Abstract
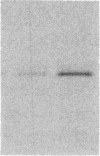
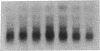
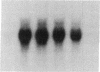
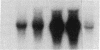
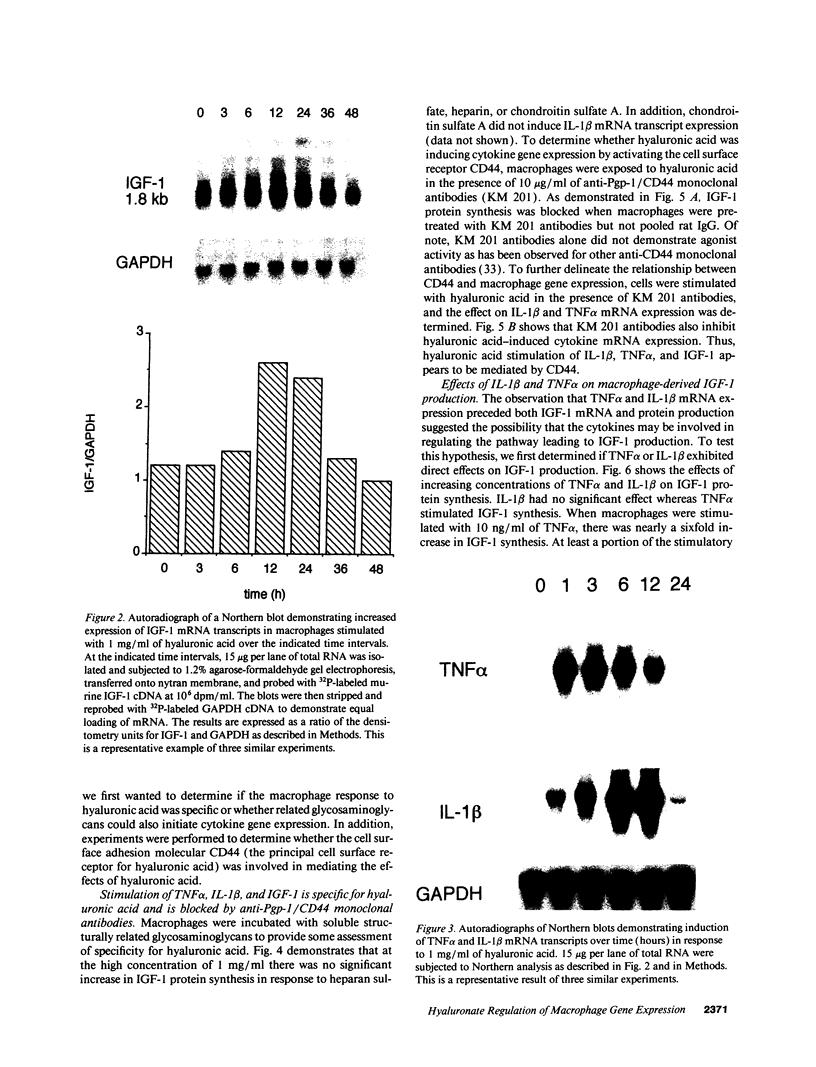
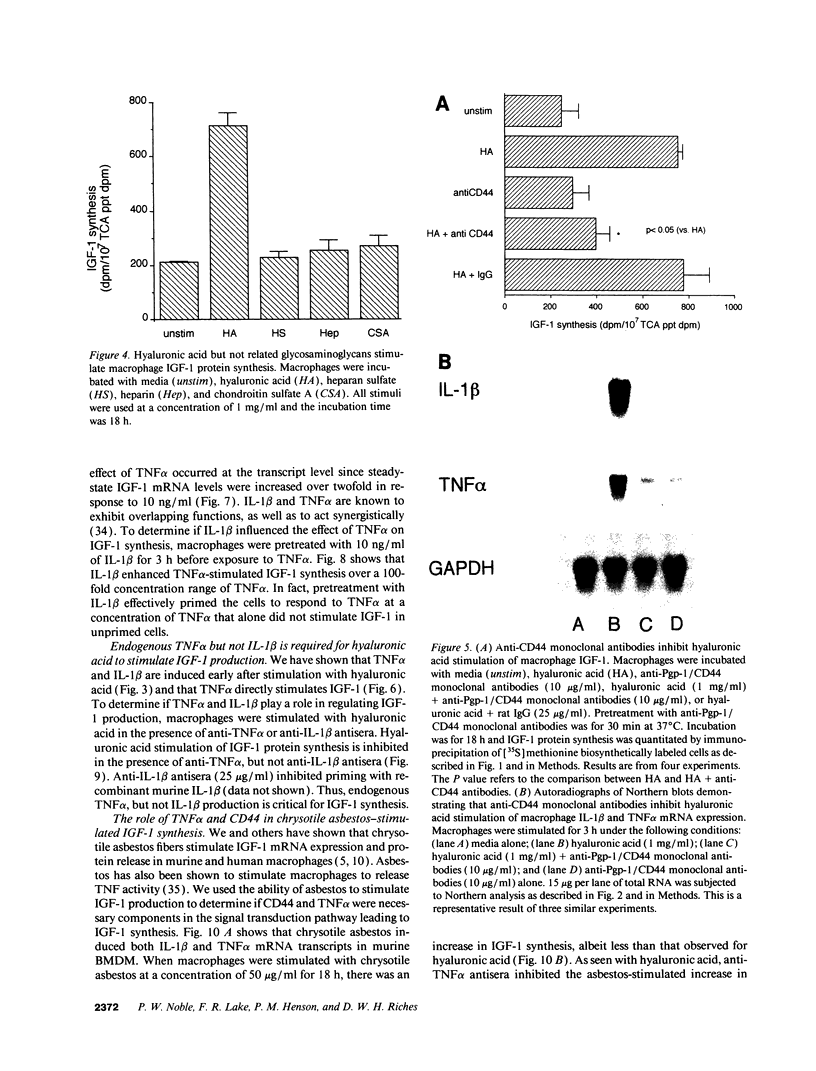
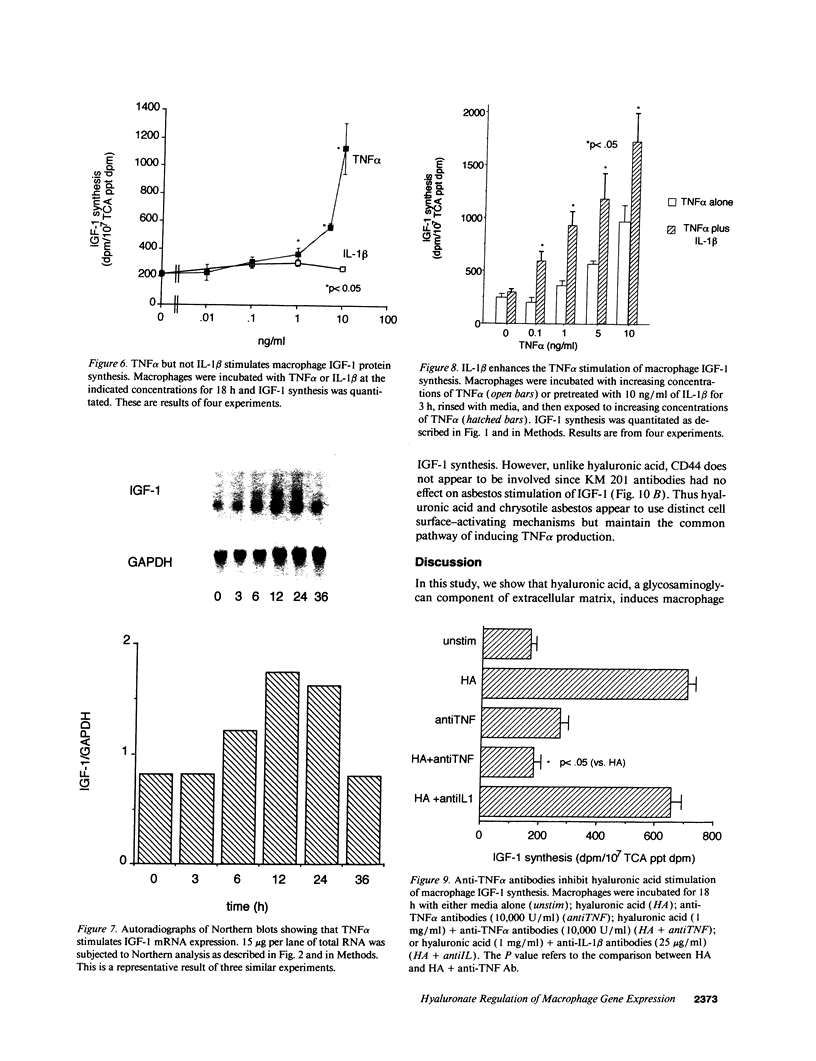
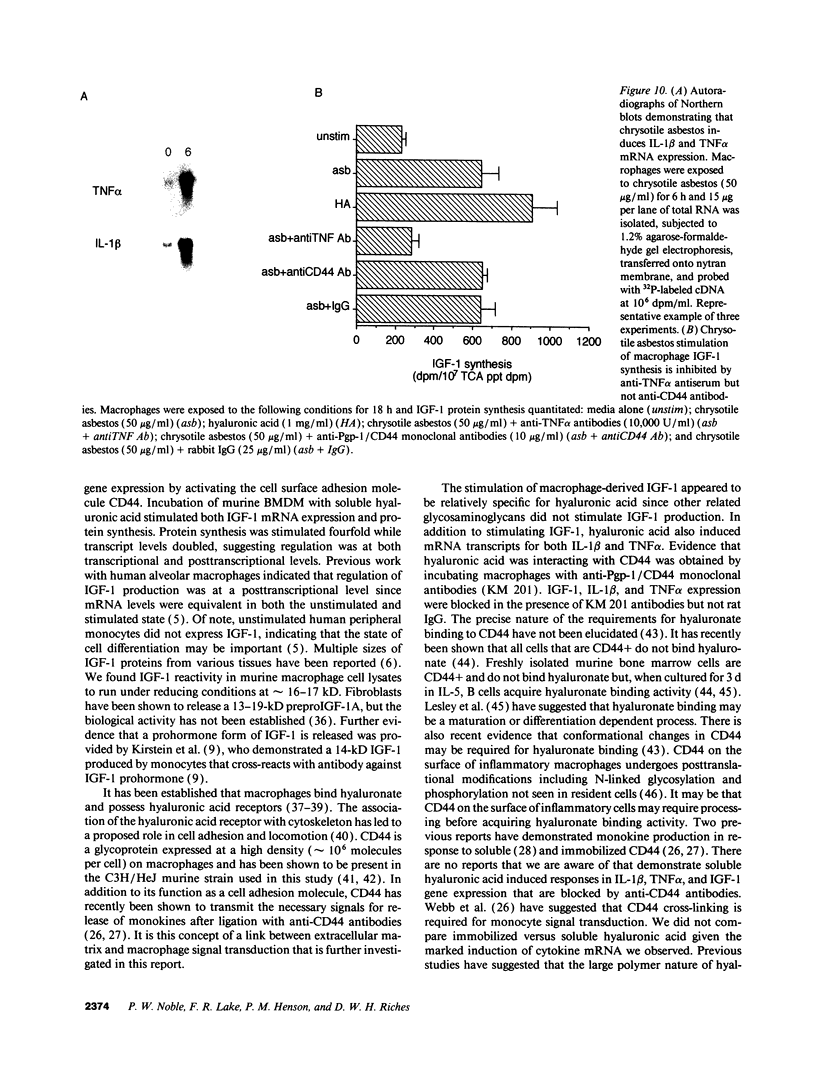
Macrophages participate in inflammatory and repair processes in part through the selective release of cytokines that contribute to tissue remodeling. Extracellular matrix components generated at inflammatory sites may influence tissue remodeling by effects on leukocyte adherence and local cytokine production. In murine bone marrow-derived macrophages, we found that soluble hyaluronic acid stimulated IL-1 beta, TNF alpha, and insulin-like growth factor-1 (IGF-1) mRNA transcript expression as well as IGF-1 protein synthesis. Monoclonal antibodies to the hyaluronic acid receptor CD44 blocked the effects of hyaluronic acid on IL-1 beta, TNF alpha, and IGF-1 expression. TNF alpha and IL-1 beta mRNA expression preceded IGF-1 protein synthesis, and TNF alpha, but not IL-1 beta, was found to directly stimulate IGF-1. Furthermore, IGF-1 induction was dependent on endogenous TNF alpha production since IGF-1 protein synthesis was inhibited in the presence of anti-TNF alpha antiserum. In addition, IL-1 beta was found to exert a regulatory role on IGF-1 production by enhancing the TNF alpha effect. IL-1 beta and TNF alpha mRNA transcript expression as well as IGF-1 protein synthesis were also stimulated by chrysotile asbestos. Anti-CD44 antibodies had no effect whereas anti-TNF alpha antiserum blocked asbestos-stimulated IGF-1 production. These results indicate that hyaluronate activation of CD44 induces cytokine expression and macrophage-derived IGF-1 production is dependent on TNF alpha expression.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud S. L., Bethel C. R., Aron D. C. Secretion of insulinlike growth factor I and insulinlike growth factor-binding proteins by murine bone marrow stromal cells. J Clin Invest. 1991 Aug;88(2):470–475. doi: 10.1172/JCI115327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander C. M., Werb Z. Proteinases and extracellular matrix remodeling. Curr Opin Cell Biol. 1989 Oct;1(5):974–982. doi: 10.1016/0955-0674(89)90068-9. [DOI] [PubMed] [Google Scholar]
