activation of IL-1β from murine macrophages via the cytosolic PRR NLRP3 (17). To test if hemolysin-stimulated IL-1β production in THP-1 cells is also due to NLRP3, we tested NLRP3def THP-1 cells. These cells were confirmed to lack NLRP3-dependent signaling by the lack of CaCl2-inducible IL-1β production in response to LPS (see Fig. S6 in the supplemental material). Using NLRP3def THP-1 cells, we showed that the IL-1β secretion induced by hemolysin from wild-type El Tor strain P27459 is reduced by the absence of hlyA and that the effect is dependent on NLRP3 (Fig. 2F).
NLRP3 inflammasome activation is a two-step process (30). TLR and NOD2 ligands prime the cell by inducing NF-κB activation, and this activation is independent of the secreted toxins (see Fig. S1 in the supplemental material). Activated NF-κB then mediates the expression of NLRP3, pro-caspase-1, and pro-IL-1β. A second signal is required for assembly of the inflammasome complex, thus leading to the activation of caspase-1. However, in the absence of hemolysin, caspase-1 is not activated (see Fig. S7 in the supplemental material), indicating that hemolysin activates NLRP3 by acting as the second signal that is required for the cleavage of caspase-1. Additionally, deletion of hlyA had no effect on tumor necrosis factor alpha (TNF-α) secretion from THP-1 cells (see Fig. S8 in the supplemental material), further demonstrating that hemolysin specifically activates the inflammasome, as opposed to exerting a nonspecific induction of cytokine expression.
Thus, our studies show that rapid induction of IL-1β secretion by El Tor strains in THP-1 cells is due solely to the pore-forming hemolysin via NLRP3 and ASC and is independent of CT, the MARTX toxin, HA/protease, and all other bacterial factors. This is in contrast to previously published studies with mouse macrophages that had, in addition to hemolysin, linked inflammasome activation to CTB (18, 22) and the MARTX toxin (17).
Classical V. cholerae strains induce low levels of IL-1β secretion dependent on CT and ASC but independent of NLRP3.
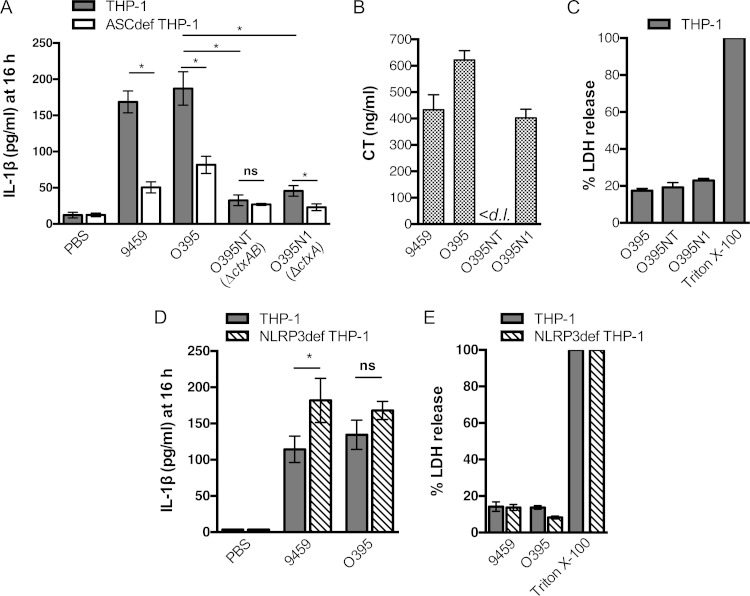
Despite clarity that only hemolysin is linked to rapid NLRP3/ASC-dependent IL-1β secretion from THP-1 cells by El Tor strains, multiple independent studies using murine macrophages have reported that classical V. cholerae strains can activate inflammasomes (18, 20), even though these strains do not produce either hemolysin (11–13) or the MARTX toxin (14) due to naturally occurring inactivating mutations. In addition, studies with phorbol ester-activated THP-1 cells show that CT alone can induce low-level IL-1β secretion (31). However, after 3 h of infection of THP-1 cells, our studies show that classical strains 9459 and O395 induced relatively modest IL-1β secretion compared to an El Tor strain, although the low level of IL-1β was statistically significantly higher than that in ASCdef THP-1 control cells (Fig. 1B). In previous studies that demonstrated the ability of classical strains to induce inflammasome activation, cells were either incubated overnight in purified toxins and LPS (18, 31) or incubated with bacteria for 3 h as ours were, but then the cells were washed and cytokine secretion was assayed after 13 h of further incubation in antibiotic-containing medium to amplify the detection of cytokine accumulation over time (20). Similarly, to amplify the detection of IL-1β secretion in this study, THP-1 cells were incubated with classical strains 9459 and O395 at an MOI of 25 for 3 h, followed by 13 h in gentamicin-containing medium. After the additional 13 h of incubation, high levels of IL-1β secretion were observed. The predominance of the activation was ASC dependent, although ASC-independent activation was also evident (Fig. 3A). The presence of ASC-independent activation in this assay also is evidence that the ASC knockout cells are responsive to V. cholerae stimuli under these conditions.
FIG 3.
