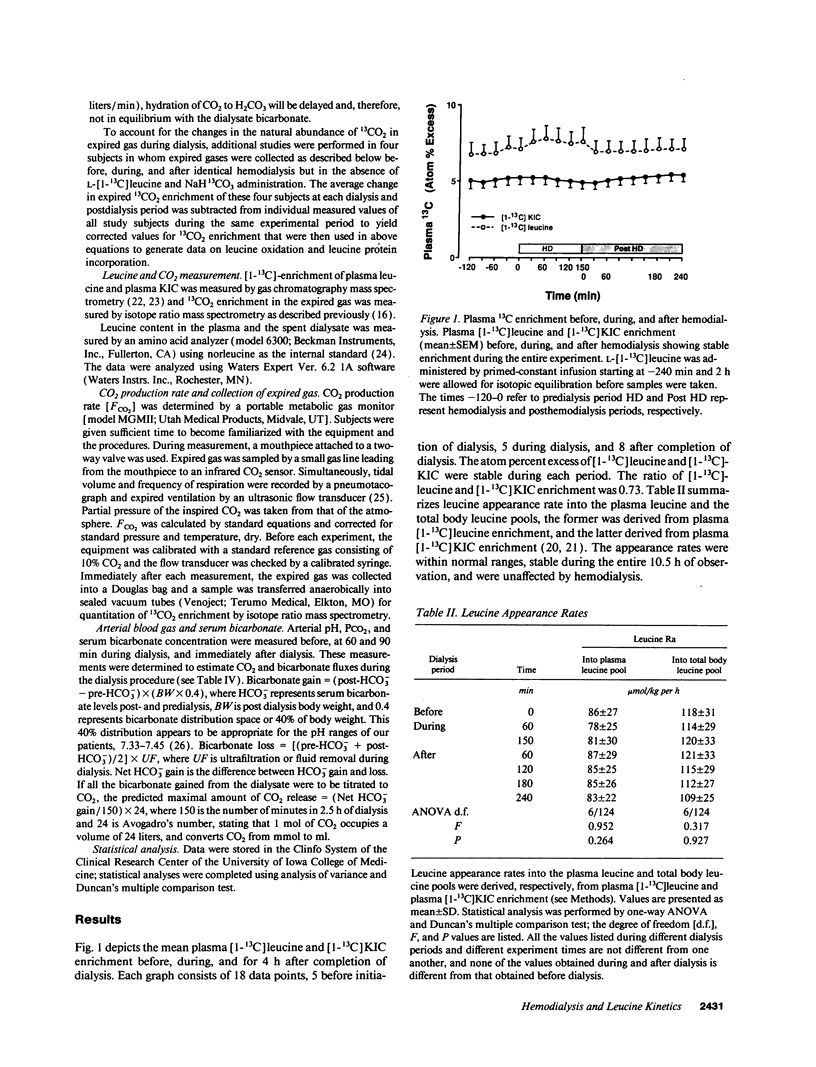
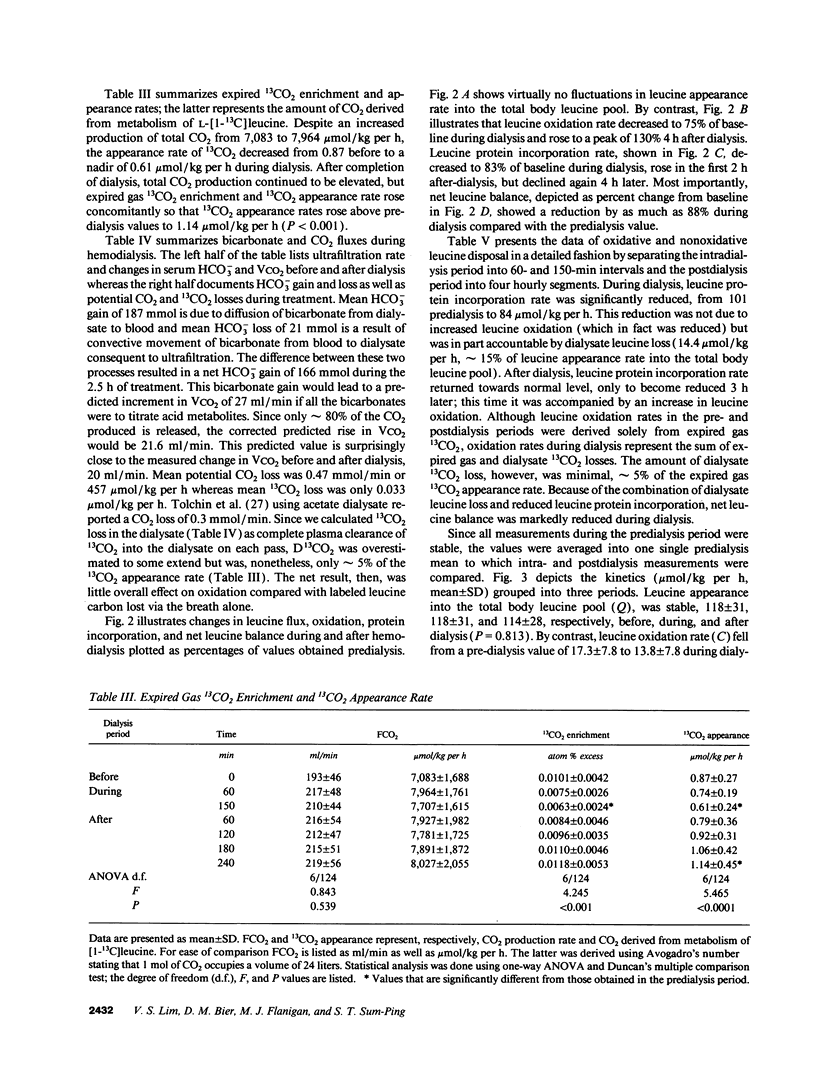
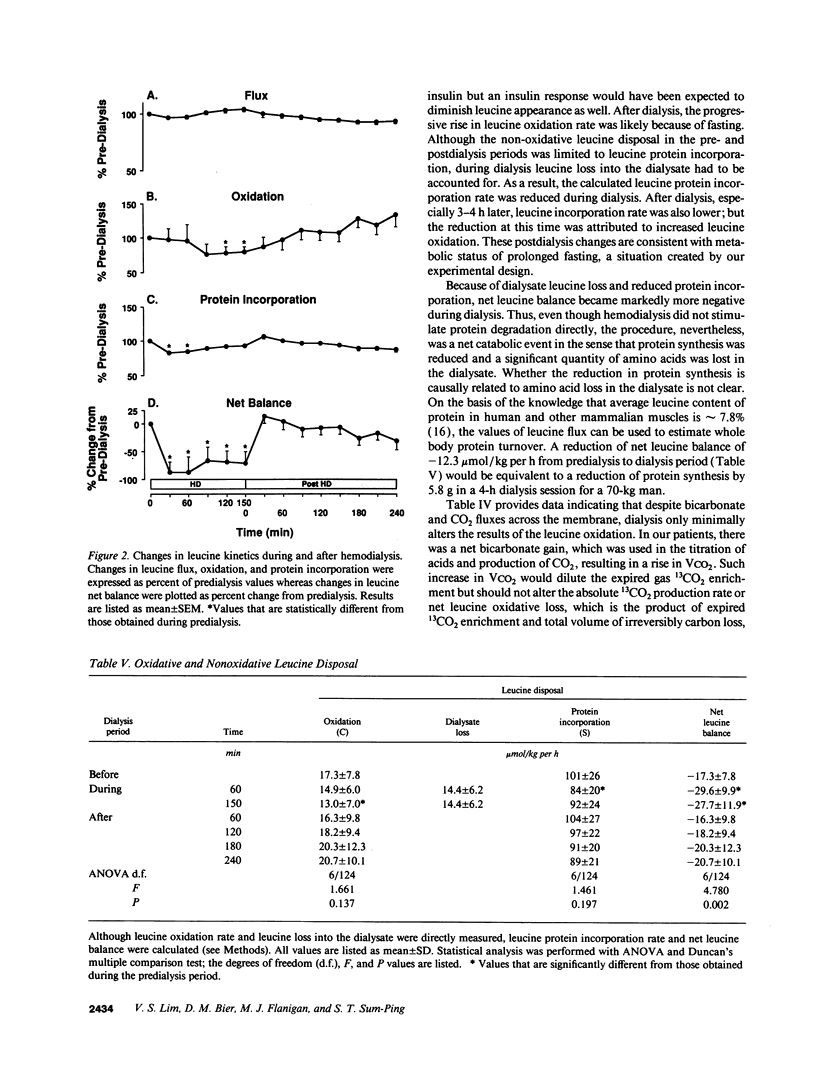
Abstract
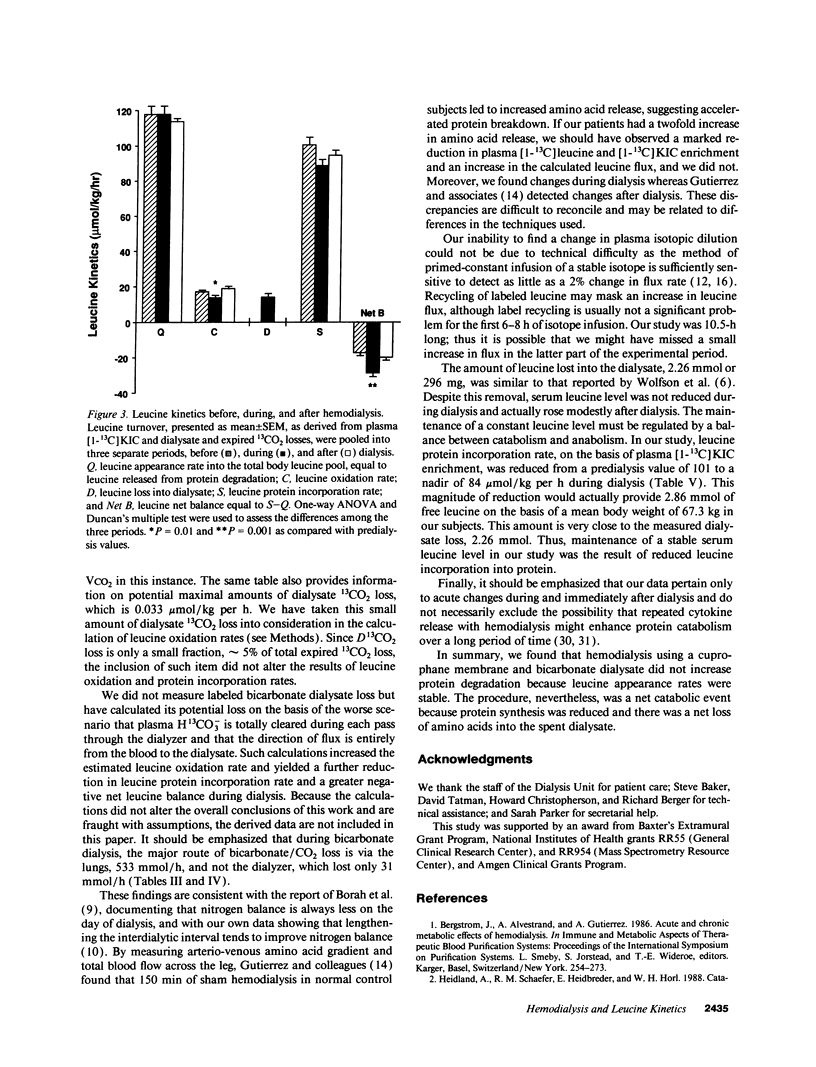
To assess the effect of hemodialysis on protein metabolism, leucine flux was measured in seven patients before, during, and after high efficiency hemodialysis using cuprophane dialyzers and bicarbonate dialysate during a primed-constant infusion of L-[1-13C]leucine. The kinetics [mumol/kg per h, mean +/- SD] are as follows: leucine appearance into the plasma leucine pool was 86 +/- 28, 80 +/- 28, and 85 +/- 25, respectively, before, during, and after dialysis. Leucine appearance into the whole body leucine pool, derived from plasma [1-13C]alpha-ketoisocaproate enrichment, was 118 +/- 31, 118 +/- 31, and 114 +/- 28 before, during, and after dialysis, respectively. In the absence of leucine intake, appearance rate reflects protein degradation, which was clearly unaffected by dialysis. Leucine oxidation rate was 17.3 +/- 7.8 before, decreased to 13.8 +/- 7.8 during, and increased to 18.9 +/- 10.3 after dialysis (P = 0.027). Leucine protein incorporation was 101 +/- 26 before, was reduced to 89 +/- 23 during, and returned to 95 +/- 23 after dialysis (P = 0.13). Leucine net balance, the difference between leucine protein incorporation and leucine release from endogenous degradation, was -17.3 +/- 7.8 before, decreased to -28.5 +/- 11.0 during, and returned to -18.9 +/- 10.3 after dialysis (P < 0.0001). This markedly more negative leucine balance during dialysis was accountable by dialysate leucine