Summary
Although spiral waves are ubiquitous features of nature, and have been observed in many biological systems, their existence and potential function in mammalian cerebral cortex remains uncertain. Using voltage-sensitive dye imaging, we found that spiral waves occur frequently in the neocortex in vivo, both during pharmacologically induced oscillations and during sleep-like states. While their lifespan is limited, spiral waves can modify ongoing cortical activity by influencing oscillation frequencies and spatial coherence, and by reducing amplitude in the area surrounding the spiral phase singularity. During sleep-like states, the rate of occurrence of spiral waves varies greatly depending on brain states. These results support the hypothesis that spiral waves, as an emergent activity pattern, can organize and modulate cortical population activity on the mesoscopic scale and may contribute to both normal cortical processing and to pathological patterns of activity such as those found in epilepsy.
Keywords: Spiral wave, voltage-sensitive dye, propagating wave, frequency modulation, cortex in vivo, cortical oscillation, sleep-like states
Introduction
Propagating waves of neuronal activity (also known as traveling waves, Ermentrout and Kleinfeld, 2001; Wu et al., 2008) are frequently found when a large number of cortical neurons are activated during sensory or motor events (Benucci et al., 2007; Dorries and Kauer, 2000; Ferezou et al., 2006; Ferezou et al., 2007; Freeman and Barrie, 2000; Han et al., 2008; Jancke et al., 2004; Lam et al., 2000; London et al., 1989; Lubenov and Siapas, 2009; Roland et al., 2006; Rubino et al., 2006; Senseman and Robbins, 1999; Sharon and Grinvald, 2002; Sharon et al., 2007; Slovin et al., 2002; Xu et al., 2007). A sensory-evoked propagating wave starts at the cortical sensory representation, and propagates into a larger area including the whole primary and secondary cortical areas (Benucci et al., 2007; Chen et al., 2006; Ferezou et al., 2006; Ferezou et al., 2007; Jancke et al., 2004; Roland et al., 2006; Sharon and Grinvald, 2002; Sharon et al., 2007; Slovin et al., 2002; Xu et al., 2007). Individual neurons may only be mildly depolarized during the wave, but with increased probability of firing action potentials (Ferezou et al., 2006; Petersen et al., 2003). When a large population of neurons is coherently depolarized by such a wave, local interneuronal transmission may be increased. Thus a propagating wave might contribute to cortical processing by determining when and where the cortex is depolarized, in relation to a sensory or motor event.
Spiral waves are a particular form of propagating waves, rotating around a center point known as a rotor. A spiral rotor can emerge from free-ends of a traveling wave front. The rotor, after being created, becomes a powerful rhythmic organizer by sending robust rotating waves outward (Winfree, 2001). In this way, spiral waves may provide an essential mechanism for organizing the irregular activity of cortical neurons into rhythmic activity (Schiff et al., 2007). Spiral waves have been found to play an important role in cardiac arrhythmia (Bub et al., 2005; Bub et al., 2003; Davidenko et al., 1992a; Davidenko et al., 1992b; Jalife, 2003; Jalife and Gray, 1996). In the nervous system, while spiral waves have been observed in turtle visual cortex (Prechtl et al., 1997) and rodent brain slices (Huang et al., 2004), the existence of spiral waves in intact mammalian cortex has not been demonstrated.
Dominant local excitatory interactions are essential for sustaining spiral waves in general (Winfree, 2001), and can explain why such patterns are seen robustly in slice preparations, where local excitatory interactions dominate (Huang et al., 2004). In the cortex in vivo, long-range and non-local connections, such as thalamocortical and corticocortical connections may disrupt the spiral rotors and make spiral waves unsustainable. Therefore, spiral waves in intact cortex may occur during certain brain states when local excitatory interactions are predominant, while they are unsustainable in other brain states when long-range connections are strong.
Voltage sensitive dye imaging method provides adequate sensitivity and spatiotemporal resolution for identifying the spiral phase singularity, a hallmark of spiral waves. In this report, we examined the spiral dynamics under two conditions: In one condition, ~10 Hz oscillations were induced by epidural application of carbachol and bicuculline. With this pharmacological manipulation, cortical local excitatory connections are greatly enhanced. In the other condition, sleep-like activity was induced with low levels of pentobarbital anesthesia. In this state, the cortex exhibits alternation of theta and delta rhythms, suggesting a strong influence of subcortical rhythm generators via long-range connections to the cortex. We found that spiral waves can be generated in both conditions but the rate of occurrence and duration of spiral waves varied greatly. Established spiral waves interact extensively with ongoing cortical activity, causing significant changes in the oscillation frequency, amplitude, and spatial coherence. These results suggest that spiral waves may participate in cortical activity by imposing its distinct spatiotemporal patterns to the cortical network. Compared to the spiral waves in brain slices, spiral waves in vivo are sustained for shorter times but the spiral center (phase singularity) drifted much faster, suggesting that cortex may have mechanisms to control the duration and location of cortical spirals.
Results
Spiral waves emerged from oscillatory waves
Epidural application of carbachol and bicuculline induced spontaneous oscillations with a frequency range from 3 to 22 Hz. The oscillations are organized as epochs, each epoch containing 3 −100 cycles. During the oscillations, spiral waves occurred alternately with other wave patterns within each oscillation epoch (Figure 1A). The occurrence of spiral waves was frequent, observed in about 40% of the 433 recording trials (12-16 seconds each trial, 24 animals). Most spiral waves (81%) were short-lived, with a lifespan of 1-3 rotations, while the remainders ranged from 4 to 25 rotations (Figure S4).
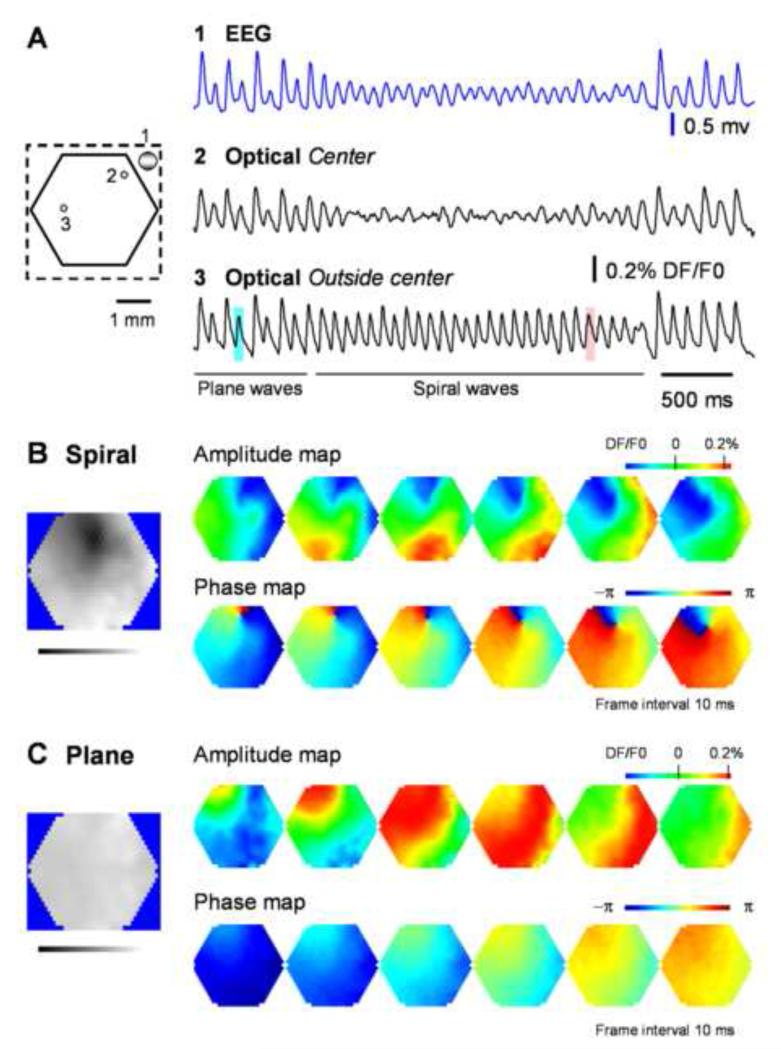
Figure 1. Spiral waves during carbachol/bicuculline induced oscillations.