- Alexander S. A., Donoff R. B. The glycosaminoglycans of open wounds. J Surg Res. 1980 Nov;29(5):422–429. doi: 10.1016/0022-4804(80)90055-4. [DOI] [PubMed] [Google Scholar]
- Amiri P., Locksley R. M., Parslow T. G., Sadick M., Rector E., Ritter D., McKerrow J. H. Tumour necrosis factor alpha restores granulomas and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992 Apr 16;356(6370):604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- Aruffo A., Stamenkovic I., Melnick M., Underhill C. B., Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990 Jun 29;61(7):1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- Bazil V., Horejsí V. Shedding of the CD44 adhesion molecule from leukocytes induced by anti-CD44 monoclonal antibody simulating the effect of a natural receptor ligand. J Immunol. 1992 Aug 1;149(3):747–753. [PubMed] [Google Scholar]
- Bitterman P. B., Rennard S. I., Hunninghake G. W., Crystal R. G. Human alveolar macrophage growth factor for fibroblasts. Regulation and partial characterization. J Clin Invest. 1982 Oct;70(4):806–822. doi: 10.1172/JCI110677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermer L., Lundgren R., Hällgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989 Feb;44(2):126–131. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Warner-Lambert/Parke-Davis Award lecture. Cellular and molecular mechanisms that direct leukocyte traffic. Am J Pathol. 1990 Jan;136(1):3–11. [PMC free article] [PubMed] [Google Scholar]
- Camp R. L., Kraus T. A., Puré E. Variations in the cytoskeletal interaction and posttranslational modification of the CD44 homing receptor in macrophages. J Cell Biol. 1991 Dec;115(5):1283–1292. doi: 10.1083/jcb.115.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Conover C. A., Baker B. K., Hintz R. L. Cultured human fibroblasts secrete insulin-like growth factor IA prohormone. J Clin Endocrinol Metab. 1989 Jul;69(1):25–30. doi: 10.1210/jcem-69-1-25. [DOI] [PubMed] [Google Scholar]