Low-level release of IL-1β by classical V. cholerae is due to CT holotoxin. (A, D) IL-1β secretion from THP-1 (gray bars), ASCdef THP-1 (white bars), or NLRP3def THP-1 (striped bars) cells treated at an MOI of 25 with wild-type V. cholerae classical strains 9459 and O395 and the isogenic ΔctxAB mutant O395NT and ΔctxA mutant O395N1 for 3 h, followed by incubation in fresh medium containing gentamicin for 13 h to amplify the detection of low levels of IL-1β release. (B) CT secreted into RPMI medium measured by ELISA. (C, E) Percent lysis of THP-1 (gray bars) and NLRP3def THP-1 cells (striped bars) after 16 h as measured by LDH release. Data are presented as the mean ± the standard deviation of triplicate samples. *, P < 0.05; ns, not statistically significant; <d. l., below the limit of detection of the assay.
We thus revisited whether CT secreted by V. cholerae can activate inflammasomes under these conditions. Deletion of ΔctxAB completely abrogated caspase-1 activation and ASC-dependent secretion of IL-1β, compared to parent strain O395. Interestingly, deletion of only the ctxA gene also significantly inhibited caspase-1 activation and IL-1β release (Fig. 3A), despite ongoing secretion of CTB (Fig. 3; see Fig. S7 in the supplemental material). Thus, the CT holotoxin, but not CTB alone, is responsible for activation of caspase-1 and increased IL-1β secretion from THP-1 cells.
Next, we determined if IL-1β secretion induced by classical strains at 16 h is dependent on the NLRP3 inflammasome, as previously reported in murine macrophages (18). To evaluate this, we incubated THP-1 and NLRP3def THP-1 cells with classical strains 9459 and O395. IL-1β secretion by classical strains occurred entirely independent of NLRP3. In addition, under our assay conditions, we found that all of the classical V. cholerae strains induced only low-level lysis of cells at 16 h for (Fig. 3C), revealing that measured IL-1β secretion is not due to CTA-dependent release of pro-IL-1β from lysed cells. These results further suggest that pyroptosis does not accompany IL-1β secretion even during the 3 h of incubation with classical strains or during the subsequent 13 h of incubation in gentamicin.
El Tor V. cholerae strains also induce IL-1β secretion independent of hemolysin.
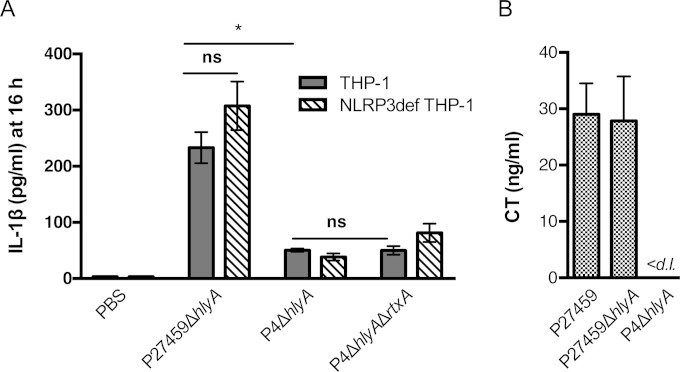
We reasoned that El Tor strains should likewise be able to activate CT-dependent induction of IL-1β secretion. Since expression of the pore-forming hemolysin is cytolytic after several hours (32), CT-dependent inflammasome activation was tested with the P27459ΔhlyA strain and the isogenic ΔctxAB strain P4ΔhlyA (Fig. 4A). Secretion of CT by the P27459ΔhlyA strain and the lack of CT secretion by the P4ΔhlyA strain during the 3 h of incubation in culture medium were confirmed by ELISA (Fig. 4B). When THP-1 and NLRP3def THP-1 cells were incubated with V. cholerae, only P27459ΔhlyA induced high levels of IL-1β secretion at 16 h and this CT-dependent secretion was, similar to studies with classical strains, independent of NLRP3. Additional deletion of the rtxA gene further demonstrated that the MARTX toxin did not contribute to inflammasome activation in THP-1 cells, even after extended incubation (Fig. 4A).
FIG 4.
Low-level release of IL-1β by El Tor V. cholerae is also due to CT. (A) IL-1β secretion from THP-1 (gray bars), ASCdef THP-1 (white bars), or NLRP3def THP-1 (striped bars) cells treated at an MOI of 25 with wild-type V. cholerae El Tor strain P27459 with additional deletions in rtxA or hlyA as indicated for 3 h, followed by incubation in gentamicin for 13 h to amplify the detection of low-level release of IL-1β. (B) CT secreted into RPMI medium measured by ELISA. Data are presented as the mean ± the standard deviation of triplicate samples. *, P < 0.05; ns, not statistically significant; <d. l., below the limit of detection of the assay.
The CTA subunit is responsible for activation of inflammasome-dependent IL-1β release.