A. EEG and voltage-sensitive dye signals during one oscillation epoch. Left schematic diagram: Broken line outlines the cranial window and the solid hexagon outlines the imaging field (4 mm in diameter, 464 optical detectors); two small green dots mark the optical detectors whose traces are plotted on the right; large gray dot marks the location of the EEG electrode. Right plots: raw data traces from the EEG (blue) and two optical detectors labeled on the left. Detector 2 (Optical spiral center) is selected from the spiral center, showing large amplitude reduction. Detector 3 is selected from a location that spiral center never swept through, showing no amplitude reduction. Wave patterns, plane or spirals, are determined by amplitude and phase maps of the voltage-sensitive dye signals shown in B and C respectively. The spatiotemporal patterns of two cycles of the oscillation marked with blue and red squares are shown in panels C and B respectively. B and C. Amplitude and phase maps during spiral (B) and plane(C) waves from the same field of view. For amplitude maps, the VSD signals on all detectors were normalized to the peak amplitude of the whole field and then were converted to pseudo-color according to a linear color scale (Top right). For phase maps, the phase on each detector was assigned a color on a linear color scale from −π to π (middle right). Phase singularity, as a point with highest spatial phase gradient in the entire field, is seen during spiral waves but not during other wave patterns. The left gray-scale images show the spatial distribution of the amplitude standard deviation (SD) during the two periods marked by blue and red squares in A. Darker color indicates smaller SD and brighter color indicates larger SD. Note that an area of small SD, indicating amplitude reduction, is seen around the spiral phase singularity. Another example of spiral waves from a different animal is shown in supplemental Figure S1. More information of spiral waves and plane waves can be obtained from supplement movies (Movie S1, S2, S6, S7).
Spiral waves have spatiotemporal patterns distinct from other waves, such as target and plane waves. Amplitude pseudo-color maps of spiral and plane waves during the same oscillation epoch are shown in Figure 1B and 1C (also in Movies S6, S7). During spiral waves, a wavefront initiated in the upper-left corner of the field of view and rotated counter-clockwise around a center in the field, while plane waves initiated in the same area but propagated in straight line across the field.
The hallmarks of spiral waves are amplitude reduction and phase singularity at the spiral center (Huang et al., 2004). Both are clearly seen in Figure 1A, B and Figure S1, indicating that the rotating waves we observed were true spiral waves. In Figure 1A, the optical signal near the spiral center shows significant amplitude reduction during spiral waves, while the optical signal from outside of the center does not. In order to show the spatial distribution of amplitude reduction, we mapped the amplitude standard deviations (SD) of all detectors within selected periods. As expected, the reduced amplitude around the spiral center results in a small SD, while outside the center, the phase modulation of a large amplitude results in a large SD. The SD map (grey-scale image) in Figure 1B clearly shows a dark area around the spiral center, indicating reduced amplitudes. In contrast, the plane wave had an evenly distributed SD (Figure 1C, grey-scale image). Amplitude reduction was observed in both short and long spirals, indicating that the reduction is exclusively linked to the development of spiral waves and is independent of the length of oscillation epochs. A phase singularity was observed in the spatial phase map (Figure 1B, bottom row, and Figure S1B), in which the phase distributions within the field of view were mapped between −π and π. During spiral waves, a distinct pinwheel pattern was seen in the phase map. At the center of the pinwheel, the spatial phase gradient was highest, and one point was surrounded by all phases (Figure 1B, Figure S1B, Movie S1). This point is referred to as “the phase singularity” (Winfree, 2001). During plane waves, phase gradients are much smaller and there is no phase singularity (Figure 1C, bottom row, and Movie S2).
Spiral waves in sleep-like states
Next we investigated whether spiral waves can also exist in neocortex during sleep-like states, without enhancement of the local excitatory connectivity by locally applied carbachol/bicuculline (Cch/bic). The sleep-like states were induced with slow withdrawal of pentobarbital anesthesia. The animals were first given an initial induction dose of pentobarbital (50 mg/kg), followed by tail vein infusion at a low rate of 6 - 9 mg/kg-hour. When the initial anesthesia wore out after 2-3 hours, an alternation of EEG patterns (theta/delta waves) occurred under the low maintenance infusion (Figure 2A). The pentobarbital infusion rate (9 - 15 mg/kg-hour) was then carefully adjusted for each individual animal to sustain the alternation of theta/delta wave patterns. The theta/delta alternations resemble the rapid eye movement (REM) state in rodent natural sleep (Montgomery et al., 2008). These alternations occur spontaneously, not a direct consequence of acute adjustment of anesthesia level. As shown in Figure 2A, a slight increase in the pentobarbital infusion rate (marked by the yellow line) can gradually shift the alternative theta/delta state to a stable delta-dominant state. Conversely, slight decreases in the infusion rate result in shifts from the delta-dominant state back to the alternating theta/delta state.
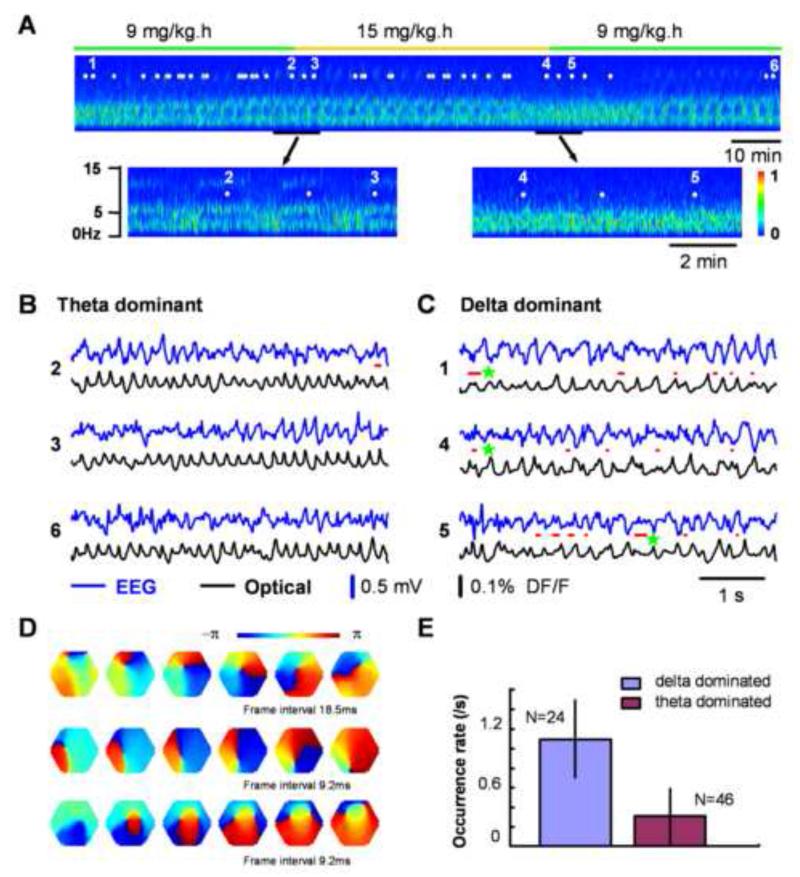
Figure 2. Spiral waves during sleep-like states.
A. EEG spectrum during sleep-like states. The EEG recording started about 4 hours after the initial injection of 50 mg/kg pentobarbital. A low level of anesthesia is maintained by continuous tail vein infusion of pentobarbital at 9 mg/kg-hour (green bar). The EEG was filtered between 1 - 40 Hz for clarity. Note that the power of the spectrum transitions periodically between theta (~ 6Hz) and delta (1-4 Hz) dominant states when pentobarbital infusion is maintained at a low constant rate. With higher infusion rate (15mg/kg-hour) of pentobarbital (marked with orange bar), the alternating patterns in the EEG shifted to the delta-dominant state. Note: there are approximately 20 min delays of drug effects because of the slow infusion of pentobarbital. Seventy imaging trials were taken during a four-hour period (43 trials during the section shown, marked as white dots) and representative trials (1-6) are presented in panels B and C. B. and C. Simultaneous EEG (blue) and optical (black) traces recorded during the theta-dominant (B, trials 2, 3, 6) and delta-dominant periods (C, trials 1, 4, 5). Spiral waves are identified from the 8,192 images of each trial and durations of spiral waves are marked by red lines under each EEG trace. Three example spiral waves (red lines next to green stars) are shown in panel D. D. Phase maps of example spiral waves in sleep-like states. Note that the bottom row images show an example of double spirals with opposite rotating directions. The imaging area is 4 mm in diameter. E. Rate of occurrence of spiral waves during sleep-like states. The occurrence rate was obtained from 70 imaging trials (202 cases of spirals) in this animal based on the EEG spectrum (Panel A shows about half of this data set). Supplemental Figure S2 shows another example from a different animal during sleep-like states.
Spiral waves occurred frequently during sleep-like states and the rate of occurrence varied substantially with different EEG patterns, and demonstrated a higher rate during the delta-dominant state (0.9±0.36 /sec, mean±SD, N = 92, from 5 animals) and a lower rate during the theta-dominant state (0.28 ±0.32 /sec, mean±SD, N = 122, from 5 animals). In the experiment shown in Figure 2, the EEG was recorded continuously and imaging trials were taken intermittently (timing marked by white dots in Figure 2A), during both the theta-dominant state (e.g., 2, 3, 6 in Figure 2A, B) and the delta-dominant state (e.g., 1, 4, 5 in Figure 2A, C). Simultaneously recorded raw EEG (blue) and VSD (black) signals are shown in Figure 2B, C. Spiral waves were identified with imaging analysis, and their occurrence and duration are marked by red lines between the raw signal traces. The rate of occurrence of spiral waves differed significantly between the two states, as demonstrated in the example trials (Figure 2B, 2C). Of all 70 imaging trials performed on this animal, 24 were taken during delta-dominant state and 132 spiral waves are identified. In contrast, in the 46 imaging trials taken during the theta-dominant state, only 70 spiral waves were found. The occurrence frequency of spiral waves during the delta-dominant state was about 4 times that during theta-dominant state (Figure 2E). Another example from a different animal is shown in Figure S2.