- Denning S. M., Le P. T., Singer K. H., Haynes B. F. Antibodies against the CD44 p80, lymphocyte homing receptor molecule augment human peripheral blood T cell activation. J Immunol. 1990 Jan 1;144(1):7–15. [PubMed] [Google Scholar]
- Dubois C. M., Bissonnette E., Rola-Pleszczynski M. Asbestos fibers and silica particles stimulate rat alveolar macrophages to release tumor necrosis factor. Autoregulatory role of leukotriene B4. Am Rev Respir Dis. 1989 May;139(5):1257–1264. doi: 10.1164/ajrccm/139.5.1257. [DOI] [PubMed] [Google Scholar]
- Eierman D. F., Johnson C. E., Haskill J. S. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrates. J Immunol. 1989 Mar 15;142(6):1970–1976. [PubMed] [Google Scholar]
- Forrester J. V., Balazs E. A. Inhibition of phagocytosis by high molecular weight hyaluronate. Immunology. 1980 Jul;40(3):435–446. [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Tarone G., Underhill C. B. Aggregation of macrophages and fibroblasts is inhibited by a monoclonal antibody to the hyaluronate receptor. Exp Cell Res. 1988 Oct;178(2):224–232. doi: 10.1016/0014-4827(88)90393-x. [DOI] [PubMed] [Google Scholar]
- Green S. J., Tarone G., Underhill C. B. Distribution of hyaluronate and hyaluronate receptors in the adult lung. J Cell Sci. 1988 May;90(Pt 1):145–156. doi: 10.1242/jcs.90.1.145. [DOI] [PubMed] [Google Scholar]
- Gruber M. F., Webb D. S., Gerrard T. L. Stimulation of human monocytes via CD45, CD44, and LFA-3 triggers macrophage-colony-stimulating factor production. Synergism with lipopolysaccharide and IL-1 beta. J Immunol. 1992 Feb 15;148(4):1113–1118. [PubMed] [Google Scholar]
