Abstract
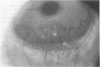
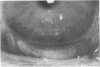
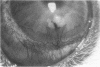
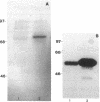
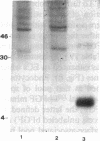
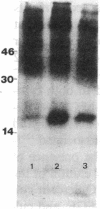
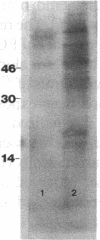
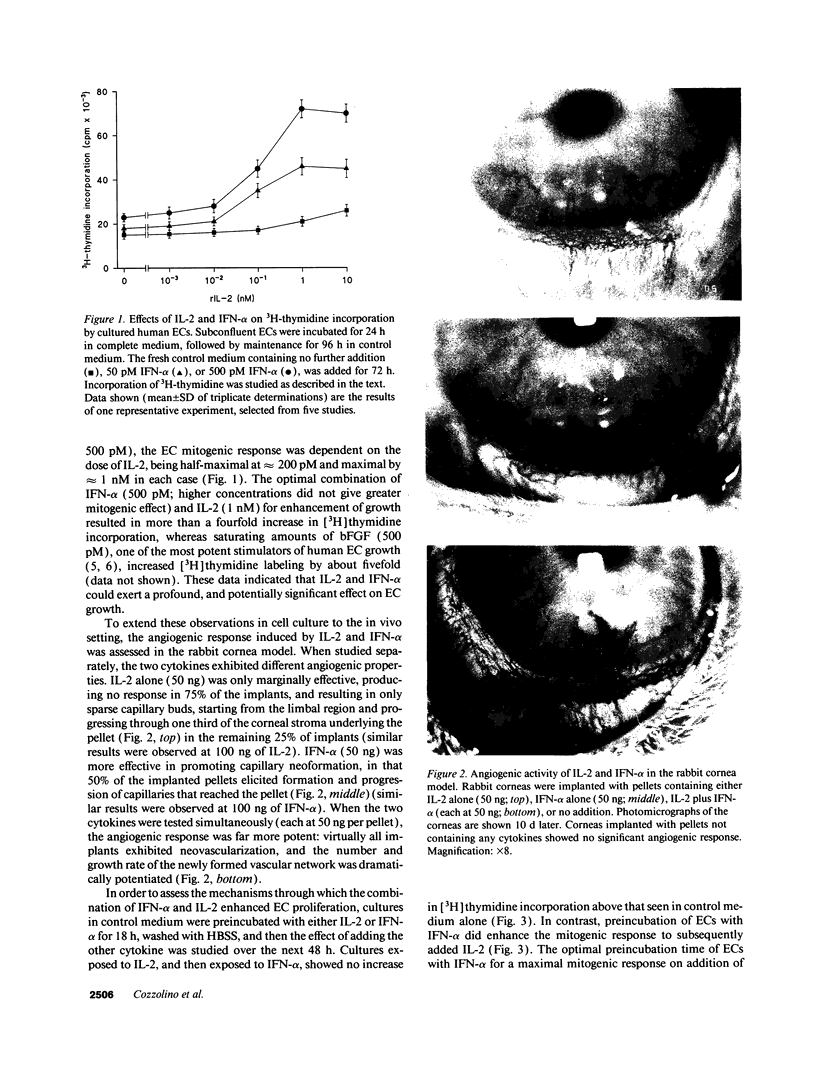
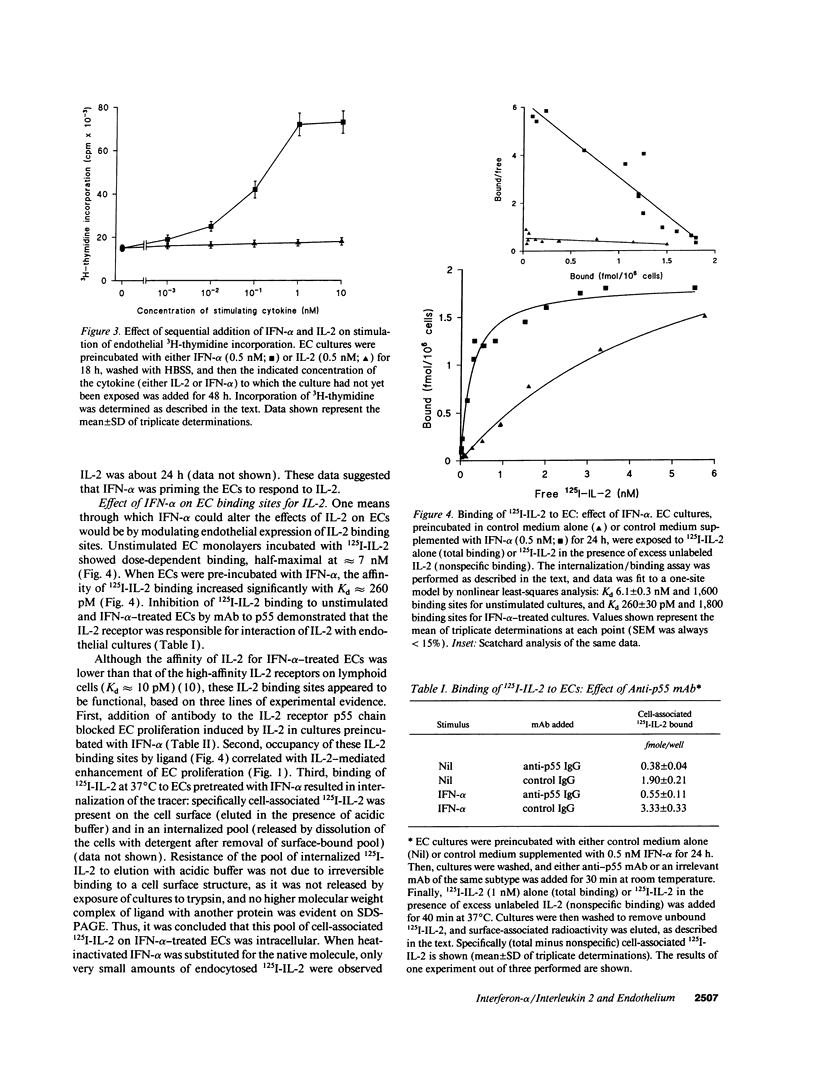
To elucidate mechanisms underlying neovascularization that accompanies certain chronic immune/inflammatory disorders, the effects of interferon-alpha (IFN-alpha) and interleukin 2 (IL-2) on endothelial cell (EC) growth in vitro and angiogenesis in vivo were studied. Preincubation of cultured human ECs with IFN-alpha, followed by exposure to IL-2, resulted in effective stimulation of cell growth, whereas either cytokine alone had only a slight effect. The combination of IFN-alpha/IL-2 induced an angiogenic response in the rabbit cornea. IL-2 receptor expression was enhanced on IFN-alpha-treated ECs: p55 was increased and p70 was induced. 125I-IL-2 binding to ECs treated with IFN-alpha was enhanced (Kd from approximately 7 nM to approximately 260 pM with IFN-alpha), and anti-p55 IgG blocked 125I-IL-2/EC interaction as well as IL-2-mediated EC proliferation. Consistent with these findings in cell culture, immunohistologic studies demonstrated p55 and p70 antigen in the vasculature of rheumatoid joints, but not in normal joint tissue. Exposure of cultured ECs to IFN-alpha increased levels of intracellular EC basic fibroblast growth factor (bFGF), and subsequent addition of IL-2 led to bFGF release into the medium. The observation that anti-bFGF IgG largely blocked EC proliferation in response to IFN-alpha/IL-2 suggested that bFGF was a critical agent in this setting. These data suggest a mechanism rendering ECs responsive to IL-2 which may be relevant in immune/inflammatory disorders: IFN-alpha-mediated induction of functional EC receptors for IL-2, which drives cell proliferation by a mechanism dependent on increased synthesis and release of bFGF.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burgess W. H., Bizik J., Mehlman T., Quarto N., Rifkin D. B. Direct evidence for methylation of arginine residues in high molecular weight forms of basic fibroblast growth factor. Cell Regul. 1991 Feb;2(2):87–93. doi: 10.1091/mbc.2.