During these sleep-like states, spiral waves were only sustained for a short duration. In 762 spiral waves seen in 5 animals, only 6 cases were more than 2 rotations in duration; the majority (88%) contained less than one cycle (cases with less than 1/6 rotations were disregarded as uncertain). Three examples of spiral waves are shown in Figure 2D. Double spirals with opposite rotation directions were often seen during sleep-like states (Figure 2D, bottom row images). In all animals examined, double spirals were seen in ~40% of the cases. Breaking of plane waves is a possible mechanism for generating double spirals (discussed in Figure 8C).
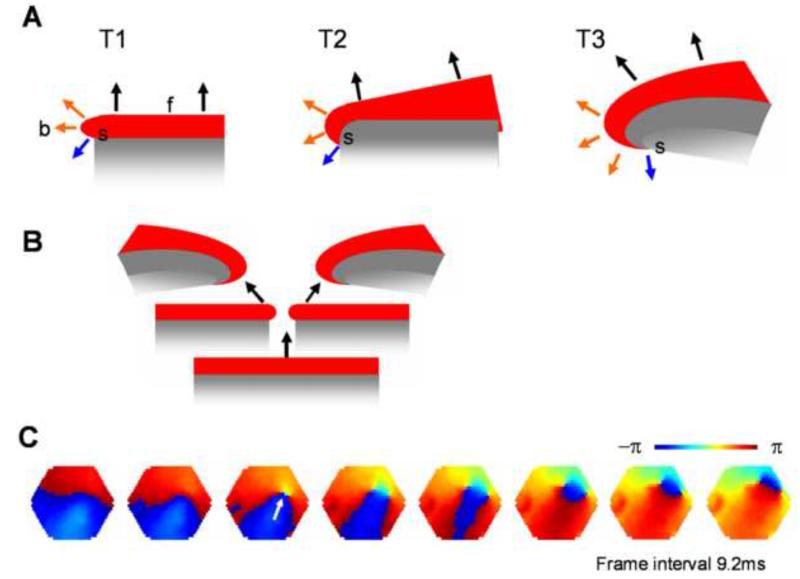
Figure 8. Mechanism for initiating and sustaining spiral waves.
A. How does a spiral wave emerge from the free-end of a propagating wave? The propagating direction (black arrows) at the planar portion of the wave (f) is perpendicular to the front edge of the excitation and pointing away from the refractory tail (gray). In contrast, around the free-end of the wave (b), propagating directions (orange arrows) are different, because the excitation also spread to the side. With time (T1 - T3), the difference in propagating directions creates a curvature to the wave front. The curvature is largest at the point (s), where a largest difference in the propagation direction (blue arrow) occurs. This is an inevitable dynamical process that converts a planar wave into a spiral. B. A plane wave may break in the middle, creating a pair of spirals rotating in opposite directions. C. During sleep-like states, broken in the wave front edge is frequently seen (white arrow), resulting in a pair of spirals. Imaging data were collected during delta-dominant periods of sleep-like state, similar to the condition of Figure 2C. The imaging area is 4 mm in diameter. In supplement Movie S5, we show that wave collision is another possible mechanism for spiral formation.
Spiral waves correspond with changes in the frequency of ongoing activity
The emergence of spiral waves corresponds with changes in the frequency of cortical activity. This change is most obvious in long-lasting spiral waves during Cch/bic oscillation. Frequency-time analysis showed that long-lasting spiral waves have prominent modulation effects on the oscillation frequencies (Figures 3, S3). In the example in Figure 3A, a segment of oscillation signal containing spiral waves are shown with a corresponding frequency spectrum. Frequency differences can be clearly seen in the trace before and during spiral waves. Before the development of a spiral wave, there were multiple frequency components in the signals and the major component was low of frequency; however, when the spiral wave was fully developed, there was only one single frequency component left and this component had a higher frequency. When the spiral phase singularity disappeared (marked by a black arrow), the single frequency component broke up into multiple components. Similar influences on the frequency can also be seen in other examples shown in Figure S3 A-C. These examples suggest that established spiral waves have a powerful modulating effect on cortical oscillation frequencies. The major frequency components during spiral waves are similar in different animals, suggesting that spiral waves in the cortex may have a preferred intrinsic frequency, probably determined by their rotation angular velocity. Interestingly, the spiral waves are not a high power event as we can see in Figure 3A, S3A-C. Apparently, the powerful modulating effect on the overall frequency was not caused by a high level of neuronal activity that overwhelmed the system. Instead, a spiral singularity is a dynamically stable event, which might recruit neurons into its dynamical regime without increasing the power. The system entrained by spiral waves was not only seen in these individual examples, but also seen in statistical analyses. In 394 spiral waves observed, 89% occurred in a frequency range between 9 Hz to 16 Hz (Figure 3C). The overall frequencies of spiral waves are higher (Figure 3C) than those of control wave patterns (segments of signals longer than 300 ms, randomly chosen from each trial regardless of the presence or absence of spiral waves in the section).
Figure 3. Frequency modulation effects of spiral waves.
In A and B, top trace: Raw recording signal from a representing optical detector. Middle image: the frequency-time map (frequency spectrum) made from that detector. Fast Fourier transform was used to analyze the power spectrum of signals in a sliding window of 300 ms. Pseudocolor scale: relative power of the frequency components, red for high (1) and blue for low (0) power. Bottom images: selected from the phase movie for identifying wave patterns. Broken vertical lines indicate the times for these images. The red broken lines mark the first cycle of spiral waves and black arrows mark the end of spiral waves. A. An oscillation segment contains a long-lasting spiral waves (7 rotations). The frequency-time map shows that the oscillation frequency increases to a higher frequency during spiral waves. Three additional examples are shown in Figure S3A-C. B. Ongoing cortical activity during the theta-dominant period of sleep-like state, a pair of short-lived spiral waves was identified by phase maps (bottom images). Similar effects are observed during spiral waves. Three additional examples are shown in Figure S3D-F. C. Distribution of frequencies during spiral waves and all wave patterns in Cch/bic induced oscillations. Fast Fourier transform was used to analyze the power spectrum of signals with spiral waves or randomly chosen segments of signals, and the frequency corresponding to the major peak was used. D. Frequency distribution of spiral waves and other periods during sleep-like states.
Short-lived spiral waves during sleep-like states are also associated with changes in cortical frequency components. In examples shown in Figure 3B and S3D-F, the emergence of a spiral phase singularity showed similar effects of reducing system power and shifting the dominant frequency. While the short spirals had less obvious effects in individual cases, as compared to the Cch/bic condition, a statistical analysis with pooled data did show significant shift in average frequency (Figure 3D). In this analysis, we chose all the short-lived spiral waves from the same animal, and randomly selected a control section for each spiral case. The frequency spectrum during spiral waves was significantly different from control patterns (Figure 3D). Spiral waves showed a significantly higher frequency peak (Figure 3D, blue curve), similar to that seen under Cch/bic induced oscillations (Figure 3C).
