Abstract
Background
The optimal post-operative care regimen after surgically fixed Lauge Hansen supination exorotation injuries remains to be established. This study compares whether unprotected weight bearing as tolerated is superior to protected weight bearing and unprotected non-weight bearing in terms of functional outcome and safety.
Methods/Design
The WOW! Study is a prospective multicenter clinical trial. Patients between 18 and 65 years of age with a Lauge Hansen supination exorotation type 2, 3 or 4 ankle fractures requiring surgical treatment are eligible for inclusion. An expert panel validates the classification and inclusion eligibility. After surgery, patients are randomized to either the 1) unprotected non-weight-bearing, 2) protected weight-bearing, or 3) unprotected weight-bearing group.
The primary outcome measure is ankle-specific disability measured by the Olerud-Molander ankle score. Secondary outcomes are 1) quality of life (e.g., return to work and resumption of sport), 2) complications, 3) range of motion, 4) calf wasting, and 5) maximum pressure load after 3 months and 1 year.
Discussion
This trial is designed to compare the effectiveness and safety of unprotected weight bearing with two commonly used post-operative treatment regimens after internal fixation of specified, intrinsically stable but displaced ankle fractures. An expert panel has been established to evaluate every potential subject, which ensures that every patient is strictly screened according to the inclusion and exclusion criteria and that there is a clear indication for surgical fixation.
Trial registration
The WOW! Study is registered in the Dutch Trial Register (NTR3727). Date of registration: 28-11-2012.
Keywords: Ankle, Ankle fracture, Lauge Hansen, Post-operative care regimen, Weight bearing
Background and rationale
Ankle fractures in adults occur frequently with an annual incidence of approximately 100 to 180 fractures per 100,000 people each year [1-3]. Most ankle fractures occur after inversion or eversion twisting trauma and sports injuries. For stable non-displaced ankle fractures, a conservative treatment with a splint or cast is indicated [4,5]. However, when the congruity of the ankle fork or the joint stability is compromised, open reduction and internal fixation is required to attain full function of the ankle joint [6,7].
While indications for surgical treatment are rather well defined, controversy exists with regard to the optimal postoperative care regimen [8]. Post-operative care regimens vary widely from plaster casts and functional bracing to unprotected non-weight bearing and weight bearing [9-15]. Prior studies show a possible advantage of (protected) weight bearing over (protected) non-weight bearing and of functional mobilization over non-functional mobilization with crutches [11,16]. Some recent, small retrospective and matched control studies even suggested that early (unprotected) weight bearing results in faster recovery and better ankle function [11-13].
The objective of this trial is to compare functional outcome and safety after three different post-operative care regimens: 1) unprotected non weight bearing, 2) protected weight bearing, and 3) unprotected weight bearing.
Methods and design
Study design
This is a prospective multicenter randomized controlled trial involving six hospitals in the Netherlands. The study has been approved by the local Institutional Review Board under protocol number WOW-01/NL40835.100.12. This study compares three different post-operative care regimens after ankle surgery; all patients with a Lauge Hansen supination exorotation type 2, 3 or 4 ankle fractures requiring surgical treatment are eligible for inclusion.
After registration of the patient, an anonymous X-ray is sent to an expert panel consisting of six experienced orthopedic trauma surgeons. The fracture is classified according to the Lauge Hansen classification and advice on whether or not operative fixation is indicated is provided within 24 hours. A patient is eligible for inclusion when a majority of the expert panel agrees that operative fixation is necessary and the fracture type meets the inclusion criteria. When votes are split equally, the chairman (LL) is the tiebreaker. A flow chart of the study is shown in Figure 1.
Figure 1.
