Abstract
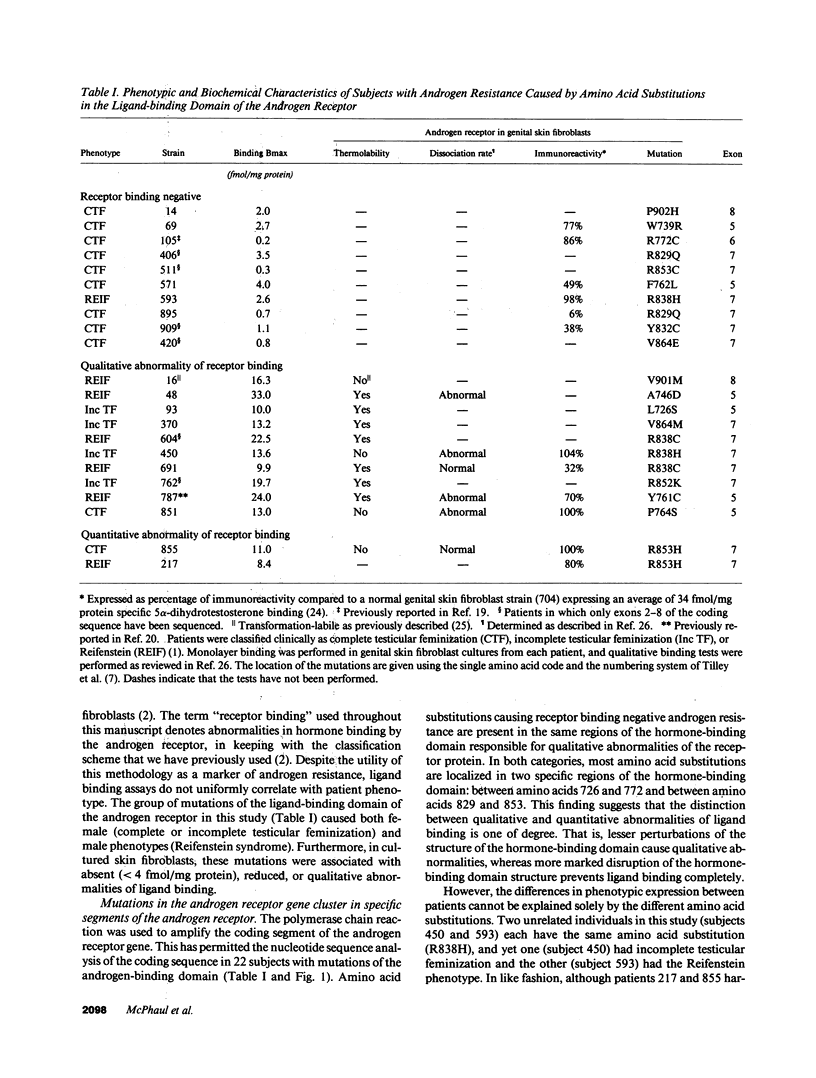
We have analyzed the nucleotide sequence of the androgen receptor from 22 unrelated subjects with substitution mutations of the hormone-binding domain. Eleven had the phenotype of complete testicular feminization, four had incomplete testicular feminization, and seven had Reifenstein syndrome. The underlying functional defect in cultured skin fibroblasts included individuals with absent, qualitative, or quantitative defects in ligand binding. 19 of the 21 substitution mutations (90%) cluster in two regions that account for approximately 35% of the hormone-binding domain, namely, between amino acids 726 and 772 and between amino acids 826 and 864. The fact that one of these regions is homologous to a region of the human thyroid hormone receptor (hTR-beta) which is a known cluster site for mutations that cause thyroid hormone resistance implies that this localization of mutations is not a coincidence. These regions of the androgen receptor may be of particular importance for the formation and function of the hormone-receptor complex.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown T. R., Lubahn D. B., Wilson E. M., French F. S., Migeon C. J., Corden J. L. Functional characterization of naturally occurring mutant androgen receptors from subjects with complete androgen insensitivity. Mol Endocrinol. 1990 Dec;4(12):1759–1772. doi: 10.1210/mend-4-12-1759. [DOI] [PubMed] [Google Scholar]
- Brufsky A. M., Malchoff D. M., Javier E. C., Reardon G., Rowe D., Malchoff C. D. A glucocorticoid receptor mutation in a subject with primary cortisol resistance. Trans Assoc Am Physicians. 1990;103:53–63. [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Structural analysis of complementary DNA and amino acid sequences of human and rat androgen receptors. Proc Natl Acad Sci U S A. 1988 Oct;85(19):7211–7215. doi: 10.1073/pnas.85.19.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giguère V., Hollenberg S. M., Rosenfeld M. G., Evans R. M. Functional domains of the human glucocorticoid receptor. Cell. 1986 Aug 29;46(5):645–652. doi: 10.1016/0092-8674(86)90339-9. [DOI] [PubMed] [Google Scholar]
- Griffin J. E., Punyashthiti K., Wilson J. D. Dihydrotestosterone binding by cultured human fibroblasts. Comparison of cells from control subjects and from patients with hereditary male pseudohermaphroditism due to androgen resistance. J Clin Invest. 1976 May;57(5):1342–1351. doi: 10.1172/JCI108402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grino P. B., Isidro-Gutierrez R. F., Griffin J. E., Wilson J. D. Androgen resistance associated with a qualitative abnormality of the androgen receptor and responsive to high dose androgen therapy. J Clin Endocrinol Metab. 1989 Mar;68(3):578–584. doi: 10.1210/jcem-68-3-578. [DOI] [PubMed] [Google Scholar]