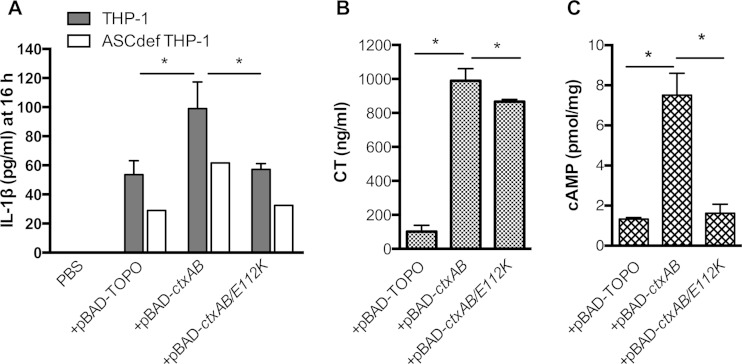
Data on classical strains indicate that induction of the inflammasome in THP-1 cells by CT is linked to the CTA, and not the CTB, subunit (Fig. 3). To further demonstrate this also in El Tor strains, we cloned the ctxAB genes onto a plasmid to express CT under the control of the arabinose-inducible PBAD promoter. Under arabinose induction, KFV101 expressing ctxAB produced high levels of CT, up to 100 times the natural levels. When transformed to express ctxAB, induction of IL-1β release from KFV101 was stimulated, in contrast to a vector-only control (Fig. 5A). As induction was detectable, we could test if the CT contribution to increased IL-1β secretion was due to the structure of CTA or due specifically to its catalytic activity by modifying ctxA via site-directed mutagenesis to produce CTA E112K, a well-characterized catalytically inactive mutant form of CTA (33). We confirmed that CT E112K is secreted from KFV101-transformed cells by ELISA (Fig. 5B) but does not induce cAMP production from THP-1 cells (Fig. 5C). This mutant form did not stimulate an increase in IL-1β release over the level measured in the vector control, linking inflammasome induction to catalytically active CTA.
FIG 5.
Catalytically active CT is required for induction of IL-1β secretion. (A) IL-1β secretion from THP-1 (gray bars), ASCdef THP-1 (white bars), or NLRP3def THP-1 (striped bars) cells treated at an MOI of 25 with V. cholerae multitoxin-deficient strain KFV101 transformed with a PBAD-TOPO plasmid or plasmids for the expression of CT (ctxAB) or catalytically inactive CT (ctxAB/E112K) under the control of the araBAD promoter for 3 h, followed by incubation in gentamicin for 13 h to amplify the detection of low-level release of IL-1β. (B) CT secretion into RPMI medium measured by ELISA. (C) cAMP levels in THP-1 cells. Data are presented as the mean ± the standard deviation of triplicate samples. *, P < 0.05; ns, not statistically significant; <d. l., below the limit of detection of the assay.
Further, THP-1 cells treated with purified CT holotoxin, followed by 3 h of incubation with KFV101, were able to secrete IL-1β robustly (see Fig. S9 in the supplemental material). In contrast, treatment with CTB alone did not induce IL-1β secretion. Thus, similar to our findings with bacterial toxins secreted endogenously (Fig. 3) or exogenously (Fig. 5), it is the CT holotoxin that potently induces inflammasome activation. In order to test further whether any stimulus of cAMP might induce activation, we treated THP-1 cells with a concentration of forskolin sufficient to induce cAMP production to a level comparable to that of CT (see Fig. S9). However, forskolin, in combination with KFV101 infection, did not induce IL-1β secretion (see Fig. S9). Therefore, these studies show that the sustained modified signaling environment of covalent modification of the adenylate cyclase by the CTA subunit is responsible for inflammasome activation.
Primary human monocytes also secrete IL-1 β in response to hemolysin and CT during V. cholerae infection.
THP-1 cells have been used extensively to study human inflammasomes and have been shown to require priming signals similar to primary human macrophages (16, 34). THP-1 cells are also an attractive model system for inflammasome activation, as they allow the development of stable cell lines in which specific inflammasome components are knocked down. However, THP-1 cells are not a perfect model of primary human monocytes, demonstrating some differences in cytokine profiles in response to stimuli (35, 36).
In order to demonstrate that our observations of inflammasome activation pathways in THP-1 cells are relevant to primary human cells, we incubated PBMCs with different strains of V. cholerae and measured IL-1β secretion. Similar to our observations on THP-1 cells, PBMCs secreted IL-1β in response to infection with El Tor strain P27459 after 3 h of infection and this secretion was dependent on hemolysin (Fig. 6A). Secretion of the unrelated cytokine TNF-α was evident after 3 h of infection, independent of the action of hemolysin (Fig. 6D). Also similar to THP-1 cells, El Tor V. cholerae did not induce cell lysis or pyroptosis in primary human monocytes under these incubation conditions (Fig. 6G).
FIG 6.
Primary human monocytes also secrete IL-1β in response to hemolysin and CT during V. cholerae infection. Shown is the IL-1β (A) and TNF-α (B) secretion from PBMCs treated at an MOI of 25 with a wild-type V. cholerae El Tor strain or a mutant with a deletion in hlyA. Also shown is the IL-1β (D) and TNF-α (E) secretion from PBMCs treated at an MOI of 25 with wild-type V. cholerae classical strain O395 and its isogenic ΔctxAB mutant O395NT and ΔctxA mutant O395N1 for 3 h. (C, F) Percent lysis of PBMCs after 3 h as measured by LDH release. Data are presented as the mean ± the standard deviation of triplicate samples. *, P < 0.05; ns, not statistically significant; <d. l., below the limit of detection of the assay.