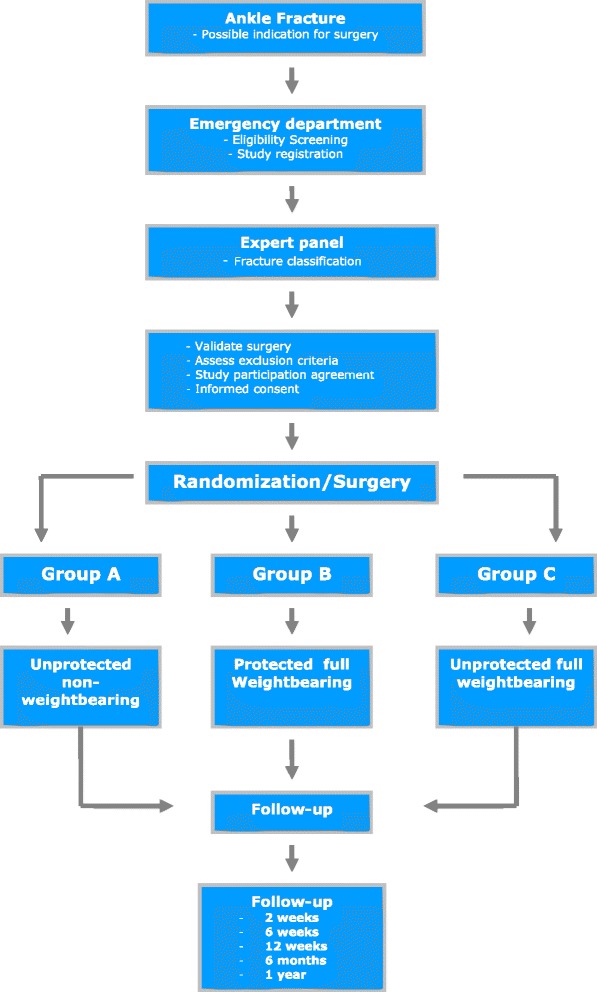
Flowchart WOW! Study.
Patient population
Non-pregnant, Dutch speaking patients are being recruited from the participating hospitals in the emergency room or pre-operatively during an outpatient department visit. Patients are screened for eligibility according to the inclusion and exclusion criteria (Table 1). The treating physician or study staff explains the study and informed consent is obtained from each patient. Retrospective data of the participating hospitals shows that approximately 395 patients per year undergo surgery for ankle fractures. Approximately 20% of these patients would be eligible for enrollment according to our inclusion criteria. If this trend continues, we anticipate enrollment of 79 patients per year (Table 1).
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Exclusion criteria |
|---|---|
| Age ranging from 18–65 years | Pre-existent impaired mobility |
| Fractures classified as Lauge Hansen supination eversion type 2–3 or 4 | Expected insufficient stable fracture fixation with standard surgical technique |
| Articular discongruity of >2 mm on X-ray | Pre-existent cognitive disability |
| Necessity for a syndesmosis screw | |
| Tertius fragment requiring operative fixation | |
| Body mass index >30 | |
| Diabetes mellitus | |
| Polytrauma patients (ISS a >16 or >2 AIS b regions involved) | |
| Gustillo 2 and 3 open fractures | |
| Inability to comply with non-weight-bearing mobilization | |
| Inability to comply with follow-up |
aInjury Severity Score.
bAbbreviated Injury Scale.
Inclusion and exclusion criteria
All patients between 18 and 65 years with a Lauge Hansen supination exorotation type 2, 3 and 4 ankle fractures are eligible for inclusion (Figure 2). Exclusion criteria include pre-existent impaired morbidity, cognitive disability, body mass index >30, and diabetes mellitus. All inclusion and exclusion criteria are listed in Table 1.
Figure 2.