- Hasty K. A., Smith G. N., Jr, Kang A. H. Studies on glycosaminoglycans of regenerating rabbit ear cartilage. Dev Biol. 1981 Aug;86(1):198–205. doi: 10.1016/0012-1606(81)90330-4. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Hale L. P., Patton K. L., Martin M. E., McCallum R. M. Measurement of an adhesion molecule as an indicator of inflammatory disease activity. Up-regulation of the receptor for hyaluronate (CD44) in rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1434–1443. doi: 10.1002/art.1780341115. [DOI] [PubMed] [Google Scholar]
- Haynes B. F., Telen M. J., Hale L. P., Denning S. M. CD44--a molecule involved in leukocyte adherence and T-cell activation. Immunol Today. 1989 Dec;10(12):423–428. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- He Q., Lesley J., Hyman R., Ishihara K., Kincade P. W. Molecular isoforms of murine CD44 and evidence that the membrane proximal domain is not critical for hyaluronate recognition. J Cell Biol. 1992 Dec;119(6):1711–1719. doi: 10.1083/jcb.119.6.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiro D., Ito A., Matsuta K., Mori Y. Hyaluronic acid is an endogenous inducer of interleukin-1 production by human monocytes and rabbit macrophages. Biochem Biophys Res Commun. 1986 Oct 30;140(2):715–722. doi: 10.1016/0006-291x(86)90790-4. [DOI] [PubMed] [Google Scholar]
- Hughes E. N., Colombatti A., August J. T. Murine cell surface glycoproteins. Purification of the polymorphic Pgp-1 antigen and analysis of its expression on macrophages and other myeloid cells. J Biol Chem. 1983 Jan 25;258(2):1014–1021. [PubMed] [Google Scholar]
- Hughes E. N., Mengod G., August J. T. Murine cell surface glycoproteins. Characterization of a major component of 80,000 daltons as a polymorphic differentiation antigen of mesenchymal cells. J Biol Chem. 1981 Jul 10;256(13):7023–7027. [PubMed] [Google Scholar]
- Kirstein M., Aston C., Hintz R., Vlassara H. Receptor-specific induction of insulin-like growth factor I in human monocytes by advanced glycosylation end product-modified proteins. J Clin Invest. 1992 Aug;90(2):439–446. doi: 10.1172/JCI115879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacy B. E., Underhill C. B. The hyaluronate receptor is associated with actin filaments. J Cell Biol. 1987 Sep;105(3):1395–1404. doi: 10.1083/jcb.105.3.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le J., Vilcek J. Tumor necrosis factor and interleukin 1: cytokines with multiple overlapping biological activities. Lab Invest. 1987 Mar;56(3):234–248. [PubMed] [Google Scholar]