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess W. H., Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Cozzolino F., Torcia M., Aldinucci D., Ziche M., Almerigogna F., Bani D., Stern D. M. Interleukin 1 is an autocrine regulator of human endothelial cell growth. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6487–6491. doi: 10.1073/pnas.87.17.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensoli B., Nakamura S., Salahuddin S. Z., Biberfeld P., Larsson L., Beaver B., Wong-Staal F., Gallo R. C. AIDS-Kaposi's sarcoma-derived cells express cytokines with autocrine and paracrine growth effects. Science. 1989 Jan 13;243(4888):223–226. doi: 10.1126/science.2643161. [DOI] [PubMed] [Google Scholar]
- Eschenfeldt W. H., Manrow R. E., Krug M. S., Berger S. L. Isolation and partial sequencing of the human prothymosin alpha gene family. Evidence against export of the gene products. J Biol Chem. 1989 May 5;264(13):7546–7555. [PubMed] [Google Scholar]
- Ezekowitz R. A., Mulliken J. B., Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992 May 28;326(22):1456–1463. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- Folkman J., Klagsbrun M. Angiogenic factors. Science. 1987 Jan 23;235(4787):442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Folkman J., Watson K., Ingber D., Hanahan D. Induction of angiogenesis during the transition from hyperplasia to neoplasia. Nature. 1989 May 4;339(6219):58–61. doi: 10.1038/339058a0. [DOI] [PubMed] [Google Scholar]
- Frasier-Scott K., Hatzakis H., Seong D., Jones C. M., Wu K. K. Influence of natural and recombinant interleukin 2 on endothelial cell arachidonate metabolism. Induction of de novo synthesis of prostaglandin H synthase. J Clin Invest. 1988 Dec;82(6):1877–1883. doi: 10.1172/JCI113805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii M., Sugamura K., Sano K., Nakai M., Sugita K., Hinuma Y. High-affinity receptor-mediated internalization and degradation of interleukin 2 in human T cells. J Exp Med. 1986 Mar 1;163(3):550–562. doi: 10.1084/jem.163.3.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gospodarowicz D. Biological activities of fibroblast growth factors. Ann N Y Acad Sci. 1991;638:1–8. doi: 10.1111/j.1749-6632.1991.tb49012.x. [DOI] [PubMed] [Google Scholar]