loss, which was 14.4 +/- 6.2 mumol/kg per h. These data suggest that hemodialysis using a cuprophane membrane did not acutely induce protein degradation. It was, nevertheless, a net catabolic event because protein synthesis was reduced and amino acid was lost into the dialysate.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrogué H. J., Brensilver J., Cohen J. J., Madias N. E. Influence of steady-state alterations in acid-base equilibrium on the fate of administered bicarbonate in the dog. J Clin Invest. 1983 Apr;71(4):867–883. doi: 10.1172/JCI110841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvestrand A., Fürst P., Bergström J. Intracellular amino acids in uremia. Kidney Int Suppl. 1983 Dec;16:S9–16. [PubMed] [Google Scholar]
- Alvestrand A., Fürst P., Bergström J. Plasma and muscle free amino acids in uremia: influence of nutrition with amino acids. Clin Nephrol. 1982 Dec;18(6):297–305. [PubMed] [Google Scholar]
- Bier D. M. Intrinsically difficult problems: the kinetics of body proteins and amino acids in man. Diabetes Metab Rev. 1989 Mar;5(2):111–132. doi: 10.1002/dmr.5610050203. [DOI] [PubMed] [Google Scholar]
- Borah M. F., Schoenfeld P. Y., Gotch F. A., Sargent J. A., Wolfsen M., Humphreys M. H. Nitrogen balance during intermittent dialysis therapy of uremia. Kidney Int. 1978 Nov;14(5):491–500. doi: 10.1038/ki.1978.154. [DOI] [PubMed] [Google Scholar]
- Farrell P. C., Hone P. W. Dialysis-induced catabolism. Am J Clin Nutr. 1980 Jul;33(7):1417–1422. doi: 10.1093/ajcn/33.7.1417. [DOI] [PubMed] [Google Scholar]