Spiral waves associated with reduced spatial coherence
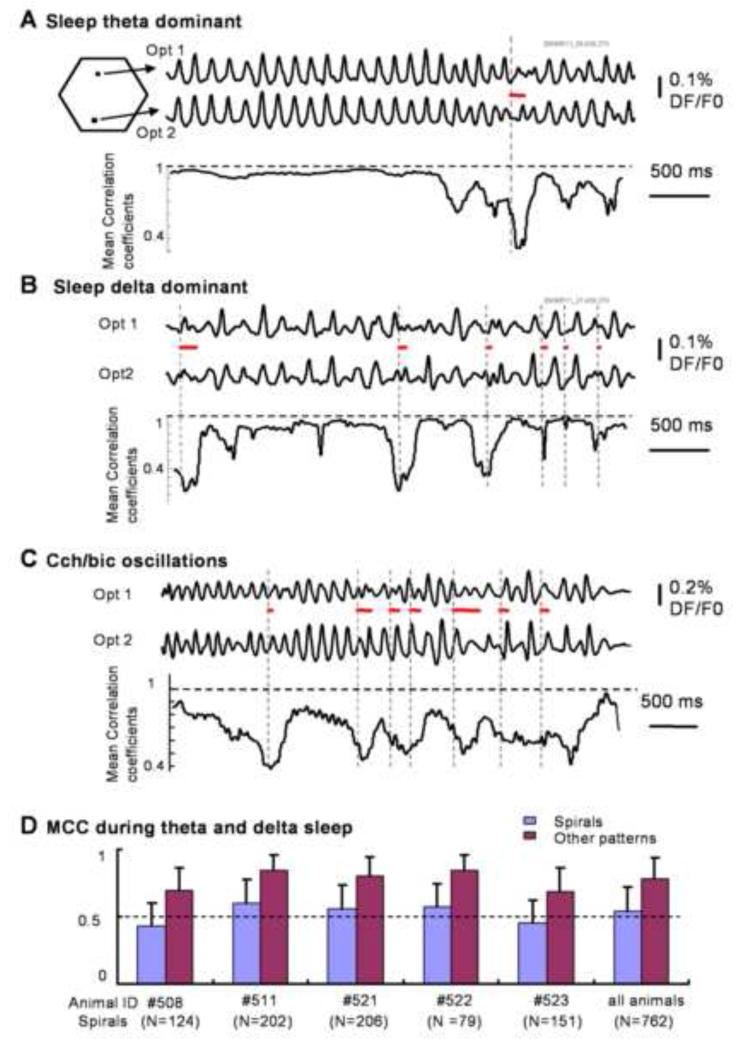
The emergence of spiral waves is associated with significant reduction in the spatial coherence of cortical activity. Such reduction can be observed in both raw optical recording traces and the plot of mean correlation coefficient (MCC, see methods). During the theta-dominant phase of the sleep-like state, cortical activity manifests as plane propagating waves. Optical signals on different detectors were well correlated (Figure 4A, upper traces), and the spatial coherence measured by MCC from all detectors was high due to a small spatial phase gradient (Figure 4A bottom trace). When a spiral wave emerged (marked by the red bars in Figure 4A), the variations between optical traces became larger. This was manifested as a sharp drop in the MCC, indicating that spiral phase singularities are associated with lower spatial coherence. Similar changes in the optical signals and the MCC during spiral waves were also seen during delta-dominant sleep-like state and Cch/bic induced oscillations (Figure 4B, C respectively).
Figure 4. Spiral waves and spatial coherence.
A-C, top two traces are raw optical signals from two detectors (Opt1, Opt2, 2.5 mm apart). The bottom trace is the Mean Correlation Coefficient (MCC, methods section), calculated from all 464 detectors with a sliding window of 300 ms. The onset time and duration of the spiral waves are marked by the vertical broken lines and the red lines respectively. Spiral waves are correlated with decreases of MCC. D. Average of MCC measured from 5 animals during 762 spiral and non-spiral patterns. From each spiral wave (“spirals”), a 300 ms recording segment was selected. The “other patterns” were 300 ms of a recording segment without spiral waves randomly selected in the same recording trial.
Analysis of data from 5 animals with 762 spiral wave events during sleep-like states is shown on Figure 4D. In the MCC calculation, we selected a 300 ms recording segment from each spiral wave (“spirals”) along with another randomly selected 300 ms segment without spiral waves (“other patterns”). The MCC from the two groups show significant differences (p < 0.0001), suggesting a large decrease in spatial coherence associated with spiral events (Figure 4D).
Short-lived spiral waves and their impact on the local frequency
Spiral waves in the intact cortex were not as stable as in slices. Most spiral waves during sleep-like states were shorter than 2 cycles. Even during Cch/bic induced oscillations, most spiral waves (81%) were only sustained for 1-3 cycles, as shown in the distribution of lifespan of spiral waves (Figure S4). Ongoing background activity and long-range connections may contribute to this short duration and instability. Short-lived spiral waves do not change frequency over a large range or a long period. However, they may still have a profound impact on local frequency. We often observed that oscillation frequencies were not evenly distributed spatially, and the frequency often fluctuated during short-lived spiral waves.
Short-lived spirals often temporarily increase the frequency in a local area. Figure 5A shows the spatial distribution of frequencies during a short-lived spiral wave in Cch/bic induced oscillations. Over a duration of 400 ms, there were two cycles of spiral waves rotating counter-clockwise followed by two cycles of plane waves propagating from the right to the left of the imaging field. During spiral waves, one triangular area in the field of view showed a higher frequency (Fig 5A upper image, yellow-red region) than the rest of the field. In contrast, during plane waves the entire field exhibited little fluctuation (Fig 5A bottom image, all green). The power spectrum of the signals from two selected detectors in the imaging field are plotted to demonstrate this difference. The peak frequency of detectors in the triangular area is about 16 Hz during spiral waves and 11 Hz during plane waves. The peak frequency at detectors outside of the triangular area was ~11 Hz, and did not change significantly during spiral or plane waves. Figure 5B shows more examples from 6 different animals. High-frequency patches were shown when spiral phase singularities were identified (one example shown in Figure 5B, bottom row images). Similar changes in spatial frequency distribution are also seen during spiral waves observed in sleep-like states, where most of spiral waves are shorter than 1 rotation (Figure 5C). When double spirals were identified (Figure 5C bottom row images), two areas of frequency change were observed (Fig 5C upper row, examples 1, 3, 5), suggesting that the changes in frequency are related to the sustaining of spiral phase singularities.
Figure 5. Spatial distribution of frequency during spiral waves.
A. left panel: The images are pseudo-color maps for frequency. Fast Fourier transform was used to analyze the power spectrum of signals during spiral waves and plane waves. The frequency with peak power from FFT analysis on each detector was converted to a pseudo-color according to a linear color scale (shown in the bottom). During spiral waves (top image), there is a triangular area (red-yellow) showing higher frequencies than other areas in the field of view, while during plane waves (bottom) all the areas show similar frequencies. Circled arrow indicates propagating direction (counter-clockwise) of spiral waves, and straight arrow indicates the propagating direction of plane waves. Right panel: power spectrums from two detectors (red line for red square and blue line for blue square) during spiral waves and planes waves are shown. During spiral waves, the detector from the triangular area has a peak frequency of 16 Hz and the detector from outside the area has a peak frequency of 11 Hz. During plane waves, these two detectors both show a peak frequency of 11 Hz. Two inserts in up-left corners show the raw traces of these two detectors during spiral waves and plane waves. B. Upper row of images: Frequency maps of six short-lived spirals (from 6 animals) during Cch/bic induced oscillations. The color bar was calibrated according to different animals (blue, 3-5 Hz; red, 18-21 Hz). Bottom row of images: Phase maps of one spiral wave in the first example in the upper row (marked by the star). C. Upper row of images: Frequency maps of 6 short-lived spirals (from 3 animals) during sleep-like states. Note that examples 1, 3, 5 are double spirals. Bottom row of images: Phase maps of the first example in the upper row (marked by the star).
Amplitude reduction during spiral waves
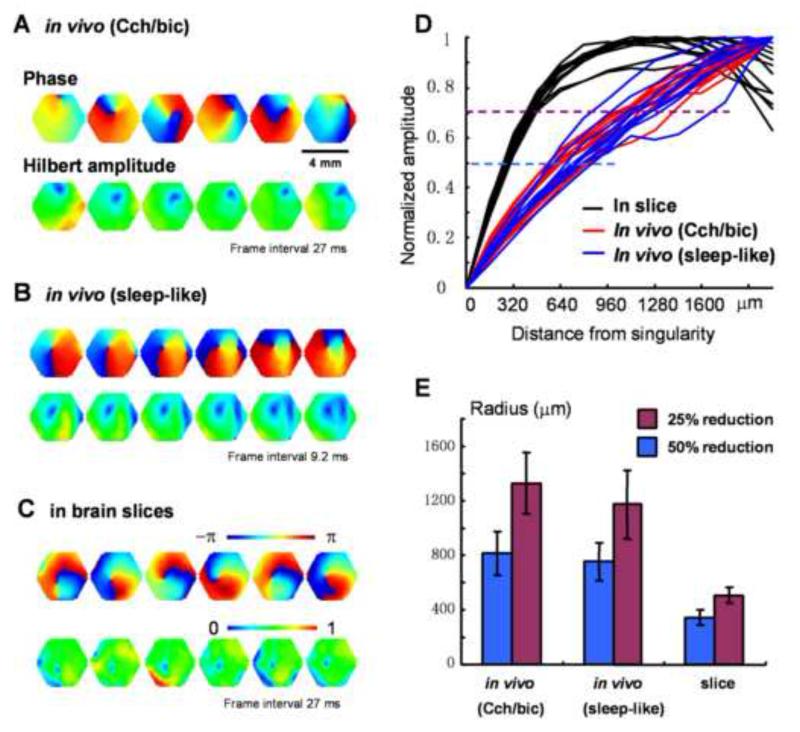
Amplitude reduction surrounding a phase singularity is the hallmark of true spiral waves (Winfree, 2001). In cortical slices and in computational models, amplitude reduction only occurs in a small area around the phase singularity (Huang et al., 2004). In contrast, the area of amplitude reduction is much larger for spiral waves in cortex in vivo during both sleep-like states and Cch/bic oscillations (Figure 1B, Figure S1B, S2C). To quantify the spatial profile of the amplitude reduction, we employed the Hilbert transform to estimate the “instantaneous amplitude” (Hilbert amplitude), so that the modulation of the phase is excluded. Hilbert amplitude maps in Figure 6A-C clearly show areas with amplitude reduction (blue) surrounding the phase singularities, demonstrating that the reduction in the amplitude is real, and not the result of phase modulation. The profiles of amplitude reduction show significant differences between the in vitro and in vivo conditions: in slices, the amplitude drops sharply from the periphery to the spiral center (Figure 6D, black curves), while in vivo, it drops gradually (Figure 6D, red and blue curves). No significant difference was seen between the two in vivo conditions. To confirm these findings, we examined 40 cases of spiral waves (containing at least 2 rotations) from 8 animals during Cch/bic induced oscillations and 31 short-lived spiral waves during sleep-like states from 5 animals. We found that in both conditions, on average, an area of ~1600 μm in diameter around the phase singularity exhibited an amplitude reduction of over 50% (Figure 6E). In comparison, we measured the amplitude reduction in 26 spiral waves in cortical slices from 5 animals, and found that the area of amplitude reduction is only ~600 μm in diameter, significantly smaller (p< 0.0001) than that of in vivo spirals (Figure 6E). The difference between in vivo and in vitro spirals suggests the involvement of thalamocortical and other non-local connections during in vivo spiral waves. The similarity between the two in vivo conditions (with and without bicuculline) suggests that GABAergic inhibitions do not play a significant role in this amplitude reduction.