- Lesley J., He Q., Miyake K., Hamann A., Hyman R., Kincade P. W. Requirements for hyaluronic acid binding by CD44: a role for the cytoplasmic domain and activation by antibody. J Exp Med. 1992 Jan 1;175(1):257–266. doi: 10.1084/jem.175.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesley J., Hyman R. CD44 can be activated to function as an hyaluronic acid receptor in normal murine T cells. Eur J Immunol. 1992 Oct;22(10):2719–2723. doi: 10.1002/eji.1830221036. [DOI] [PubMed] [Google Scholar]
- Lesley J., Schulte R., Hyman R. Binding of hyaluronic acid to lymphoid cell lines is inhibited by monoclonal antibodies against Pgp-1. Exp Cell Res. 1990 Apr;187(2):224–233. doi: 10.1016/0014-4827(90)90085-o. [DOI] [PubMed] [Google Scholar]
- Lynch S. E., Colvin R. B., Antoniades H. N. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. J Clin Invest. 1989 Aug;84(2):640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Medina K. L., Hayashi S., Ono S., Hamaoka T., Kincade P. W. Monoclonal antibodies to Pgp-1/CD44 block lympho-hemopoiesis in long-term bone marrow cultures. J Exp Med. 1990 Feb 1;171(2):477–488. doi: 10.1084/jem.171.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake K., Underhill C. B., Lesley J., Kincade P. W. Hyaluronate can function as a cell adhesion molecule and CD44 participates in hyaluronate recognition. J Exp Med. 1990 Jul 1;172(1):69–75. doi: 10.1084/jem.172.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami S., Miyake K., June C. H., Kincade P. W., Hodes R. J. IL-5 induces a Pgp-1 (CD44) bright B cell subpopulation that is highly enriched in proliferative and Ig secretory activity and binds to hyaluronate. J Immunol. 1990 Dec 1;145(11):3618–3627. [PubMed] [Google Scholar]