In contrast to THP-1 cells, classical V. cholerae strain O395 induced a much more robust secretion of IL-1β from primary human monocytes within 3 h of infection, perhaps indicating a more potent effect of CT on primary cells. This IL-1β secretion was largely dependent on CT, as the levels of IL-1β measured from ΔctxA mutant strain O395N1and ΔctxAB mutant strain O395NT were significantly lower than those of the wild type. However, there appears to be a CT-independent pathway of activation also present in primary human monocytes, as the mutant strains did induce a low level of IL-1β secretion above that of mock-infected controls.
In sum, these studies show that V. cholerae induces both NLRP3-dependent activation of the inflammasome dependent upon the hemolysin and NLRP3-independent activation due to catalytically active CT and this occurs both in culture THP-1 monocytic-like cells and in primary human monocytes. Thus, when considering V. cholerae-induced inflammasome activity, it is important to consider that multiple activation pathways are stimulated with differing kinetics, with variations occurring due to the strain of bacteria, the species of immune cell, and the context.
DISCUSSION
Extensive work has connected secreted factors of V. cholerae to modulation of the innate immune response. In particular, numerous studies have shown a linkage between V. cholerae activation of the inflammasome in innate immune cells resulting in release of the proinflammatory cytokine IL-1β (17, 18, 20, 31). Both V. cholerae and purified secreted components of V. cholerae have been examined recently as activators of noncanonical inflammasomes (18, 20), wherein CTB is found to function as a carrier of E. coli O111:B4 LPS into the cytosol to stimulate caspase-11 (22). However, previous work on CTB had shown that it does not induce IL-1β release from THP-1 cells when coincubated with E. coli O111:B4 LPS, suggesting that different pathways function in THP-1 cells compared to murine macrophages (31). The distinction in these results suggests that the pathways that drive the stimulation of innate immune responses in THP-1 cells may be different from those induced in murine macrophages, although caspase-4/5 can also function as an LPS receptor in THP-1 cells, similar to caspase-11 in murine macrophages (23). Further, studies with murine macrophages have been done in some cases with El Tor strains (17), while others used classical strains (18, 20) without noting that they should activate different innate immune pathways because of differences in their toxin repertoires.
In this study, we used a singular readout of IL-1β release to facilitate the screening of our extensive collection of V. cholerae strains and mutants to define which secreted factors identified in murine macrophages as contributing to inflammasome activation are required for induction of IL-1β release from human monocytic cells dependent upon core components of the canonical inflammasome complexes. A second advantage of using IL-1β release as a readout for inflammasome activation is that it allows us to directly quantify altered responses from both canonical and noncanonical inflammasomes. Furthermore, most studies are conducted with LPS-primed cells, although V. cholerae is a Gram-negative bacterium that is known to have an LPS that potently activates TNF-α in both murine macrophages and THP-1 cells (37). The absence of priming allowed us to exclude any priming artifacts and thereby to directly assess the ability of V. cholerae strains to promote IL-1β release. After the initial screening of a large number of strains via IL-1β release, a smaller number of activating strains could then be further tested for cell lysis and caspase-1 activation.
Using these methods, we found that classical strains such as 9459 and O395, which are by definition nonhemolytic (38), do not induce the inflammasome via NLRP3. In contrast, the pore-forming hemolysin produced by strains of the El Tor biotype does induce the inflammasome via NLRP3 in either murine macrophages (17) or THP-1 cells (Fig. 3). It is notable that, together, these results deviate from those of some studies that have reported that classical V. cholerae induces the inflammasome via NLRP3. It is therefore important to recognize that V. cholerae stimulation of NLRP3 is highly dependent on the biotype and does not include strain 9459, which is commonly used for inflammasome studies (18, 20).
Similar to hemolysin, classical strains also do not produce the MARTX toxin (14), while some, but not all (39), El Tor biotype strains do produce the MARTX toxin. In murine macrophages, it was found that the MARTX toxin does induce processing of IL-1β and rapid lysis of cells (17). In contrast, this toxin is active and induces actin cross-linking in THP-1 cells but it does not induce IL-1β secretion or lysis of cells (Fig. 2). Indeed, mutants with a deletion in the hlyA gene but with the rtxA gene intact did not lyse THP-1 cells during 3 h of incubation with bacteria or at any point during a subsequent 13 h of incubation and showed no induction of IL-1β release during this period (Fig. 4). Thus, there is clearly a difference in the response to the MARTX toxin in murine macrophages and that in human THP-1 cells. The MARTX toxin is known not to induce lysis of human epithelial cells (40, 41) and thus is not considered a pore-forming cytolysin, so it is not surprising that it would not induce NLRP3 in all cell lines. It does, however, transfer effector domains to the target cell cytosol, where actin dynamics are modified, including inactivation of Rho GTPases (41). One possible difference between the two systems is that pyrin domains have recently been identified as activating the inflammasome complexes by responding to changes in cytoskeletal dynamics, especially modification of Rho GTPases (42). However, THP-1 cells have pyrin-only proteins (POPs) that can fine-tune the activation of pyrin domain-containing Nod-like receptor (PYD-NLR) inflammasomes (43, 44). These POPs are not present in mouse cells (45). In addition, human and mouse macrophages differ in the expression of NLR and ALR genes (27, 46). Thus, the difference in the system may reflect actual variation in the signaling of inflammasome activation, but this will need to be examined further. An alternative explanation is that the MARTX toxin either fails to activate or actively suppresses signaling pathways. While the actin cross-linking activity of the actin cross-linking domain of the V. cholerae MARTX toxin is well characterized (47), the detailed mechanism of action of the other two effector domains remains unknown. It has recently been suggested that these domains function in part to manipulate either cytokine production or activation and that this manipulation may vary by cell type (41). This must occur downstream of NF-κB activation, as the MARTX toxin did not influence this step (see Fig. S1 in the supplemental material). Testing of the linkage of any particular domain to a signaling response requires extensive genetic modification of the toxins to unlink the putative signaling activities from the predominant activity of actin cross-linking.