Figure 6. Amplitude reduction around spiral phase singularity.
A-C Examples from spiral waves in vivo during Cch/bic oscillations, in vivo during sleep-like states and in brain slices respectively. In each panel the top row images are phase maps, identifying the location of the singularity. The bottom row images are the Hilbert amplitude, each from the same moment as the phase map in the top row. The blue area surrounding the phase singularity indicates the range of amplitude reduction. Note that the area of amplitude reduction is larger for in vivo spirals. D. Normalized amplitude at different distances from the phase singularity. 12 cases of in vivo spirals from one animal applied with Cch/bic (red), 10 cases of in vivo spirals from one animal during sleep-like states (blue), and 12 cases from one slice (black) are plotted. Broken lines mark the amplitude reduction of 50% (blue) and 25% (purple) respectively. E. The area of amplitude reduction (radius, mean ± SD) from 40 in vivo spirals under Cch/bic application (8 animals), 31 in vivo spirals during sleep-like states (5 animals) and 26 spirals from slices (5 animals). The plot indicates that during in vivo spirals, an area of ~1600 μm in diameter around the spiral phase singularity has an amplitude reduction 50%; In contrast during spirals in brain slices the amplitude reduction area is much smaller, only about 600 μm in diameter.
Drifting of spiral phase singularity
In our previous studies in brain slices (Huang et al., 2004), we observed that the spiral phase singularity drifted in the cortex, suggesting that the spiral wave does not rotate around a column or other anatomical structure. The drift of the spiral phase singularity was much larger and faster under in vivo conditions, both in the long spiral waves during Cch/bic induced oscillations and in short-lived spirals during sleep-like states (Figure 7, Movie S3). Figure 7A-C and Figure S5A show examples of the trajectory of spiral phase singularities during spiral waves in different conditions. In cortical slices, the spiral phase singularity drifted a short distance progressively in each rotation and between rotations. In contrast, in the cortex in vivo, phase singularities in both Cch/bic induced oscillations and sleep-like states drifted a longer distance per rotation, at faster speeds and covering a larger area. In order to quantify the difference between slices and in vivo, we calculated the traveling distance of spiral phase singularities per unit time for both types of preparation. As shown in Figure 7D and Figure S5B, there was a large difference in average drifting speed for spiral centers in vivo and in slices. Because of the large standard deviations caused by the inconsistencies in the drifting velocity of the phase singularity, we used an unpaired t-test, Welch’s test, to calculate the difference between data from in vivo and in vitro. The results show that on average, the phase singularities of in vivo spirals drifted much faster than that of in vitro (p<0.001), and drifted faster in sleep-like states than in Cch/bic induced oscillations (p<0.001). Since we used visual cortical area for imaging in both slices and in vivo, the local connections should be similar. The large drift in intact brain is therefore likely due to non-local connections, which may selectively activate various local areas, creating spatial unevenness and an excitability gradient that could facilitate the drift of spiral waves.
Figure 7. Drifting of spiral phase singularities.
A. Trajectory of spiral phase singularity during a 12-cycle spiral waves in cortical slices. B. Trajectory of spiral phase singularity during an 11-cycle spiral waves in vivo under Cch/bic application. Hexagon shows the field of view and each color represents one cycle of spiral wave. C. Trajectory of spiral phase singularities during 2 spiral waves (red and cyan, each with ~1.5 turn) during sleep-like states. Additional examples during sleep-like states are shown in Figure S5A. D. Comparison of drifting speed of spiral phase singularity for slices and in vivo. Five examples from in vivo under Cch/bic, in vivo during sleep-like states and slices respectively are shown. Columns with stars on top are from the examples in A-C. The standard deviation is large because the drifting of spiral phase singularity is not consistent and there are large variations from time to time. The difference between in vivo and slices is statistically significant (Welch’s test, P<0.001, 25 t-tests). The difference between in vivo (Cch/bic) and in vivo (sleep-like) is also significant. Average of traveling distance at unit time under these three conditions was shown in Figure S5B.
Discussion
This report demonstrates the existence of spiral dynamics in the intact mammalian brain during pharmacologically induced oscillations and sleep-like states. Intact cortex exhibits rhythmic activities generated from cortical and subcortical sources. Spiral waves may act as a robust rhythm generator and spatial pattern organizer, interacting with cortical rhythms. We found that the emergence of spiral waves has a large impact on the oscillation frequency, spatial coherence and amplitude of cortical activity. Even short spiral waves can have a significant impact. Compared to spiral waves previously observed in brain slices, spiral waves in the intact cortex have shorter duration, the phase singularity drifts much faster, and the spatial extent of amplitude reduction is much larger. These main findings suggest that cortical networks are able to generate and sustain spiral waves, and that the duration and spatial location of spiral waves are controlled in intact cortex by a number of mechanisms. Spiral waves might participate in cortical processing as either rhythm generators or as spatial organizers of population neuronal activity.
Mechanisms for generating cortical spiral waves
We propose that cortical spiral waves are not generated by specialized “spiral circuits”, but instead, by a dynamic mechanism involved in the propagation of an excitation wave. In this sense, the process for generating cortical spirals is similar to that involved in spiral generation in the excitatory continua during Belousov-Zhabotinsky reactions (Keener and Tyson, 1986), calcium waves on the surface of fertilizing Xenopus oocytes (Lechleiter, 1991), and spreading depression waves in the retina (Gorelova and Bures, 1983). This process is illustrated in Figure 8A, where the propagation direction (black arrows) of neuronal excitation is pointing away from the refractory region (gray area). At the free end of the wave, however, the excitation can spread to both the front and side directions, resulting in combined propagating directions that are different from the direction in the planar portion of the wave-front (Figure 8A, orange arrows). With time (Figure 8A, T1 - T3), the change in propagating directions creates a curvature to the front edge of the wave, converting a planar wave into a spiral wave. The curvature is largest at the point (s), where the largest difference in the propagation direction (blue arrow) occurs. Creating free ends in a propagating wave is thus the key for generating spiral waves.
Free ends can be created by the collision of two waves, and some of these free ends will develop into spiral waves (Palsson and Cox, 1996; Steinbock and Muller, 1993; Winfree, 1989). Such a mechanism may contribute to the initiation of spiral waves in slices (Movie S5), and may also be involved in the generation of in vivo spiral waves. Free ends can also be created by the breaking of plane waves, which was frequently seen during sleep-like states. The break-up point in a planar wave-front generates two free ends and these two free ends can evolve into a pair of spirals with opposite rotation directions, as illustrated in Figure 8B. Double spirals with opposite rotating directions were frequently seen (~40%) during sleep-like states. A close examination of initiation of double spirals revealed the process of the breaking of a plane wave (Figure 8C). In this sense, breaking of a wave-front appears to be a common mechanism involved in the generation of spiral waves during sleep-like states.
Spiral waves and network structure
The formation and maintenance of spiral waves requires strong local excitatory interactions. It is known in several biological systems that once a spiral rotor (phase singularity) is generated, it is protected from external disruptions by this strong interaction of surrounding rotating waves (Bub et al., 2005; Bub et al., 2003; Davidenko et al., 1992a; Davidenko et al., 1992b; Gorelova and Bures, 1983; Harris-White et al., 1998; Lechleiter et al., 1991; Siegert and Weijer, 1995; Verkhratsky et al., 1998). This unique protection of the rotor makes it difficult to extinguish sustained spiral waves, which leads to harmful reentry patterns underlying cardiac fibrillation (Jalife, 2003). However, the situation in the cortex is different. Cortical networks contain both local and long-range connections, resembling a network structure with “small world” topography (Strogatz, 2001; Watts and Strogatz, 1998). We hypothesize that modulations arising from a third dimension by long-range thalamocortical and corticocortical connections may change the excitability of the local network, and by doing so, control the emergence, drifting and extinction of spiral waves. This hypothesis is supported by experimental data in three scenarios: in neocortical slices, in intact cortex during Cch/bic oscillations and during sleep-like states.