- Gotch F. A. Kinetics of hemodialysis. Artif Organs. 1986 Aug;10(4):272–281. doi: 10.1111/j.1525-1594.1986.tb02564.x. [DOI] [PubMed] [Google Scholar]
- Gutierrez A., Alvestrand A., Wahren J., Bergström J. Effect of in vivo contact between blood and dialysis membranes on protein catabolism in humans. Kidney Int. 1990 Sep;38(3):487–494. doi: 10.1038/ki.1990.230. [DOI] [PubMed] [Google Scholar]
- Heidland A., Hörl W. H., Heller N., Heine H., Neumann S., Heidbreder E. Proteolytic enzymes and catabolism: enhanced release of granulocyte proteinases in uremic intoxication and during hemodialysis. Kidney Int Suppl. 1983 Dec;16:S27–S36. [PubMed] [Google Scholar]
- Heidland A., Schaefer R. M., Heidbreder E., Hörl W. H. Catabolic factors in renal failure: therapeutic approaches. Nephrol Dial Transplant. 1988;3(1):8–16. [PubMed] [Google Scholar]
- Kopple J. D., Swendseid M. E., Shinaberger J. H., Umezawa C. Y. The free and bound amino acids removed by hemodialysis. Trans Am Soc Artif Intern Organs. 1973;19:309–313. doi: 10.1097/00002480-197301900-00052. [DOI] [PubMed] [Google Scholar]
- Lim V. S., Flanigan M. J., Fangman J. Effect of hematocrit on solute removal during high efficiency hemodialysis. Kidney Int. 1990 Jun;37(6):1557–1562. doi: 10.1038/ki.1990.149. [DOI] [PubMed] [Google Scholar]
- Lim V. S., Flanigan M. J. The effect of interdialytic interval on protein metabolism: evidence suggesting dialysis-induced catabolism. Am J Kidney Dis. 1989 Aug;14(2):96–100. doi: 10.1016/s0272-6386(89)80183-0. [DOI] [PubMed] [Google Scholar]