As noted, classical strains do not induce the NLRP3 inflammasome but do induce inflammasomes in an NLRP3-independent manner, as do El Tor strains when hlyA is deleted. In contrast to extensive studies performed with CTB added to cells in combination with purified LPS (31, 37, 48), we found that CT produced by both classical and El Tor strains stimulated IL-1β release from THP-1 cells dependent upon the A, and not the B, subunit. Further, a catalytically active CTA subunit was essential. Although the activation occurs independent of NLRP3, this activation is through a canonical inflammasome pathway, as THP-1 cells incubated with classical strain O395 demonstrated activation of caspase-1 (see Fig. S7 in the supplemental material).
Although we demonstrate the necessity of a catalytically active CTA subunit, it is surprising that CTA-mediated inflammasome activation cannot simply be attributed to an increase in intracellular cAMP. THP-1 cells primed with a multitoxin-deficient strain and treated with forskolin did not secrete IL-1β, despite robust cAMP production. One possible explanation for this is that it is the sustained presence of high levels of cAMP due to constitutive activation of the cellular adenylate cyclase by covalent modification that is important, possible resulting in long-term changes in ion flux, signaling, or even gene expression that can then influence inflammasome activation.
While these studies were conducted with human THP-1 cells, an examination of published results reveal that our data are consistent with observations from a limited number of studies wherein classical V. cholerae was added to primary murine macrophages. In fact, classical strain 9459 is known to activate murine macrophages dependent on ASC (18, 20), and activation by V. cholerae bacteria, as opposed to by LPS plus CTB, was at least partially NLRP3 independent (18). Here, in human cells, we demonstrate the same and further show that activation is dependent on CTA and not CTB.
Although THP-1 cells provide a unique opportunity to ascertain the role of specific inflammasome complexes, we also sought to determine if the same V. cholerae toxins that activate in THP-1 cells are required for IL-1β secretion in primary human PBMCs (Fig. 6). We found that, similar to THP-1 cells, PBMCs respond to hemolysin of El Tor strains by secreting mature IL-1β. Studies of classical V. cholerae show that IL-1β release occurs in response to CT, but this occurs more rapidly than in THP-1 cells, with robust IL-1β secretion measured after 3 h. Similar to our findings on THP-1 cells, there is no difference in IL-1β secretion after PBMC incubation with a ΔctxB mutant strain versus a ΔctxAB mutant strain, indicating the same critical role for CTA in inflammasome activation. There is some background level of IL-1β released by PBMCs incubated with the ΔctxAB strain, and further experimentation is required to determine if there is an alternative activation pathway that has a minor contribution to inflammasome activation by classical V. cholerae in PBMCs.
Overall, in this study, we have shown that the different biotypes of V. cholerae can induce inflammasome-dependent release of IL-1β. In THP-1 cells, both classical and El Tor strains induce a low level of IL-1β that requires long incubation to increase detection and this low-level induction depends on the CT holotoxin, with human PBMCs being more potently and rapidly activated. However, El Tor strains also produce accessory toxins, including hemolysin, which induce much more rapid and robust NLRP3-dependent activation in both THP-1 cells and PBMCs. Thus, in the hemolytic El Tor strains that are responsible for the ongoing seventh cholera pandemic, parallel inflammasome activation mechanisms exist for the detection of V. cholerae by the innate immune system. Future studies in this field need to include consideration of the strain that is being used, as well as appropriate genetic mutants to properly assign an inducing toxin to a specific pathway when examining the contribution of bacterial components to inflammasome activation.
Supplementary Material
ACKNOWLEDGMENTS
We thank Verena Olivier for the construction of strain VOV8. We thank John Mekalanos for providing O395NT and E4. We thank Lucia Maria V. De Almeida, Sonal Khare, and Vanderlene Kung for input on the experimental design. Kevin Ziolo is thanked for technical assistance.
This work was supported by an Investigators in the Pathogenesis of Infectious Disease award from the Burroughs Wellcome Fund (awarded to K.J.F.S.); by National Institutes of Health grants R01 AI051490, R21 AI072461 (awarded to K.J.F.S.), and R01 AI099009 (awarded to C.S.); and by Ruth L. Kirschstein Research Service Award 1F30 DK084623 (awarded to J.Q.).