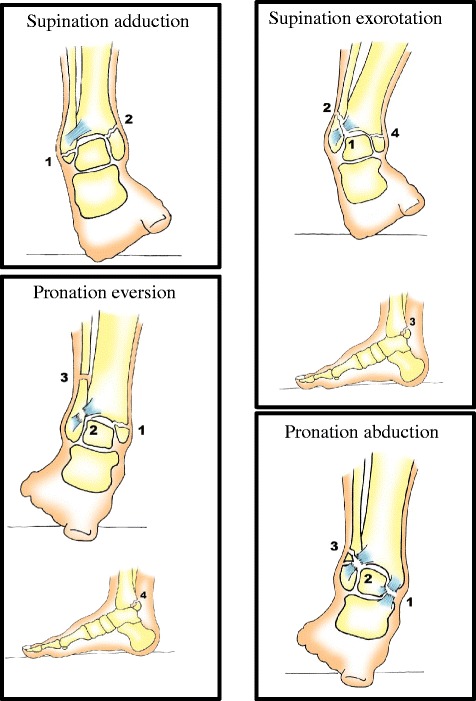
Lauge Hansen classification. Trauma mechanism of ankle fractures. Supination abduction: 1. Talofibular ligament sprain or fibular avulsion; 2. Vertical medial malleolus fracture. Supination eversion: 1. anterior tibiofibular ligament sprain; 2. Lateral oblique fibular fracture; 3. Avulsion of posterior malleolus or ligament rupture; 4. Transverse medial malleolus fracture or disruption of deltoid ligament. Pronation abduction: 1. Transverse medial malleolus fracture or deltoid ligament; 2. Anterior tibiofibular ligament sprain; 3. Transverse comminuted fracture of the fibula. Pronation exorotation: 1. Transverse medial malleolus fracture or deltoid ligament disruption; 2. Anterior tibiofibular ligament disruption; 3. Oblique or spiral fracture of the fibula; 4. Avulsion of posterior malleolus of posterior tibiofibular ligament.
Interventions
After enrollment, patients are scheduled for surgery. The ankle fixation technique is ultimately determined by the treating physician; however, advice is provided by the expert panel based on the X-ray. After surgery, all patients are randomized to one of three post-operative care regimens: 1) unprotected non-weight-bearing group – functional weight bearing as tolerated; 2) protected weight-bearing group – weight bearing as tolerated with a below knee cast for 6 weeks; 3) unprotected weight-bearing group – mobilization with crutches, active ankle exercises. All patients see a physiotherapist post-operatively or after cast removal to learn exercises and receive advice on how to start mobilizing according to their specific post-operative care regimen. The physiotherapists have been instructed by the investigators. Patients in the protected weight-bearing group receive low molecular weight heparin as thrombosis prophylaxis for the entire duration of immobilization. For the unprotected non-weight-bearing and unprotected weight-bearing groups, anti-thrombotic treatment is not indicated.
Randomization process
Patients are randomized using computerized block randomization to either protected weight bearing, unprotected non-weight bearing, or unprotected weight bearing. The randomization is stratified by the participating hospitals. The blocks for randomization consist of 21 patients with the three post-operative care regimens equally represented in each block. Follow-up is by the intention-to-treat principle. Following surgical fixation, the treating surgeon logs into the private website where the patient is randomized to one of three post-operative care regimens.
Postoperative management and follow-up
Patients are treated during same day admission if possible. According to their post-operative care regimen, patients receive a cast, exercise instructions, and a physiotherapist referral letter. All patients are reviewed in the outpatient clinic by the treating surgeon and/or investigator at 2 weeks, 6 weeks, 3 months, 6 months, and 1 year after surgery. At all post-operative outpatient visits, a standardized clinical exam is conducted including three standardized patient reported outcome questionnaires; the Olerud Molander Ankle Score (OMAS), the Short Form-36 (SF-36), and the Visual Analogue Scale (Table 2).
Table 2.
Study assessments by time-point
| Time point | Intake | 2 weeks | 6 weeks | 12 weeks | 6 months | 1 year |
|---|---|---|---|---|---|---|
| OMASa | X | X | X | X | X | |
| SF-36b | X | X | X | |||
| Wound inspection | X | X | ||||
| Calf circumference | X | X | X | X | ||
| Range of motion | X | X | X | X | ||
| Demographics | X |
aOlerud Molander Ankle Score.
bShort Form 36.
