Abstract
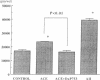
Vascular renin angiotensin system (RAS) has been reported to exist in vascular wall. However, there is no direct evidence whether the vascular RAS per se can modulate growth of vascular smooth muscle cells (VSMC), because there is no suitable method to investigate the effect of endogenously produced vasoactive substances on growth of these cells. In this study, we transferred angiotensin-converting enzyme (ACE) and/or renin cDNAs into cultured VSMC using the efficient Sendai virus (hemagglutinating virus of Japan) liposome-mediated gene transfer method, to examine their relative roles in VSMC growth in vitro. Within 35 min or 6 h, the transfection of ACE cDNA into VSMC by hemagglutinating virus of Japan method resulted in a twofold higher ACE activity than control vector, whereas a cationic liposome (Lipofectin)-mediated method failed to show any effect. This in vitro system provided us with the opportunity to investigate the influence of endogenous vascular RAS on VSMC growth. Transfection of ACE or renin cDNA resulted in increased DNA and RNA synthesis, which was inhibited with the specific angiotensin II receptor antagonist (DuP 753: 10(-6) M). Angiotensin I added to ACE-transfected VSMC increased RNA synthesis in a dose-dependent manner. Cotransfection of renin and ACE cDNAs stimulated further RNA synthesis as compared to ACE or renin cDNA alone. These results showed that transfected components of RAS can modulate VSMC growth through the endogenous production of vascular angiotensin II, and that ACE as well as renin are rate limiting in determining the VSMC RAS activity. We conclude that the hemagglutinating virus of Japan liposome-mediated gene transfer technique provides a new and useful tool for study of endogenous vascular modulators such as vascular RAS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell D. J., Habener J. F. Angiotensinogen gene is expressed and differentially regulated in multiple tissues of the rat. J Clin Invest. 1986 Jul;78(1):31–39. doi: 10.1172/JCI112566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dzau V. J., Burt D. W., Pratt R. E. Molecular biology of the renin-angiotensin system. Am J Physiol. 1988 Oct;255(4 Pt 2):F563–F573. doi: 10.1152/ajprenal.1988.255.4.F563. [DOI] [PubMed] [Google Scholar]
- Dzau V. J. Vascular renin-angiotensin: a possible autocrine or paracrine system in control of vascular function. J Cardiovasc Pharmacol. 1984;6 (Suppl 2):S377–S382. [PubMed] [Google Scholar]
- Geisterfer A. A., Peach M. J., Owens G. K. Angiotensin II induces hypertrophy, not hyperplasia, of cultured rat aortic smooth muscle cells. Circ Res. 1988 Apr;62(4):749–756. doi: 10.1161/01.res.62.4.749. [DOI] [PubMed] [Google Scholar]
- Griffin S. A., Brown W. C., MacPherson F., McGrath J. C., Wilson V. G., Korsgaard N., Mulvany M. J., Lever A. F. Angiotensin II causes vascular hypertrophy in part by a non-pressor mechanism. Hypertension. 1991 May;17(5):626–635. doi: 10.1161/01.hyp.17.5.626. [DOI] [PubMed] [Google Scholar]
- Itoh H., Pratt R. E., Dzau V. J. Atrial natriuretic polypeptide inhibits hypertrophy of vascular smooth muscle cells. J Clin Invest. 1990 Nov;86(5):1690–1697. doi: 10.1172/JCI114893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda Y., Iwai K., Uchida T. Increased expression of DNA cointroduced with nuclear protein in adult rat liver. Science. 1989 Jan 20;243(4889):375–378. doi: 10.1126/science.2911748. [DOI] [PubMed] [Google Scholar]
- Kaneda Y., Iwai K., Uchida T. Introduction and expression of the human insulin gene in adult rat liver. J Biol Chem. 1989 Jul 25;264(21):12126–12129. [PubMed] [Google Scholar]
- Kato K., Nakanishi M., Kaneda Y., Uchida T., Okada Y. Expression of hepatitis B virus surface antigen in adult rat liver. Co-introduction of DNA and nuclear protein by a simplified liposome method. J Biol Chem. 1991 Feb 25;266(6):3361–3364. [PubMed] [Google Scholar]
- Mendelsohn F. A. Localization and properties of angiotensin receptors. J Hypertens. 1985 Aug;3(4):307–316. doi: 10.1097/00004872-198508000-00002. [DOI] [PubMed] [Google Scholar]
- Murphy T. J., Alexander R. W., Griendling K. K., Runge M. S., Bernstein K. E. Isolation of a cDNA encoding the vascular type-1 angiotensin II receptor. Nature. 1991 May 16;351(6323):233–236. doi: 10.1038/351233a0. [DOI] [PubMed] [Google Scholar]
- Naftilan A. J., Pratt R. E., Dzau V. J. Induction of platelet-derived growth factor A-chain and c-myc gene expressions by angiotensin II in cultured rat vascular smooth muscle cells. J Clin Invest. 1989 Apr;83(4):1419–1424. doi: 10.1172/JCI114032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naftilan A. J., Zuo W. M., Inglefinger J., Ryan T. J., Jr, Pratt R. E., Dzau V. J. Localization and differential regulation of angiotensinogen mRNA expression in the vessel wall. J Clin Invest. 1991 Apr;87(4):1300–1311. doi: 10.1172/JCI115133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamura T., Miyazaki M., Inagami T., Toda N. Vascular renin-angiotensin system in two-kidney, one clip hypertensive rats. Hypertension. 1986 Jul;8(7):560–565. doi: 10.1161/01.hyp.8.7.560. [DOI] [PubMed] [Google Scholar]
- Owens G. K., Loeb A., Gordon D., Thompson M. M. Expression of smooth muscle-specific alpha-isoactin in cultured vascular smooth muscle cells: relationship between growth and cytodifferentiation. J Cell Biol. 1986 Feb;102(2):343–352. doi: 10.1083/jcb.102.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquet J. L., Baudouin-Legros M., Brunelle G., Meyer P. Angiotensin II-induced proliferation of aortic myocytes in spontaneously hypertensive rats. J Hypertens. 1990 Jun;8(6):565–572. doi: 10.1097/00004872-199006000-00010. [DOI] [PubMed] [Google Scholar]
- Re R., Fallon J. T., Dzau V., Quay S. C., Haber E. Renin synthesis by canine aortic smooth muscle cells in culture. Life Sci. 1982 Jan 4;30(1):99–106. doi: 10.1016/0024-3205(82)90641-5. [DOI] [PubMed] [Google Scholar]
- van Kleef E. M., Smits J. F., De Mey J. G., Cleutjens J. P., Lombardi D. M., Schwartz S. M., Daemen M. J. Alpha 1-adrenoreceptor blockade reduces the angiotensin II-induced vascular smooth muscle cell DNA synthesis in the rat thoracic aorta and carotid artery. Circ Res. 1992 Jun;70(6):1122–1127. doi: 10.1161/01.res.70.6.1122. [DOI] [PubMed] [Google Scholar]