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.02461-14.
REFERENCES
- 1.Harris JB, LaRocque RC, Qadri F, Ryan ET, Calderwood SB. 2012. Cholera. Lancet 379:2466–2476. doi: 10.1016/S0140-6736(12)60436-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sack DA, Sack RB, Nair GB, Siddique AK. 2004. Cholera. Lancet 363:223–233. doi: 10.1016/S0140-6736(03)15328-7. [DOI] [PubMed] [Google Scholar]
- 3.Wernick NL, Chinnapen DJ, Cho JA, Lencer WI. 2010. Cholera toxin: an intracellular journey into the cytosol by way of the endoplasmic reticulum. Toxins (Basel) 2:310–325. doi: 10.3390/toxins2030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satchell KJF. 2003. Activation and suppression of the proinflammatory immune response by Vibrio cholerae toxins. Microbes Infect 5:1241–1247. doi: 10.1016/j.micinf.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Queen J, Satchell KJ. 2013. Promotion of colonization and virulence by cholera toxin is dependent on neutrophils. Infect Immun 81:3338–3345. doi: 10.1128/IAI.00422-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fullner KJ. 2002. Toxins of Vibrio cholerae: consensus and controversy, p 481-502. In Hecht G. (ed), Microbial pathogenesis and the intestinal epithelial cell. ASM Press, Washington, DC. [Google Scholar]
- 7.Olivier V, Haines GK 3rd, Tan Y, Satchell KJ. 2007. Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains. Infect Immun 75:5035–5042. doi: 10.1128/IAI.00506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Queen J, Satchell KJ. 2012. Neutrophils are essential for containment of Vibrio cholerae to the intestine during the proinflammatory phase of infection. Infect Immun 80:2905–2913. doi: 10.1128/IAI.00356-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olivier V, Salzman NH, Satchell KJ. 2007. Prolonged colonization of mice by Vibrio cholerae El Tor O1 depends on accessory toxins. Infect Immun 75:5043–5051. doi: 10.1128/IAI.00508-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Olivier V, Queen J, Satchell KJ. 2009. Successful small intestine colonization of adult mice by Vibrio cholerae requires ketamine anesthesia and accessory toxins. PLoS One 4:e7352. doi: 10.1371/journal.pone.0007352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lesmana M, Subekti D, Tjaniadi P, Pazzaglia G. 1994. Modified CAMP test for biogrouping Vibrio cholerae O1 strains and distinguishing them from strains of V. cholerae non-O1. J Clin Microbiol 32:235–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alm RA, Mayrhofer G, Kotlarski I, Manning PA. 1991. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine 9:588–594. doi: 10.1016/0264-410X(91)90247-4. [DOI] [PubMed] [Google Scholar]
- 13.Alm RA, Stroeher UH, Manning PA. 1988. Extracellular proteins of Vibrio cholerae: nucleotide sequence of the structural gene (hlyA) for the haemolysin of the haemolytic El Tor strain O17 and characterization of the hlyA mutation in the non-haemolytic classical strain 569B. Mol Microbiol 2:481–488. doi: 10.1111/j.1365-2958.1988.tb00054.x. [DOI] [PubMed] [Google Scholar]
- 14.Lin W, Fullner KJ, Clayton R, Sexton JA, Rogers MB, Calia KE, Calderwood SB, Fraser C, Mekalanos JJ. 1999. Identification of a Vibrio cholerae RTX toxin gene cluster that is tightly linked to the cholera toxin prophage. Proc Natl Acad Sci U S A 96:1071–1076. doi: 10.1073/pnas.96.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martinon F, Mayor A, Tschopp J. 2009. The inflammasomes: guardians of the body. Annu Rev Immunol 27:229–265. doi: 10.1146/annurev.immunol.021908.132715. [DOI] [PubMed] [Google Scholar]
- 16.Netea MG, Simon A, van de Veerdonk F, Kullberg BJ, Van der Meer JW, Joosten LA. 2010. IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog 6:e1000661. doi: 10.1371/journal.ppat.1000661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Toma C, Higa N, Koizumi Y, Nakasone N, Ogura Y, McCoy AJ, Franchi L, Uematsu S, Sagara J, Taniguchi S, Tsutsui H, Akira S, Tschopp J, Nunez G, Suzuki T. 2010. Pathogenic Vibrio activate NLRP3 inflammasome via cytotoxins and TLR/nucleotide-binding oligomerization domain-mediated NF-kappaB signaling. J Immunol 184:5287–5297. doi: 10.4049/jimmunol.0903536. [DOI] [PubMed] [Google Scholar]
- 18.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM. 2011. Non-canonical inflammasome activation targets caspase-11. Nature 479:117–121. doi: 10.1038/nature10558. [DOI] [PubMed] [Google Scholar]