Primary outcome measures
The OMAS is a scoring scale for symptom evaluation in patients with an ankle fracture and in patients with other acute ankle injuries [17]. This score ranges from 0 to 100%, with 100% representing normal ankle function [17]. It was developed specifically as a comparative research measure to improve consistency and uniformity in ankle injury reporting [17]. A difference of 5 to 10 points between two groups on the OMAS is defined as a clinically relevant result.
Secondary outcome measures
The SF-36 is one of the most widely used generic health questionnaires. It was developed as a medical outcome score to measure the functional health status of a patient [18,19]. It has been translated into Dutch and validated as a useful questionnaire to assess a broad array of health-related quality-of-life issues [19].
Other secondary measures of function include range of motion (ROM) (plantar and dorso-flexion), weight-bearing pressure load, and calf wasting (difference in calf circumference at enrollment and at 12 weeks post-operative) of the injured ankle. Pain is measured by the Visual Analogue Scale on an 11-point Likert scale. Return to work and sports is also recorded. The pressure load of the injured and non-injured ankle is measured by a scale at every visit, starting at 6 weeks.
Post-operative complications are defined as 1) wound healing problems (no interventions required), 2) superficial wound infections (requiring oral antibiotic treatment based on a wound culture), 3) infection near hardware (requiring surgical debridement and intravenous antibiotic treatment based on a deep tissue culture), 4) hardware failure (requiring re-operation), 5) mal-union or non-union (clinically and radiographically confirmed), and 6) deep venous thrombosis (confirmed by ultrasound).
Sample size and power
Our hypothesis is that ankle-specific disability assessed with the OMAS is less for unprotected weight bearing when compared to protected weight bearing and unprotected non-weight bearing. An a priori power analysis for superiority of treatment with unprotected weight bearing has been conducted for this hypothesis. To detect a clinically significant 7-point difference on the OMAS at 12 weeks follow-up between unprotected non-weight bearing and unprotected weight bearing with a standard deviation of 10, α = 0.05, β = 0.90, two-sided test (based on superiority of unprotected weight bearing), and a maximum loss to follow-up of 20%, a sample size of 75 patients per group is necessary. Therefore, a total of 225 patients are needed for this study.
Statistical analysis
Analysis will be conducted according to the intention-to-treat principle. In bivariable analysis, Pearson’s correlation will be used for continuous variables, Student’s t-test for dichotomous variables such as gender, and ANOVA for categorical variables such as the post-operative care regimen. Multivariate analyses will only be performed post hoc and are considered hypothesis-inducing as opposed to hypothesis-testing and will be ascribed as such.
Data and safety monitoring board (DSMB) and interim analysis
Participation in this trial does not elicit additional risks besides the standard complications of ankle surgery, such as wound infections, deep venous thrombosis, and hardware failure [12,13]. Although all three post-operative care regimens are independently investigated, unprotected weight bearing by unrestricted mobilization as tolerated has not yet been investigated in a randomized trial. Therefore, strict criteria for premature termination are implemented.
A DSMB has been established. An interim analysis will be conducted after every serious adverse event and after half of the target enrollment is reached. The DSMB consists of two independent physicians and one clinical epidemiologist. The members are not committed to this trial. The DSMB will provide advice that will be disclosed to the Institutional Review Board. The steering committee may terminate the study prematurely if advised by the DSMB. In addition, statistically significant, sufficiently powered, and clinically relevant results during interim analysis may be compelling to terminate the trial prematurely. For this trial, the following termination criteria have been established:
Ethical approval
This study will be conducted according to the principles of the Declaration of Helsinki (version 9, October 2008, Seoul) and in accordance with the Medical Research Involving Human Subjects Act. The Verenigde Commissie Mensgebonden Onderzoek (VCMO) approved the study in the St. Antonius Hospital and Diakonessenhuis. The Centrale Commissie Mensgebonden Onderzoek approved the study in Medisch Centrum Haaglanden. The Medisch Ethische Toetsingscommissie approved the study in the St. Elisabeth hospital. Ethical Approval from the Twee Steden hospital was obtained from the board of directors based on VCMO approval.