- Gospodarowicz D., Cheng J., Lui G. M., Baird A., Böhlent P. Isolation of brain fibroblast growth factor by heparin-Sepharose affinity chromatography: identity with pituitary fibroblast growth factor. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6963–6967. doi: 10.1073/pnas.81.22.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Hicks C., Cooley M. A., Penny R. Investigation of interleukin 2 receptors on human endothelial cells. Growth Factors. 1991;5(3):201–208. doi: 10.3109/08977199109000284. [DOI] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., D'Amore P. A. Regulators of angiogenesis. Annu Rev Physiol. 1991;53:217–239. doi: 10.1146/annurev.ph.53.030191.001245. [DOI] [PubMed] [Google Scholar]
- Kuchler K., Thorner J. Membrane translocation of proteins without hydrophobic signal peptides. Curr Opin Cell Biol. 1990 Aug;2(4):617–624. doi: 10.1016/0955-0674(90)90102-k. [DOI] [PubMed] [Google Scholar]
- Mignatti P., Morimoto T., Rifkin D. B. Basic fibroblast growth factor released by single, isolated cells stimulates their migration in an autocrine manner. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11007–11011. doi: 10.1073/pnas.88.24.11007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norioka K., Hara M., Kitani A., Hirose T., Hirose W., Harigai M., Suzuki K., Kawakami M., Tabata H., Kawagoe M. Inhibitory effect of human recombinant interleukin-1 alpha and beta on growth of human vascular endothelial cells. Biochem Biophys Res Commun. 1987 Jun 15;145(2):969–975. doi: 10.1016/0006-291x(87)91060-6. [DOI] [PubMed] [Google Scholar]
- Okamoto Y., Minamoto S., Shimizu K., Mogami H., Taniguchi T. Interleukin 2 receptor beta chain expressed in an oligodendroglioma line binds interleukin 2 and delivers growth signal. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6584–6588. doi: 10.1073/pnas.87.17.6584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfäffle M., Ruggiero F., Hofmann H., Fernández M. P., Selmin O., Yamada Y., Garrone R., von der Mark K. Biosynthesis, secretion and extracellular localization of anchorin CII, a collagen-binding protein of the calpactin family. EMBO J. 1988 Aug;7(8):2335–2342. doi: 10.1002/j.1460-2075.1988.tb03077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouët J., Gospodarowicz D. Transforming growth factor beta-1 positively modulates the bioactivity of fibroblast growth factor on corneal endothelial cells. J Cell Physiol. 1989 Nov;141(2):392–399. doi: 10.1002/jcp.1041410221. [DOI] [PubMed] [Google Scholar]