In neocortical slices, most non-local connections are removed during slice preparation. When local excitatory interactions were enhanced by carbachol and GABAa inhibition was eliminated by bicuculline, stable spiral waves with longer lifespan and less drifting rotor were frequently observed (Huang et al., 2004, and Figure 7).
In intact cortex with epidurally applied carbachol/bicuculline, while the local excitatory connections are enhanced, there are still abundant long-range connections between cortex and sub-cortical structures and between different cortical areas. Long-range inputs to the cortical local network may disrupt spiral waves by modulating the excitability of the network or by inducing other rhythms. Compared to slices, most in vivo spiral waves are short-lived. Long-range inputs to the local network may also change the spatial homogeneity of the network excitability. Inhomogeneity is suggested to underlie the drifting of singularities in many systems (Davidenko et al., 1992a; Davidenko et al., 1992b; Gorelova and Bures, 1983; Gottwald et al., 2001; Kheowan et al., 2004; Mikhailov and Showalter, 2006; Steinbock and Muller, 1993). The large drift of the phase singularity (Figure 7) in vivo is probably due to the dynamic inhomogeneity introduced by the long-range inputs.
Long-range connections between cortex and subcortical structures might also disturb the fine geometry of the spiral rotor, causing a larger area of amplitude reduction around the phase singularity (Figure 6). Around the phase singularity, there is a large spatial phase gradient. This phase gradient may be projected to a subcortical network by long-range interactions without a spatial point-to-point relationship, resulting in reduced activity in a larger area.
In sleep-like states, the local excitatory interactions should be weaker and inhibition stronger than that with carbachol and bicuculline. Under this condition, propagating waves created by ongoing activity may break frequently, and a fraction of these free ends may further develop into spiral waves (Figure 8). The rate of occurrence of spiral waves varies significantly during different sleep-like states (Figure 2). As stated above, spiral waves occur frequently during the delta-dominant state and much less during “tonic theta” episodes during theta-dominant states. During “tonic theta” episodes, the cortex is strongly influenced by subcortical rhythms (Montgomery et al., 2008), via long-range projections. Such external modulations may form a less favorable condition in the cortical local circuits for spirals to generate and be sustained. In contrast, delta waves are more flexible, and are usually separated by activities with multiple frequencies. The unsynchronized period, which is advantageous for generation of spiral waves, is much longer in delta-dominant state than that in theta-dominant state. This probably results in the higher occurrence rate of spiral waves in delta-dominated state. The lifespan of spiral waves is shorter during sleep-like states compared to under Cch/bic condition, probably because the weaker local excitatory interactions and stronger inhibitions allow spiral waves to be easily disrupted by ongoing activity.
Spiral waves and cortical rhythms
Spiral waves in intact cortex have a large impact on rhythmic cortical activity (Figure 3, S3). A spiral wave can organize an oscillation over space, with a frequency equal to the rotation rate and phase distributed around the phase singularity. An established long-lasting spiral wave may attract the cortical rhythms to the frequency of that spiral wave (Figures 3A, S3A-C). Such attractor dynamics have been predicted by theories based on data from cortical slices (Schiff et al., 2007), and is consistent with the data in this report from the intact brain. While the modification to cortical frequency is less obvious in short-lived spirals, a shift of the overall frequency is statistically significant in grouped data (Figure 3D). We also noticed that the network frequency exhibited a significant reduction immediately after a long lasting spiral wave (Figure S3H), suggesting that the network oscillation was temporarily entrained at a higher frequency due to the engagement with spiral waves. Analysis of hundreds of spiral waves strongly supports the notion that the emergence of spiral waves can entrain the oscillations into a higher frequency range. Since spiral waves occur frequently during certain brain states, the contribution of the spiral waves to the overall cortical frequency may be significant.
In intact brain, subcortical generators can be strong and dominate cortical rhythms (Contreras et al., 1996; Steriade et al., 1993; Steriade and Timofeev, 2003). In rodent, thalamocortical delta and hippocampal theta rhythms are dominant during different phases of sleep (Montgomery et al., 2008). We found that the occurrence of spiral waves is strongly correlated to the cortical rhythms (Figure 2). During a train of theta waves (“tonic theta”, (Montgomery et al., 2008), there were almost no spiral waves seen (Figures 2B, S2B). In contrast, during delta-dominant states, the oscillations in the cortex are less regular and having a high rate of spiral waves (Figure 2C, Figure S2B).
Possible functions of cortical spiral waves
Spiral waves may participate in cortical processing by organizing neuronal activity over space. Such a mechanism may reduce the frequency components in the cortical activity and the spatiotemporal complexity, as shown in our data (Figure 3, S3) and suggested by theoretical analysis (Schiff et al., 2007).
The robust feature of spiral waves may be harmful and lead to pathological oscillations. Spiral waves are known to underlie the mechanism of fibrillations in cardiac ventricles and atria (Jalife, 2003). Long-lasting spiral waves in the cortex may contribute to seizures and other abnormal rhythms. Robust spiral waves lasting for hundreds of cycles were observed in the cortex of epileptic Mongolian gerbils, which serve as a model of focal epilepsy (Takagaki and Ohl, unpublished data).
Since robust spiral dynamics are often catastrophic, the normal cortex should possess mechanisms to prevent persistent spiral waves from disturbing normal function. We found that most cortical spirals are short-lived (Figure S4), suggesting that cortical spiral waves can be effectively controlled. The activity from long-range connections may penetrate the protecting zone surrounding the spiral phase singularity, making the singularity unstable. The existence of such a mechanism is strongly supported by our experimental data from sleep-like states, during which the emergence of spiral waves was strongly correlated with the brain states (Figures 2E).
Short-lived spiral waves may participate in cortical processing. In the turtle cortex, short-lived spiral waves were observed during visual stimulation, suggesting that spiral waves may play a role in visual processing (Prechtl et al., 1997). Spiral dynamics may be involved in prolonging sensory-evoked activity for interacting with subsequent inputs, suggested by our network models (Huang et al., 2004). Spiral waves, as a locally generated event, may also help a local cortical circuit to quickly disengage from globally synchronized rhythms, as suggested by the spatial coherence analysis (Figure 4). Such disengagement may play a role in switching between sleep states. Large phase changes in ongoing cortical oscillations can facilitate the sensory responses in multi-sensory processing (Lakatos et al., 2007). Spiral waves can create large spatial phase gradients (Figures 3, 4). These phase changes may participate in sensory processing by setting the ongoing activity in either high excitable phases to enhance the response to further inputs or in a low excitable phase to reduce the sensory response.
Sensory responses are known to trigger propagating waves in the cortex (Ferezou et al., 2006; Freeman and Barrie, 2000; Han et al., 2008; Lam et al., 2003; Prechtl et al., 2000; Prechtl et al., 1997; Senseman and Robbins, 1999; Xu et al., 2007) and collisions between two waves at certain timing and angle are known to generate spiral waves (Palsson and Cox, 1996; Steinbock and Muller, 1993; Winfree, 1989). Visual stimulation at two different locations in the visual field triggers two waves in the visual cortex, and interaction between these two waves occasionally generate short-lived spirals (data not shown). Exploring sensory-induced spiral waves would be the next step toward understanding the involvement of spiral dynamics in cortical processing.
In conclusion, while our understanding of the functional relevance of cortical spatiotemporal patterns is in its early stage, spiral waves provide a dynamic pattern that emerges from cortical population interactions and may have a powerful influence on the cortical oscillations and cortical processing.
Experimental procedures
Sprague-Dawley rats (250 - 400g, n=29) were used in the experiments. Surgical procedures were approved by Georgetown University Animal Care and Use Committee following NIH guidelines. Briefly, animals were initially anesthetized to surgical level with intraperitoneal (IP) injections of urethane (1.25 g/kg) or pentobarbital sodium (60 mg/kg). A craniotomy window of ~ 6 mm in diameter was opened over the visual cortex in the left hemisphere. After opening the skull, the dura mater was not removed; and voltage-sensitive dye solution (RH-1691 or RH-1838, (Shoham et al., 1999) Optical Imaging, 1mg/mL in Ringer solution) was applied transdurally for 2 hours. A silver ball electrode was used for local EEG recording. Visual cortex was imaged by a 5× macroscope. The field of view is about 4 mm in diameter and the image of the cortex is projected onto a 464-photodiode array (WuTech Instruments). Optical signals were digitized concurrently with local EEG and ECG signals. The voltage-sensitive dye signals have excellent signal-to-noise ratio (~10), enough to identify the amplitude reduction and phase singularities of spiral waves. For additional information regarding the VSD method see Wu and Cohen (1993) and Lippert et al. (2007).