- Lindsay R. M., Spanner E. A hypothesis: the protein catabolic rate is dependent upon the type and amount of treatment in dialyzed uremic patients. Am J Kidney Dis. 1989 May;13(5):382–389. doi: 10.1016/s0272-6386(89)80021-6. [DOI] [PubMed] [Google Scholar]
- Luger A., Kovarik J., Stummvoll H. K., Urbanska A., Luger T. A. Blood-membrane interaction in hemodialysis leads to increased cytokine production. Kidney Int. 1987 Jul;32(1):84–88. doi: 10.1038/ki.1987.175. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Ben-Galim E., Bier D. M. Determination of stable isotopic enrichment in individual plasma amino acids by chemical ionization mass spectrometry. Anal Chem. 1979 Jan;51(1):80–84. doi: 10.1021/ac50037a028. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Bier D. M., Rennie M. J., Edwards R. H., Halliday D., Millward D. J., Clugston G. A. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981 Dec 4;214(4525):1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Motil K. J., Rohrbaugh D. K., Burke J. F., Young V. R., Bier D. M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980 May;238(5):E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- Motil K. J., Matthews D. E., Bier D. M., Burke J. F., Munro H. N., Young V. R. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981 Jun;240(6):E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Pedrini L. A., Zereik S., Rasmy S. Causes, kinetics and clinical implications of post-hemodialysis urea rebound. Kidney Int. 1988 Dec;34(6):817–824. doi: 10.1038/ki.1988.255. [DOI] [PubMed] [Google Scholar]