- Nathan C., Sporn M. Cytokines in context. J Cell Biol. 1991 Jun;113(5):981–986. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nettelbladt O., Bergh J., Schenholm M., Tengblad A., Hällgren R. Accumulation of hyaluronic acid in the alveolar interstitial tissue in bleomycin-induced alveolitis. Am Rev Respir Dis. 1989 Mar;139(3):759–762. doi: 10.1164/ajrccm/139.3.759. [DOI] [PubMed] [Google Scholar]
- Nettelbladt O., Hällgren R. Hyaluronan (hyaluronic acid) in bronchoalveolar lavage fluid during the development of bleomycin-induced alveolitis in the rat. Am Rev Respir Dis. 1989 Oct;140(4):1028–1032. doi: 10.1164/ajrccm/140.4.1028. [DOI] [PubMed] [Google Scholar]
- Newman S. L., Tucci M. A. Regulation of human monocyte/macrophage function by extracellular matrix. Adherence of monocytes to collagen matrices enhances phagocytosis of opsonized bacteria by activation of complement receptors and enhancement of Fc receptor function. J Clin Invest. 1990 Sep;86(3):703–714. doi: 10.1172/JCI114766. [DOI] [PMC free article] [PubMed] [Google Scholar]
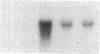
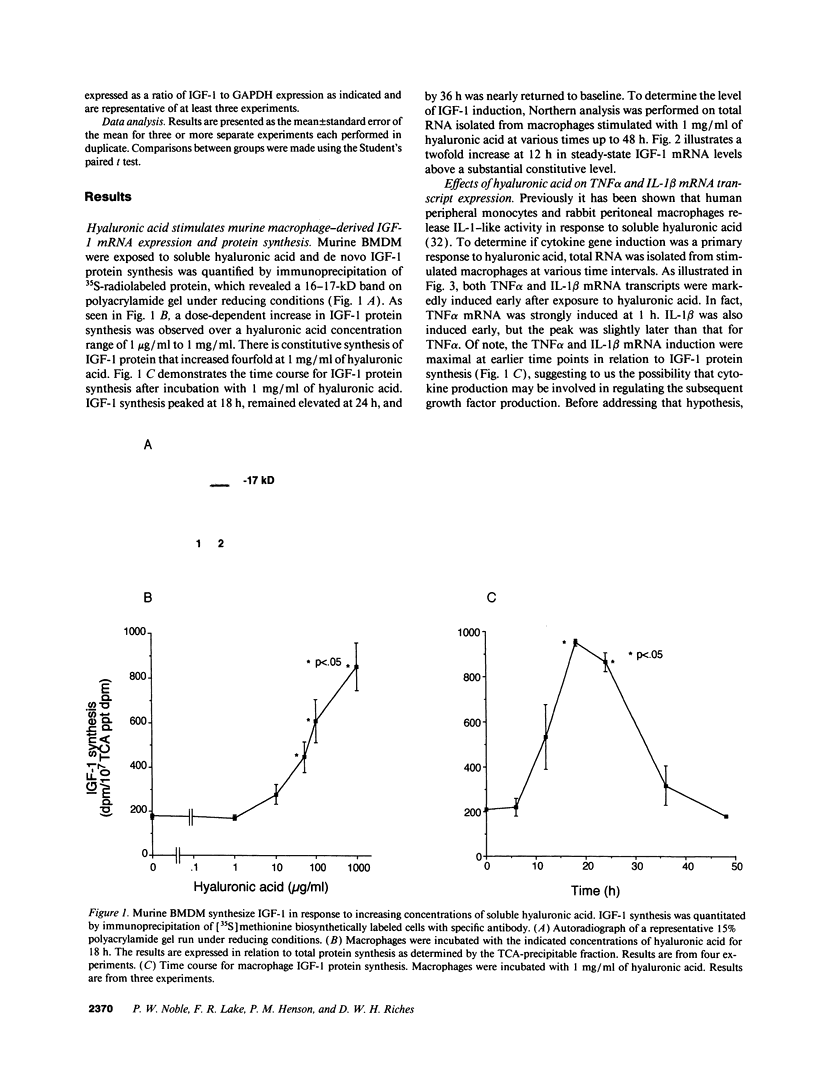
- Noble P. W., Henson P. M., Riches D. W. Insulin-like growth factor-1 (IGF-1) mRNA expression in bone marrow derived macrophages is stimulated by chrysotile asbestos and bleomycin. A potential marker for a reparative macrophage phenotype. Chest. 1991 Mar;99(3 Suppl):79S–80S. doi: 10.1378/chest.99.3_supplement.79s-a. [DOI] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Kapanci Y., Vassalli P. Tumor necrosis factor/cachectin plays a key role in bleomycin-induced pneumopathy and fibrosis. J Exp Med. 1989 Sep 1;170(3):655–663. doi: 10.1084/jem.170.3.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piguet P. F., Collart M. A., Grau G. E., Sappino A. P., Vassalli P. Requirement of tumour necrosis factor for development of silica-induced pulmonary fibrosis. Nature. 1990 Mar 15;344(6263):245–247. doi: 10.1038/344245a0. [DOI] [PubMed] [Google Scholar]