Inducing oscillations
Bicuculline (0.05 mM in saline) and carbachol (1 mM in saline) were applied epidurally to induce oscillations in the visual cortex. In some experiments, a bicuculline pre-treatment procedure was used to induce additional wave initiation sites in order to increase the chance of wave interaction. Two tiny filter paper pieces soaked with bicuculline (1 mM) were placed onto the cortex at different locations for 10 min before inducing the oscillation.
Inducing sleep-like states
Anesthesia was induced with 2.5% of isoflurane (in O2) via a nose mask. A tail vein catheter is then quickly established and an initial dose of pentobarbital (50-60 mg/kg) is infused within 30 sec. During infusion, isoflurane is gradually reduced to 0% and the mask is removed. With the EEG (EEG electrode: 3.0 mm posterior to bregma, 1 mm lateral to midline) and heart rate closely monitored, tail vein infusion of pentobarbital is maintained at a constant low rate of 6-9 mg/kg-hour through the surgery and staining procedures. Under the low infusion rate the initial anesthesia would wear out in 2-3 hours and alternations of EEG theta/delta patterns occur. The pentobarbital infusion rate (9 - 15 mg/kg-hour) is then carefully adjusted for each individual animal to sustain the alternation of theta/delta patterns.
Slice experiment
Cortical slices (500 μm) sectioned at tangential plane were obtained from P21-35 Sprague Dawley rats (described in Huang et al., 2004). Slices were stained with 5-10 μg/ml of voltage-sensitive dye NK3630 (Nippon Kankoh-Shikiso Kenkyusho, Okayama, Japan) for 60-120 min (26°C) and perfused in a submersion chamber during imaging experiment at 28°C. Imaging was performed with a photodiode array on an upright microscope with transillumination (absorption) arrangement (Huang et al., 2004). Episodic oscillations (4-20 Hz) occurred spontaneously when the slices were perfused with 100 μM carbachol and 10 μM bicuculline in the ACSF, and the oscillations manifested spatiotemporally into spiral and other forms of wave patterns.
Data analysis
The optical data were analyzed using the program NeuroPlex (RedShirtImaging) and programs written in MatLab. A mathematical algorithm was used to remove the heart beat artifact (Lippert et al., 2007). Spirals were identified by phase singularities as described in the previously (Huang et al., 2004). Briefly, raw optical signals are first band-pass filtered between 3 - 50Hz. Single value decomposition (SVD) method (Precthl et al., 1997) was also used to remove the noises in the data set obtained during sleep-like states.
Hilbert amplitude
Hilbert transformation (MatLab Signal Processing Tool Box, function “hilbert”) was used to decompose the signal on each detector. The analytic signal Sa(t)(Rosenblum et al., 1996; Sun et al., 2008) is defined as:
| (1) |
where the real part s(t) is the original data obtained in experiments, and the imaginary part was given by the Hilbert transform of s(t),
| (2) |
(P means the Hilbert transform is defined using the Cauchy Principal Value). Then the “Hilbert amplitude” A(t) and “instantaneous phase” φ(t) are given by:
| (3) |
| (4) |
Here φ(t) and A(t) were obtained from each individual detector, independent from the wave pattern and spatial relationship among detectors. The spatial maps of φ(t) from all detectors at given time points t1, t2… were used for identifying a phase singularity (a point surrounded by all φ values from −π to π). Spirals were defined by the occurrence of singularity for at least 30 consecutive frames. The spatial maps of A(t) were used for demonstrating the amplitude reduction near spiral center (Figure 6).
Mean Correlation Coefficient (MCC)
The correlation coefficient between all pairs of the 464 detectors was calculated in the standard manner (Matlab function “corrcoef”), resulting in a matrix (464 × 464) of correlation coefficients. The values at the diagonal of the matrix, which are auto-correlation coefficients, were excluded. The mean correlation coefficient was defined as the mean value of other correlation coefficients with a total number of 463 × 464.
Supplementary Material
Acknowledgement
We thank Drs. S.J. Schiff, L.B. Cohen, G Bard Ermentrout, S.D. Van Hooser, D. Fitzpatrick and S.H. Strogatz for helpful discussion and comments on the manuscript. Supported by NIH grant NS036447, NS059034 (JYW), fellowships from the American Epilepsy Society and the Lennox Trust Fund (to XH), the Humboldt Foundation/Stiftung (to KT) and Georgetown-China Scholarship council (to JL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The supplemental materials include five figures and seven movies.
Reference
- Benucci A, Frazor RA, Carandini M. Standing waves and traveling waves distinguish two circuits in visual cortex. Neuron. 2007;55:103–117. doi: 10.1016/j.neuron.2007.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bub G, Shrier A, Glass L. Global organization of dynamics in oscillatory heterogeneous excitable media. Phys Rev Lett. 2005;94:028105. doi: 10.1103/PhysRevLett.94.028105. [DOI] [PubMed] [Google Scholar]
- Bub G, Tateno K, Shrier A, Glass L. Spontaneous initiation and termination of complex rhythms in cardiac cell culture. J Cardiovasc Electrophysiol. 2003;14:S229–236. doi: 10.1046/j.1540.8167.90315.x. [DOI] [PubMed] [Google Scholar]
- Chen Y, Geisler WS, Seidemann E. Optimal decoding of correlated neural population responses in the primate visual cortex. Nat Neurosci. 2006;9:1412–1420. doi: 10.1038/nn1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras D, Destexhe A, Sejnowski TJ, Steriade M. Control of spatiotemporal coherence of a thalamic oscillation by corticothalamic feedback. Science. 1996;274:771–774. doi: 10.1126/science.274.5288.771. [DOI] [PubMed] [Google Scholar]
- Davidenko JM, Pertsov AM, Salomonsz R, Baxter WP, Jalife J. Spatiotemporal irregularities of spiral wave activity in isolated ventricular muscle. J Electrocardiol. 1992a;24(Suppl):113–122. doi: 10.1016/s0022-0736(10)80029-9. [DOI] [PubMed] [Google Scholar]
- Davidenko JM, Pertsov AV, Salomonsz R, Baxter W, Jalife J. Stationary and drifting spiral waves of excitation in isolated cardiac muscle. Nature. 1992b;355:349–351. doi: 10.1038/355349a0. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Kauer JS. Relationships between odor-elicited oscillations in the salamander olfactory epithelium and olfactory bulb. J Neurophysiol. 2000;83:754–765. doi: 10.1152/jn.2000.83.2.754. [DOI] [PubMed] [Google Scholar]
- Ermentrout GB, Kleinfeld D. Traveling electrical waves in cortex: insights from phase dynamics and speculation on a computational role. Neuron. 2001;29:33–44. doi: 10.1016/s0896-6273(01)00178-7. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Bolea S, Petersen CC. Visualizing the cortical representation of whisker touch: voltage-sensitive dye imaging in freely moving mice. Neuron. 2006;50:617–629. doi: 10.1016/j.neuron.2006.03.043. [DOI] [PubMed] [Google Scholar]
- Ferezou I, Haiss F, Gentet LJ, Aronoff R, Weber B, Petersen CC. Spatiotemporal dynamics of cortical sensorimotor integration in behaving mice. Neuron. 2007;56:907–923. doi: 10.1016/j.neuron.2007.10.007. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Barrie JM. Analysis of spatial patterns of phase in neocortical gamma EEGs in rabbit. J Neurophysiol. 2000;84:1266–1278. doi: 10.1152/jn.2000.84.3.1266. [DOI] [PubMed] [Google Scholar]
- Gorelova NA, Bures J. Spiral waves of spreading depression in the isolated chicken retina. J Neurobiol. 1983;14:353–363. doi: 10.1002/neu.480140503. [DOI] [PubMed] [Google Scholar]
- Gottwald G, Pumir A, Krinsky V. Spiral wave drift induced by stimulating wave trains. Chaos. 2001;11:487–494. doi: 10.1063/1.1395624. [DOI] [PubMed] [Google Scholar]
- Han F, Caporale N, Dan Y. Reverberation of recent visual experience in spontaneous cortical waves. Neuron. 2008;60:321–327. doi: 10.1016/j.neuron.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-White ME, Zanotti SA, Frautschy SA, Charles AC. Spiral intercellular calcium waves in hippocampal slice cultures. J Neurophysiol. 1998;79:1045–1052. doi: 10.1152/jn.1998.79.2.1045. [DOI] [PubMed] [Google Scholar]