Discussion
This trial is designed to compare the effectiveness and safety of unprotected weight bearing with two commonly used post-operative treatment regimens after internal fixation of specified, intrinsically stable but displaced ankle fractures. An expert panel has been established to evaluate every potential subject, which ensures that every patient is strictly screened according to the inclusion and exclusion criteria and that there is a clear indication for surgical fixation.
Prior research reports high patient satisfaction scores and no disadvantage for early weight bearing [12,13,22]. The combination of functional treatment and early weight bearing may reduce soft tissue atrophy and development of osteoporosis and better preserve ankle ROM [13]. Therefore, direct postoperative weight bearing and early mobilization has the potential benefit of earlier functional recovery [8-14,16,23,24].
Ankle fractures in this trial are described by the Lauge Hansen classification. This classification describes the intrinsic stability, demonstrates the trauma mechanism and incorporates ligament injuries [25]. This trial aims to provide evidence for the optimal post-operative care regimen after surgical repair, solely for Lauge Hansen supination exorotation 2–3 and 4 ankle fractures. In contrast to the majority of studies that include patients with all types of ankle fractures requiring operative fixation, this study describes a relatively stable fracture, which may be more suitable for immediate unprotected weight bearing [11-13]. A limitation of the Lauge Hansen classification is its low inter-observer kappa value [26]. The expert panel has been implemented to address this and minimize inter-observer variability.
There are limitations to this study. Not all available post-operative care regimens, such as functional bracing, are studied in this trial, as it has been suggested that functional bracing is associated with an increased risk of post-operative wound healing problems [21].
The termination criteria of this trial exceed the percentages mentioned in literature; the majority of these studies are retrospective studies and small prospective trials [11,20,21]. However, the use of strict termination criteria is important to ensure the safety of the patient.
This prospective randomized controlled trial compares three different post-operative care regimens after open reduction and internal fixation of ankle fractures. By analyzing ankle disability, pain, quality of life, ROM, weight bearing, and resumption of daily activities, this trial assesses the optimal post-operative care regimen for a specific ankle fracture.
Trial status
The institutional review board has approved the study and patient enrollment has begun in five of the six participating centers. Approval is still pending in one hospital. Recruitment commenced in February 2013 and 73 patients are currently enrolled in this study. Inclusion rates are expected to increase now that most participating centers have received approval. Based on our power analysis and expected yearly inclusion of 79 patients, enrollment of the 225th patient is expected in October 2016. Analysis will be conducted 1 year later once follow-up is completed.
Acknowledgements
Charlotte S van Kessel: recruitment and enrollment of patients. Arno Teutelink: recruitment and enrollment of patients. Alice C Meijer: Digital graphic design of Lauge Hansen figures.
Abbreviations
- DSMB
Data safety monitoring board
- OMAS
Olerud Molander ankle score
- ROM
Range of motion
- SF-36
Short form 36
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
JPB, RMH, DPJS, JSP, KWL, LPHL, PZ, SWAMZ, JMH, MH, EJMMV, GWL, and MJS participated in recruiting and enrolling patients for this study. JPB, RMH, FH, EJMMV, and participated in writing the manuscript. FH, MJS, RMH, JSP, EJMMV, and LPHL conceived this study and participated in its design. JCK provided statistical assistance for the study protocol. All authors read and approved the final version of the manuscript.
Contributor Information
Jan Paul Briet, Email: jp.briet@gmail.com.
Roderick M Houwert, Email: marijnhouwert@hotmail.com.
Diederik PJ Smeeing, Email: diederiksmeeing@hotmail.com.
Janity S Pawiroredjo, Email: j.pawiroredjo@hotmail.com.
Johannes C Kelder, Email: keld01@antoniusziekenhuis.nl.
Koen W Lansink, Email: k.lansink@elisabeth.nl.