- 19.Aachoui Y, Leaf IA, Hagar JA, Fontana MF, Campos CG, Zak DE, Tan MH, Cotter PA, Vance RE, Aderem A, Miao EA. 2013. Caspase-11 protects against bacteria that escape the vacuole. Science 339:975–978. doi: 10.1126/science.1230751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rathinam VA, Vanaja SK, Waggoner L, Sokolovska A, Becker C, Stuart LM, Leong JM, Fitzgerald KA. 2012. TRIF licenses caspase-11-dependent NLRP3 inflammasome activation by gram-negative bacteria. Cell 150:606–619. doi: 10.1016/j.cell.2012.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hagar JA, Powell DA, Aachoui Y, Ernst RK, Miao EA. 2013. Cytoplasmic LPS activates caspase-11: implications in TLR4-independent endotoxic shock. Science 341:1250–1253. doi: 10.1126/science.1240988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kayagaki N, Wong MT, Stowe IB, Ramani SR, Gonzalez LC, Akashi-Takamura S, Miyake K, Zhang J, Lee WP, Muszynski A, Forsberg LS, Carlson RW, Dixit VM. 2013. Noncanonical inflammasome activation by intracellular LPS independent of TLR4. Science 341:1246–1249. doi: 10.1126/science.1240248. [DOI] [PubMed] [Google Scholar]
- 23.Shi J, Zhao Y, Wang Y, Gao W, Ding J, Li P, Hu L, Shao F. 2014. Inflammatory caspases are innate immune receptors for intracellular LPS. Nature 514:187–192. doi: 10.1038/nature13683. [DOI] [PubMed] [Google Scholar]
- 24.Fullner KJ, Boucher JC, Hanes MA, Haines GK III, Meehan BM, Walchle C, Sansonetti PJ, Mekalanos JJ. 2002. The contribution of accessory toxins of Vibrio cholerae O1 El Tor to the proinflammatory response in a murine pulmonary cholera model. J Exp Med 195:1455–1462. doi: 10.1084/jem.20020318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryan NB, Dorfleutner A, Rojanasakul Y, Stehlik C. 2009. Activation of inflammasomes requires intracellular redistribution of the apoptotic speck-like protein containing a caspase recruitment domain. J Immunol 182:3173–3182. doi: 10.4049/jimmunol.0802367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kung VL, Khare S, Stehlik C, Bacon EM, Hughes AJ, Hauser AR. 2012. An rhs gene of Pseudomonas aeruginosa encodes a virulence protein that activates the inflammasome. Proc Natl Acad Sci U S A 109:1275–1280. doi: 10.1073/pnas.1109285109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khare S, Dorfleutner A, Bryan NB, Yun C, Radian AD, de Almeida L, Rojanasakul Y, Stehlik C. 2012. An NLRP7-containing inflammasome mediates recognition of microbial lipopeptides in human macrophages. Immunity 36:464–476. doi: 10.1016/j.immuni.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gardel CL, Mekalanos JJ. 1994. Regulation of cholera toxin by temperature, pH, and osmolarity. Methods Enzymol 235:517–526. doi: 10.1016/0076-6879(94)35167-8. [DOI] [PubMed] [Google Scholar]
- 29.Boardman BK, Meehan BM, Satchell KJ. 2007. Growth phase regulation of Vibrio cholerae RTX toxin export. J Bacteriol 189:1827–1835. doi: 10.1128/JB.01766-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bauernfeind FG, Horvath G, Stutz A, Alnemri ES, MacDonald K, Speert D, Fernandes-Alnemri T, Wu J, Monks BG, Fitzgerald KA, Hornung V, Latz E. 2009. Cutting edge: NF-kappaB activating pattern recognition and cytokine receptors license NLRP3 inflammasome activation by regulating NLRP3 expression. J Immunol 183:787–791. doi: 10.4049/jimmunol.0901363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hajishengallis G, Nawar H, Tapping RI, Russell MW, Connell TD. 2004. The type II heat-labile enterotoxins LT-IIa and LT-IIb and their respective B pentamers differentially induce and regulate cytokine production in human monocytic cells. Infect Immun 72:6351–6358. doi: 10.1128/IAI.72.11.6351-6358.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zitzer A, Zitzer O, Bhakdi S, Palmer M. 1997. Potent membrane-permeabilizing and cytocidal action of Vibrio cholerae cytolysin on human intestinal cells. Infect Immun 65:1293–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamamoto S, Takeda Y, Yamamoto M, Kurazono H, Imaoka K, Yamamoto M, Fujihashi K, Noda M, Kiyono H, McGhee JR. 1997. Mutants in the ADP-ribosyltransferase cleft of cholera toxin lack diarrheagenicity but retain adjuvanticity. J Exp Med 185:1203–1210. doi: 10.1084/jem.185.7.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Netea MG, Nold-Petry CA, Nold MF, Joosten LA, Opitz B, van der Meer JH, van de Veerdonk FL, Ferwerda G, Heinhuis B, Devesa I, Funk CJ, Mason RJ, Kullberg BJ, Rubartelli A, van der Meer JW, Dinarello CA. 2009. Differential requirement for the activation of the inflammasome for processing and release of IL-1beta in monocytes and macrophages. Blood 113:2324–2335. doi: 10.1182/blood-2008-03-146720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Heil TL, Volkmann KR, Wataha JC, Lockwood PE. 2002. Human peripheral blood monocytes versus THP-1 monocytes for in vitro biocompatibility testing of dental material components. J Oral Rehabil 29:401–407. doi: 10.1046/j.1365-2842.2002.00893.x. [DOI] [PubMed] [Google Scholar]