- Huang X, Troy WC, Yang Q, Ma H, Laing CR, Schiff SJ, Wu JY. Spiral waves in disinhibited mammalian neocortex. J Neurosci. 2004;24:9897–9902. doi: 10.1523/JNEUROSCI.2705-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalife J. Rotors and spiral waves in atrial fibrillation. J Cardiovasc Electrophysiol. 2003;14:776–780. doi: 10.1046/j.1540-8167.2003.03136.x. [DOI] [PubMed] [Google Scholar]
- Jalife J, Gray R. Drifting vortices of electrical waves underlie ventricular fibrillation in the rabbit heart. Acta Physiol Scand. 1996;157:123–131. doi: 10.1046/j.1365-201X.1996.505249000.x. [DOI] [PubMed] [Google Scholar]
- Jancke D, Chavane F, Naaman S, Grinvald A. Imaging cortical correlates of illusion in early visual cortex. Nature. 2004;428:423–426. doi: 10.1038/nature02396. [DOI] [PubMed] [Google Scholar]
- Keener JP, Tyson JJ. Spiral waves in the Belousov-Zhabotinskii reaction. Physica D:Nonlinear Phenomena. 1986;21:307–324. [Google Scholar]
- Kheowan OU, Kantrasiri S, Wilairat P, Storb U, Muller SC. Spiral wave dynamics under feedback control derived from a variety of sensory domains. Phys Rev E Stat Nonlin Soft Matter Phys. 2004;70:046221. doi: 10.1103/PhysRevE.70.046221. [DOI] [PubMed] [Google Scholar]
- Lakatos P, Chen CM, O’Connell MN, Mills A, Schroeder CE. Neuronal oscillations and multisensory interaction in primary auditory cortex. Neuron. 2007;53:279–292. doi: 10.1016/j.neuron.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Cohen LB, Wachowiak M, Zochowski MR. Odors elicit three different oscillations in the turtle olfactory bulb. J Neurosci. 2000;20:749–762. doi: 10.1523/JNEUROSCI.20-02-00749.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam YW, Cohen LB, Zochowski MR. Odorant specificity of three oscillations and the DC signal in the turtle olfactory bulb. Eur J Neurosci. 2003;17:436–446. doi: 10.1046/j.1460-9568.2003.02457.x. [DOI] [PubMed] [Google Scholar]
- Lechleiter J, Girard S, Peralta E, Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991;252:123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lippert MT, Takagaki K, Xu W, Huang X, Wu JY. Methods for Voltage-Sensitive Dye Imaging of Rat Cortical Activity With High Signal-to-Noise Ratio. J Neurophysiol. 2007;98:502–512. doi: 10.1152/jn.01169.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London JA, Cohen LB, Wu JY. Optical recordings of the cortical response to whisker stimulation before and after the addition of an epileptogenic agent. J Neurosci. 1989;9:2182–2190. doi: 10.1523/JNEUROSCI.09-06-02182.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubenov EV, Siapas AG. Hippocampal theta oscillations are travelling waves. Nature. 2009 doi: 10.1038/nature08010. [DOI] [PubMed] [Google Scholar]
- Mikhailov AS, Showalter K. Control of waves, patterns and turbulence in chemical systems. Phys. Rep. 2006;425:79–194. [Google Scholar]
- Montgomery SM, Sirota A, Buzsaki G. Theta and gamma coordination of hippocampal networks during waking and rapid eye movement sleep. J Neurosci. 2008;28:6731–6741. doi: 10.1523/JNEUROSCI.1227-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palsson E, Cox EC. Origin and evolution of circular waves and spirals in Dictyostelium discoideum territories. Proc Natl Acad Sci U S A. 1996;93:1151–1155. doi: 10.1073/pnas.93.3.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen CC, Hahn TT, Mehta M, Grinvald A, Sakmann B. Interaction of sensory responses with spontaneous depolarization in layer 2/3 barrel cortex. Proc Natl Acad Sci U S A. 2003;100:13638–13643. doi: 10.1073/pnas.2235811100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtl JC, Bullock TH, Kleinfeld D. Direct evidence for local oscillatory current sources and intracortical phase gradients in turtle visual cortex. Proc Natl Acad Sci U S A. 2000;97:877–882. doi: 10.1073/pnas.97.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prechtl JC, Cohen LB, Pesaran B, Mitra PP, Kleinfeld D. Visual stimuli induce waves of electrical activity in turtle cortex. Proc Natl Acad Sci U S A. 1997;94:7621–7626. doi: 10.1073/pnas.94.14.7621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roland PE, Hanazawa A, Undeman C, Eriksson D, Tompa T, Nakamura H, Valentiniene S, Ahmed B. Cortical feedback depolarization waves: a mechanism of top-down influence on early visual areas. Proc Natl Acad Sci U S A. 2006;103:12586–12591. doi: 10.1073/pnas.0604925103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblum MG, Pikovsky AS, Kurths J. Phase synchronization of chaotic oscillators. Phys Rev Lett. 1996;76:1804–1807. doi: 10.1103/PhysRevLett.76.1804. [DOI] [PubMed] [Google Scholar]
- Rubino D, Robbins KA, Hatsopoulos NG. Propagating waves mediate information transfer in the motor cortex. Nat Neurosci. 2006;9:1549–1557. doi: 10.1038/nn1802. [DOI] [PubMed] [Google Scholar]
- Schiff SJ, Huang X, Wu JY. Dynamical evolution of spatiotemporal patterns in mammalian middle cortex. Phys Rev Lett. 2007;98:178102. doi: 10.1103/PhysRevLett.98.178102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senseman DM, Robbins KA. Modal behavior of cortical neural networks during visual processing. J Neurosci. 1999;19:RC3. doi: 10.1523/JNEUROSCI.19-10-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon D, Grinvald A. Dynamics and constancy in cortical spatiotemporal patterns of orientation processing. Science. 2002;295:512–515. doi: 10.1126/science.1065916. [DOI] [PubMed] [Google Scholar]
- Sharon D, Jancke D, Chavane F, Na’aman S, Grinvald A. Cortical response field dynamics in cat visual cortex. Cereb Cortex. 2007;17:2866–2877. doi: 10.1093/cercor/bhm019. [DOI] [PubMed] [Google Scholar]
- Shoham D, Glaser DE, Arieli A, Kenet T, Wijnbergen C, Toledo Y, Hildesheim R, Grinvald A. Imaging cortical dynamics at high spatial and temporal resolution with novel blue voltage-sensitive dyes. Neuron. 1999;24:791–802. doi: 10.1016/s0896-6273(00)81027-2. [DOI] [PubMed] [Google Scholar]
- Siegert F, Weijer CJ. Spiral and concentric waves organize multicellular Dictyostelium mounds. Curr Biol. 1995;5:937–943. doi: 10.1016/s0960-9822(95)00184-9. [DOI] [PubMed] [Google Scholar]
- Slovin H, Arieli A, Hildesheim R, Grinvald A. Long-term voltage-sensitive dye imaging reveals cortical dynamics in behaving monkeys. J Neurophysiol. 2002;88:3421–3438. doi: 10.1152/jn.00194.2002. [DOI] [PubMed] [Google Scholar]
- Steinbock O, Muller SC. Light-controlled anchoring of meandering spiral waves. Phys Rev E Stat Phys Plasmas Fluids Relat Interdiscip Topics. 1993;47:1506–1509. doi: 10.1103/physreve.47.1506. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Timofeev I. Neuronal plasticity in thalamocortical networks during sleep and waking oscillations. Neuron. 2003;37:563–576. doi: 10.1016/s0896-6273(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Strogatz SH. Exploring complex networks. Nature. 2001;410:268–276. doi: 10.1038/35065725. [DOI] [PubMed] [Google Scholar]
- Sun J, Zhang J, Zhou J, Xu X, Small M. Detecting phase synchronization in noisy data from coupled chaotic oscillators. Phys Rev E Stat Nonlin Soft Matter Phys. 2008;77:046213. doi: 10.1103/PhysRevE.77.046213. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A, Orkand RK, Kettenmann H. Glial calcium: homeostasis and signaling function. Physiol Rev. 1998;78:99–141. doi: 10.1152/physrev.1998.78.1.99. [DOI] [PubMed] [Google Scholar]
- Watts DJ, Strogatz SH. Collective dynamics of ‘small-world’ networks. Nature. 1998;393:440–442. doi: 10.1038/30918. [DOI] [PubMed] [Google Scholar]
- Winfree AT. Electrical instability in cardiac muscle: phase singularities and rotors. J Theor Biol. 1989;138:353–405. doi: 10.1016/s0022-5193(89)80200-0. [DOI] [PubMed] [Google Scholar]
- Winfree AT. The Geometry of Biological Time. Springer-Verlag; New York: 2001. [Google Scholar]
- Wu JY, Xiaoying H, Chuan Z. Propagating waves of activity in the neocortex: what they are, what they do. Neuroscientist. 2008;14:487–502. doi: 10.1177/1073858408317066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu W, Huang X, Takagaki K, Wu JY. Compression and reflection of visually evoked cortical waves. Neuron. 2007;55:119–129. doi: 10.1016/j.neuron.2007.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.