Luke PH Leenen, Email: lleenen@umcutrecht.nl.
Peer van der Zwaal, Email: peerzwaal@hotmail.com.
Stephan WAM van Zutphen, Email: s.v.zuthpen@elisabeth.nl.
Jochem M Hoogendoorn, Email: j.hoogendoorn@mchaaglanden.nl.
Mark van Heijl, Email: markvanheijl@gmail.com.
Egbert JMM Verleisdonk, Email: ejverlei@diakhuis.nl.
Guus W van Lammeren, Email: g.van.lammeren@antoniusziekenhuis.nl.
Michiel J Segers, Email: m.segers@antoniusziekenhuis.nl.
Falco Hietbrink, Email: f.hietbrink@hotmail.com.
References
- 1.Jensen SL, Andresen BK, Mencke S, Nielsen PT. Epidemiology of ankle fractures: a prospective population-based study of 212 cases in Aalborg, Denmark. Acta Orthop Scand. 1998;69:48–50. doi: 10.3109/17453679809002356. [DOI] [PubMed] [Google Scholar]
- 2.Daly PJ, Fitzgerald RH, Jr, Melton LJ, Ilstrup DM. Epidemiology of ankle fractures in Rochester, Minnesota. Acta Orthop Scand. 1987;58:539–44. doi: 10.3109/17453678709146395. [DOI] [PubMed] [Google Scholar]
- 3.Donken CC, Al-Khateeb H, Verhofstad MH, Van Laarhoven CJ. Surgical versus conservative interventions for treating ankle fractures in adults. Cochrane Database Syst Rev. 2012;8 doi: 10.1002/14651858.CD008470.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Kristensen KD, Hansen T. Closed treatment of ankle fractures: stage II supination-eversion fractures followed for 20 years. Acta Orthop Scand. 1985;56:107–9. doi: 10.3109/17453678508994330. [DOI] [PubMed] [Google Scholar]
- 5.Bauer M, Jonsson K, Nilsson B. Thirty-year follow-up of ankle fractures. Acta Orthop Scand. 1985;56:103–6. doi: 10.3109/17453678508994329. [DOI] [PubMed] [Google Scholar]
- 6.Michelson JD. Fractures about the ankle. J Bone Joint Surg Am. 1995;77:142–52. doi: 10.2106/00004623-199501000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Marsh JL, Saltzman CL. Ankle fractures. In: Bucholz RW, Heckman JD, editors. Rockwood and Green’s Fractures in Adults. 7. Philadelphia: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 8.Lin CW, Donkers NA, Refshauge KM, Beckenkamp PR, Khera K, Moseley AM. Rehabilitation for ankle fractures in adults. Cochrane Database Syst Rev. 2012;11 doi: 10.1002/14651858.CD005595.pub3. [DOI] [PubMed] [Google Scholar]
- 9.Ahl T, Dalen N, Holmberg S, Selvik G. Early weight bearing of displaced ankle fractures. Acta Orthop Scand. 1987;58:535–8. doi: 10.3109/17453678709146394. [DOI] [PubMed] [Google Scholar]
- 10.Ahl T, Dalen N, Selvik G. Mobilization after operation of ankle fractures: good results of early motion and weight bearing. Acta Orthop Scand. 1988;59:302–6. doi: 10.3109/17453678809149368. [DOI] [PubMed] [Google Scholar]
- 11.van Laarhoven CJ, Meeuwis JD, van der Werken C. Postoperative treatment of internally fixed ankle fractures: a prospective randomised study. J Bone Joint Surg Am. 1996;78:395–9. [PubMed] [Google Scholar]
- 12.Simanski CJ, Maegele MG, Lefering R, Lehnen DM, Kawel N, Riess P, et al. Functional treatment and early weightbearing after an ankle fracture: a prospective study. J Orthop Trauma. 2006;20:108–14. doi: 10.1097/01.bot.0000197701.96954.8c. [DOI] [PubMed] [Google Scholar]
- 13.Gul A, Batra S, Mehmood S, Gillham N. Immediate unprotected weight-bearing of operatively treated ankle fractures. Acta Orthop Belg. 2007;73:360–5. [PubMed] [Google Scholar]