- Postlethwaite A. E., Smith G. N., Jr, Lachman L. B., Endres R. O., Poppleton H. M., Hasty K. A., Seyer J. M., Kang A. H. Stimulation of glycosaminoglycan synthesis in cultured human dermal fibroblasts by interleukin 1. Induction of hyaluronic acid synthesis by natural and recombinant interleukin 1s and synthetic interleukin 1 beta peptide 163-171. J Clin Invest. 1989 Feb;83(2):629–636. doi: 10.1172/JCI113927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Rappolee D. A., Werb Z. Secretory products of phagocytes. Curr Opin Immunol. 1988 Sep-Oct;1(1):47–55. doi: 10.1016/0952-7915(88)90050-7. [DOI] [PubMed] [Google Scholar]
- Rennard S. I., Hunninghake G. W., Bitterman P. B., Crystal R. G. Production of fibronectin by the human alveolar macrophage: mechanism for the recruitment of fibroblasts to sites of tissue injury in interstitial lung diseases. Proc Natl Acad Sci U S A. 1981 Nov;78(11):7147–7151. doi: 10.1073/pnas.78.11.7147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riches D. W., Underwood G. A. Expression of interferon-beta during the triggering phase of macrophage cytocidal activation. Evidence for an autocrine/paracrine role in the regulation of this state. J Biol Chem. 1991 Dec 25;266(36):24785–24792. [PubMed] [Google Scholar]
- Rom W. N., Basset P., Fells G. A., Nukiwa T., Trapnell B. C., Crysal R. G. Alveolar macrophages release an insulin-like growth factor I-type molecule. J Clin Invest. 1988 Nov;82(5):1685–1693. doi: 10.1172/JCI113781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sara V. R., Hall K. Insulin-like growth factors and their binding proteins. Physiol Rev. 1990 Jul;70(3):591–614. doi: 10.1152/physrev.1990.70.3.591. [DOI] [PubMed] [Google Scholar]
- Shannon B. T., Love S. H., Myrvik Q. N. Participation of hyaluronic acid in the macrophage disappearance reaction. Immunol Commun. 1980;9(4):357–370. doi: 10.3109/08820138009052982. [DOI] [PubMed] [Google Scholar]