- Queluz T. H., Pawlowski I., Brunda M. J., Brentjens J. R., Vladutiu A. O., Andres G. Pathogenesis of an experimental model of Goodpasture's hemorrhagic pneumonitis. J Clin Invest. 1990 May;85(5):1507–1515. doi: 10.1172/JCI114597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogelj S., Weinberg R. A., Fanning P., Klagsbrun M. Basic fibroblast growth factor fused to a signal peptide transforms cells. Nature. 1988 Jan 14;331(6152):173–175. doi: 10.1038/331173a0. [DOI] [PubMed] [Google Scholar]
- Rubartelli A., Cozzolino F., Talio M., Sitia R. A novel secretory pathway for interleukin-1 beta, a protein lacking a signal sequence. EMBO J. 1990 May;9(5):1503–1510. doi: 10.1002/j.1460-2075.1990.tb08268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagaya Y., Maeda Y., Mitsui A., Kondo N., Matsui H., Hamuro J., Brown N., Arai K., Yokota T., Wakasugi H. ATL-derived factor (ADF), an IL-2 receptor/Tac inducer homologous to thioredoxin; possible involvement of dithiol-reduction in the IL-2 receptor induction. EMBO J. 1989 Mar;8(3):757–764. doi: 10.1002/j.1460-2075.1989.tb03436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuboi R., Sato Y., Rifkin D. B. Correlation of cell migration, cell invasion, receptor number, proteinase production, and basic fibroblast growth factor levels in endothelial cells. J Cell Biol. 1990 Feb;110(2):511–517. doi: 10.1083/jcb.110.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsudo M., Karasuyama H., Kitamura F., Tanaka T., Kubo S., Yamamura Y., Tamatani T., Hatakeyama M., Taniguchi T., Miyasaka M. The IL-2 receptor beta-chain (p70). Ligand binding ability of the cDNA-encoding membrane and secreted forms. J Immunol. 1990 Jul 15;145(2):599–606. [PubMed] [Google Scholar]
- Usuki K., Heldin N. E., Miyazono K., Ishikawa F., Takaku F., Westermark B., Heldin C. H. Production of platelet-derived endothelial cell growth factor by normal and transformed human cells in culture. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7427–7431. doi: 10.1073/pnas.86.19.7427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziche M., Alessandri G., Gullino P. M. Gangliosides promote the angiogenic response. Lab Invest. 1989 Dec;61(6):629–634. [PubMed] [Google Scholar]