- 14.Thomas G, Whalley H, Modi C. Early mobilization of operatively fixed ankle fractures: a systematic review. Foot Ankle Int. 2009;30:666–74. doi: 10.3113/FAI.2009.0666. [DOI] [PubMed] [Google Scholar]
- 15.Ahl T, Dalen N, Holmberg S, Selvik G. Early weight bearing of malleolar fractures. Acta Orthop Scand. 1986;57:526–9. doi: 10.3109/17453678609014785. [DOI] [PubMed] [Google Scholar]
- 16.Finsen V, Saetermo R, Kibsgaard L, Farran K, Engebretsen L, Bolz KD, et al. Early postoperative weight-bearing and muscle activity in patients who have a fracture of the ankle. J Bone Joint Surg Am. 1989;71:23–7. [PubMed] [Google Scholar]
- 17.Olerud C, Molander H. A scoring scale for symptom evaluation after ankle fracture. Arch Orthop Trauma Surg. 1984;103:190–4. doi: 10.1007/BF00435553. [DOI] [PubMed] [Google Scholar]
- 18.Aaronson NK, Muller M, Cohen PD, Essink-Bot ML, Fekkes M, Sanderman R, et al. Translation, validation, and norming of the Dutch language version of the SF-36 Health Survey in community and chronic disease populations. J Clin Epidemiol. 1998;51:1055–68. doi: 10.1016/S0895-4356(98)00097-3. [DOI] [PubMed] [Google Scholar]
- 19.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30:473–83. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 20.SooHoo NF, Krenek L, Eagan MJ, Gurbani B, Ko CY, Zingmond DS. Complication rates following open reduction and internal fixation of ankle fractures. J Bone Joint Surg Am. 2009;91:1042–9. doi: 10.2106/JBJS.H.00653. [DOI] [PubMed] [Google Scholar]
- 21.Lehtonen H, Jarvinen TL, Honkonen S, Nyman M, Vihtonen K, Jarvinen M. Use of a cast compared with a functional ankle brace after operative treatment of an ankle fracture: a prospective, randomized study. J Bone Joint Surg Am. 2003;85-A:205–11. doi: 10.2106/00004623-200302000-00004. [DOI] [PubMed] [Google Scholar]
- 22.Partenheimer A, Geerling J, Voigt C, Lill H. Early functional treatment and full weight-bearing of surgically treated isolated ankle fractures in the elderly. Unfallchirurg. 2010;113:308–12. doi: 10.1007/s00113-009-1724-9. [DOI] [PubMed] [Google Scholar]
- 23.DiStasio AJ, 2nd, Jaggears FR, DePasquale LV, Frassica FJ, Turen CH. Protected early motion versus cast immobilization in postoperative management of ankle fractures. Contemp Orthop. 1994;29:273–7. [PubMed] [Google Scholar]
- 24.Vioreanu M, Dudeney S, Hurson B, Kelly E, O’Rourke K, Quinlan W. Early mobilization in a removable cast compared with immobilization in a cast after operative treatment of ankle fractures: a prospective randomized study. Foot Ankle Int. 2007;28:13–9. doi: 10.3113/FAI.2007.0003. [DOI] [PubMed] [Google Scholar]
- 25.Lauge-Hansen N. Fractures of the ankle. II. Combined experimental-surgical and experimental-roentgenologic investigations. Arch Surg. 1950;60:957–85. doi: 10.1001/archsurg.1950.01250010980011. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen NO, Overgaard S, Olsen LH, Hansen H, Nielsen ST. Observer variation in the radiographic classification of ankle fractures. J Bone Joint Surg Am. 1991;73:676–8. doi: 10.1302/0301-620X.73B4.2071659. [DOI] [PubMed] [Google Scholar]