- Hurley D. M., Accili D., Stratakis C. A., Karl M., Vamvakopoulos N., Rorer E., Constantine K., Taylor S. I., Chrousos G. P. Point mutation causing a single amino acid substitution in the hormone binding domain of the glucocorticoid receptor in familial glucocorticoid resistance. J Clin Invest. 1991 Feb;87(2):680–686. doi: 10.1172/JCI115046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs W. J., Griffin J. E., Weaver D. D., Carlson B. R., Wilson J. D. A mutation that causes lability of the androgen receptor under conditions that normally promote transformation to the DNA-binding state. J Clin Invest. 1984 Apr;73(4):1095–1104. doi: 10.1172/JCI111295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D. B., Brown T. R., Simental J. A., Higgs H. N., Migeon C. J., Wilson E. M., French F. S. Sequence of the intron/exon junctions of the coding region of the human androgen receptor gene and identification of a point mutation in a family with complete androgen insensitivity. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9534–9538. doi: 10.1073/pnas.86.23.9534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubahn D. B., Joseph D. R., Sar M., Tan J., Higgs H. N., Larson R. E., French F. S., Wilson E. M. The human androgen receptor: complementary deoxyribonucleic acid cloning, sequence analysis and gene expression in prostate. Mol Endocrinol. 1988 Dec;2(12):1265–1275. doi: 10.1210/mend-2-12-1265. [DOI] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Wilson C. M., Wilson J. D., Griffin J. E., McPhaul M. J. A single nucleotide substitution introduces a premature termination codon into the androgen receptor gene of a patient with receptor-negative androgen resistance. J Clin Invest. 1990 May;85(5):1522–1528. doi: 10.1172/JCI114599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcelli M., Tilley W. D., Zoppi S., Griffin J. E., Wilson J. D., McPhaul M. J. Androgen resistance associated with a mutation of the androgen receptor at amino acid 772 (Arg----Cys) results from a combination of decreased messenger ribonucleic acid levels and impairment of receptor function. J Clin Endocrinol Metab. 1991 Aug;73(2):318–325. doi: 10.1210/jcem-73-2-318. [DOI] [PubMed] [Google Scholar]
- McPhaul M. J., Marcelli M., Tilley W. D., Griffin J. E., Isidro-Gutierrez R. F., Wilson J. D. Molecular basis of androgen resistance in a family with a qualitative abnormality of the androgen receptor and responsive to high-dose androgen therapy. J Clin Invest. 1991 Apr;87(4):1413–1421. doi: 10.1172/JCI115147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao R., Haji M., Yanase T., Ogo A., Takayanagi R., Katsube T., Fukumaki Y., Nawata H. A single amino acid substitution (Met786----Val) in the steroid-binding domain of human androgen receptor leads to complete androgen insensitivity syndrome. J Clin Endocrinol Metab. 1992 May;74(5):1152–1157. doi: 10.1210/jcem.74.5.1569163. [DOI] [PubMed] [Google Scholar]