- 36.Schildberger A, Rossmanith E, Eichhorn T, Strassl K, Weber V. 2013. Monocytes, peripheral blood mononuclear cells, and THP-1 cells exhibit different cytokine expression patterns following stimulation with lipopolysaccharide. Mediators Inflamm 2013:697972. doi: 10.1155/2013/697972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zughaier SM, Zimmer SM, Datta A, Carlson RW, Stephens DS. 2005. Differential induction of the Toll-like receptor 4-MyD88-dependent and -independent signaling pathways by endotoxins. Infect Immun 73:2940–2950. doi: 10.1128/IAI.73.5.2940-2950.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakazaki R. 1992. Bacteriology of Vibrio and related organisms, p 37–55. In Barua D, Gereenough WB (ed), Cholera. Plenum Medical Book Company, New York, NY. [Google Scholar]
- 39.Dolores J, Satchell KJ. 2013. Analysis of Vibrio cholerae genome sequences reveals unique rtxA variants in environmental strains and an rtxA-null mutation in recent altered El Tor isolates. mBio 4:e00624-00612. doi: 10.1128/mBio.00624-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fullner KJ, Mekalanos JJ. 2000In vivo covalent crosslinking of actin by the RTX toxin of Vibrio cholerae. EMBO J 19:5315–5323. doi: 10.1093/emboj/19.20.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dolores JS, Agarwal S, Egerer M, Satchell KJ. 2015. Vibrio cholerae MARTX toxin heterologous translocation of beta-lactamase and roles of individual effector domains on cytoskeleton dynamics. Mol Microbiol 95:590–604. doi: 10.1111/mmi.12879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu H, Yang J, Gao W, Li L, Li P, Zhang L, Gong YN, Peng X, Xi JJ, Chen S, Wang F, Shao F. 2014. Innate immune sensing of bacterial modifications of Rho GTPases by the pyrin inflammasome. Nature 513:237–241. doi: 10.1038/nature13449. [DOI] [PubMed] [Google Scholar]
- 43.Dorfleutner A, Bryan NB, Talbott SJ, Funya KN, Rellick SL, Reed JC, Shi X, Rojanasakul Y, Flynn DC, Stehlik C. 2007. Cellular pyrin domain-only protein 2 is a candidate regulator of inflammasome activation. Infect Immun 75:1484–1492. doi: 10.1128/IAI.01315-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stehlik C, Krajewska M, Welsh K, Krajewski S, Godzik A, Reed JC. 2003. The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated NF-kappaB and pro-caspase-1 regulation. Biochem J 373:101–113. doi: 10.1042/BJ20030304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stehlik C, Dorfleutner A. 2007. COPs and POPs: modulators of inflammasome activity. J Immunol 179:7993–7998. doi: 10.4049/jimmunol.179.12.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ting JP, Lovering RC, Alnemri ES, Bertin J, Boss JM, Davis BK, Flavell RA, Girardin SE, Godzik A, Harton JA, Hoffman HM, Hugot JP, Inohara N, Mackenzie A, Maltais LJ, Nunez G, Ogura Y, Otten LA, Philpott D, Reed JC, Reith W, Schreiber S, Steimle V, Ward PA. 2008. The NLR gene family: a standard nomenclature. Immunity 28:285–287. doi: 10.1016/j.immuni.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Satchell KJ. 2009. Actin crosslinking toxins of Gram-negative bacteria. Toxins 1:123–133. doi: 10.3390/toxins1020123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakharwade SC, Sharma PK, Mukhopadhaya A. 2013. Vibrio cholerae porin OmpU induces pro-inflammatory responses, but down-regulates LPS-mediated effects in RAW 264.7, THP-1 and human PBMCs. PLoS One 8:e76583. doi: 10.1371/journal.pone.0076583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fullner KJ, Mekalanos JJ. 1999. Genetic characterization of a new type IV pilus gene cluster found in both classical and El Tor biotypes of Vibrio cholerae. Infect Immun 67:1393–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mekalanos JJ. 1983. Duplication and amplification of toxin genes in Vibrio cholerae. Cell 35:252–263. [DOI] [PubMed] [Google Scholar]
- 51.Ottaviani D, Leoni F, Rocchegiani E, Canonico C, Masini L, Pianetti A, Parlani C, Luzzi I, Caola I, Paternoster C, Carraturo A. 2011. Unusual case of necrotizing fasciitis caused by Vibrio cholerae O137. J Clin Microbiol 49:757–759. doi: 10.1128/JCM.02257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mekalanos JJ, Swartz DJ, Perason GDN, Harford N, Groyne F, de Wilde M. 1983. Cholera toxin genes: nucleotide sequence, deletion analysis and vaccine development. Nature 306:551–557. doi: 10.1038/306551a0. [DOI] [PubMed] [Google Scholar]
- 53.Goldberg I, Mekalanos JJ. 1986. Effect of a recA mutation on cholera toxin gene amplification and deletion events. J Bacteriol 165:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.