- Schwarz H. P., Karl I. E., Bier D. M. The alpha-keto acids of branched-chain amino acids: simplified derivatization for physiological samples and complete separation as quinoxalinols by packed column gas chromatography. Anal Biochem. 1980 Nov 1;108(2):360–366. doi: 10.1016/0003-2697(80)90600-4. [DOI] [PubMed] [Google Scholar]
- Schwenk W. F., Beaufrere B., Haymond M. W. Use of reciprocal pool specific activities to model leucine metabolism in humans. Am J Physiol. 1985 Dec;249(6 Pt 1):E646–E650. doi: 10.1152/ajpendo.1985.249.6.E646. [DOI] [PubMed] [Google Scholar]
- Tepper T., van der Hem G. K., Klip H. G., Donker A. J. Loss of amino acids during hemodialysis: effect of oral essential amino acid supplementation. Nephron. 1981;29(1-2):25–29. doi: 10.1159/000182233. [DOI] [PubMed] [Google Scholar]
- Tolchin N., Roberts J. L., Lewis E. J. Respiratory gas exchange by high-efficiency hemodialyzers. Nephron. 1978;21(3):137–145. doi: 10.1159/000181384. [DOI] [PubMed] [Google Scholar]
- Ward R. A., Shirlow M. J., Hayes J. M., Chapman G. V., Farrell P. C. Protein catabolism during hemodialysis. Am J Clin Nutr. 1979 Dec;32(12):2443–2449. doi: 10.1093/ajcn/32.12.2443. [DOI] [PubMed] [Google Scholar]
- Westenskow D. R., Cutler C. A., Wallace W. D. Instrumentation for monitoring gas exchange and metabolic rate in critically ill patients. Crit Care Med. 1984 Mar;12(3):183–187. doi: 10.1097/00003246-198403000-00006. [DOI] [PubMed] [Google Scholar]
- Wolfson M., Jones M. R., Kopple J. D. Amino acid losses during hemodialysis with infusion of amino acids and glucose. Kidney Int. 1982 Mar;21(3):500–506. doi: 10.1038/ki.1982.52. [DOI] [PubMed] [Google Scholar]