- Parrilla R., Mixson A. J., McPherson J. A., McClaskey J. H., Weintraub B. D. Characterization of seven novel mutations of the c-erbA beta gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two "hot spot" regions of the ligand binding domain. J Clin Invest. 1991 Dec;88(6):2123–2130. doi: 10.1172/JCI115542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ris-Stalpers C., Trifiro M. A., Kuiper G. G., Jenster G., Romalo G., Sai T., van Rooij H. C., Kaufman M., Rosenfield R. L., Liao S. Substitution of aspartic acid-686 by histidine or asparagine in the human androgen receptor leads to a functionally inactive protein with altered hormone-binding characteristics. Mol Endocrinol. 1991 Oct;5(10):1562–1569. doi: 10.1210/mend-5-10-1562. [DOI] [PubMed] [Google Scholar]
- Sai T. J., Seino S., Chang C. S., Trifiro M., Pinsky L., Mhatre A., Kaufman M., Lambert B., Trapman J., Brinkmann A. O. An exonic point mutation of the androgen receptor gene in a family with complete androgen insensitivity. Am J Hum Genet. 1990 Jun;46(6):1095–1100. [PMC free article] [PubMed] [Google Scholar]
- Tilley W. D., Marcelli M., Wilson J. D., McPhaul M. J. Characterization and expression of a cDNA encoding the human androgen receptor. Proc Natl Acad Sci U S A. 1989 Jan;86(1):327–331. doi: 10.1073/pnas.86.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapman J., Klaassen P., Kuiper G. G., van der Korput J. A., Faber P. W., van Rooij H. C., Geurts van Kessel A., Voorhorst M. M., Mulder E., Brinkmann A. O. Cloning, structure and expression of a cDNA encoding the human androgen receptor. Biochem Biophys Res Commun. 1988 May 31;153(1):241–248. doi: 10.1016/s0006-291x(88)81214-2. [DOI] [PubMed] [Google Scholar]
- Trifiro M., Prior R. L., Sabbaghian N., Pinsky L., Kaufman M., Nylen E. G., Belsham D. D., Greenberg C. R., Wrogemann K. Amber mutation creates a diagnostic MaeI site in the androgen receptor gene of a family with complete androgen insensitivity. Am J Med Genet. 1991 Sep 15;40(4):493–499. doi: 10.1002/ajmg.1320400425. [DOI] [PubMed] [Google Scholar]
- Weiss R. E., Refetoff S. Thyroid hormone resistance. Annu Rev Med. 1992;43:363–375. doi: 10.1146/annurev.me.43.020192.002051. [DOI] [PubMed] [Google Scholar]
- Yarbrough W. G., Quarmby V. E., Simental J. A., Joseph D. R., Sar M., Lubahn D. B., Olsen K. L., French F. S., Wilson E. M. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990 May 25;265(15):8893–8900. [PubMed] [Google Scholar]
- Zoppi S., Marcelli M., Deslypere J. P., Griffin J. E., Wilson J. D., McPhaul M. J. Amino acid substitutions in the DNA-binding domain of the human androgen receptor are a frequent cause of receptor-binding positive androgen resistance. Mol Endocrinol. 1992 Mar;6(3):409–415. doi: 10.1210/mend.6.3.1316540. [DOI] [PubMed] [Google Scholar]