- Solis-Herruzo J. A., Brenner D. A., Chojkier M. Tumor necrosis factor alpha inhibits collagen gene transcription and collagen synthesis in cultured human fibroblasts. J Biol Chem. 1988 Apr 25;263(12):5841–5845. [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartaglia L. A., Weber R. F., Figari I. S., Reynolds C., Palladino M. A., Jr, Goeddel D. V. The two different receptors for tumor necrosis factor mediate distinct cellular responses. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9292–9296. doi: 10.1073/pnas.88.20.9292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C. B., Thurn A. L., Lacy B. E. Characterization and identification of the hyaluronate binding site from membranes of SV-3T3 cells. J Biol Chem. 1985 Jul 5;260(13):8128–8133. [PubMed] [Google Scholar]
- Underhill C. B., Toole B. P. Binding of hyaluronate to the surface of cultured cells. J Cell Biol. 1979 Aug;82(2):475–484. doi: 10.1083/jcb.82.2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilcek J., Palombella V. J., Henriksen-DeStefano D., Swenson C., Feinman R., Hirai M., Tsujimoto M. Fibroblast growth enhancing activity of tumor necrosis factor and its relationship to other polypeptide growth factors. J Exp Med. 1986 Mar 1;163(3):632–643. doi: 10.1084/jem.163.3.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb D. S., Shimizu Y., Van Seventer G. A., Shaw S., Gerrard T. L. LFA-3, CD44, and CD45: physiologic triggers of human monocyte TNF and IL-1 release. Science. 1990 Sep 14;249(4974):1295–1297. doi: 10.1126/science.1697984. [DOI] [PubMed] [Google Scholar]
- Werb Z., Tremble P. M., Behrendtsen O., Crowley E., Damsky C. H. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989 Aug;109(2):877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witsell A. L., Schook L. B. Tumor necrosis factor alpha is an autocrine growth regulator during macrophage differentiation. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4754–4758. doi: 10.1073/pnas.89.10.4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolffe E. J., Gause W. C., Pelfrey C. M., Holland S. M., Steinberg A. D., August J. T. The cDNA sequence of mouse Pgp-1 and homology to human CD44 cell surface antigen and proteoglycan core/link proteins. J Biol Chem. 1990 Jan 5;265(1):341–347. [PubMed] [Google Scholar]