Abstract
The development of functional neural circuits relies upon the coordination of various cell types. In particular, astrocytes play a crucial role in orchestrating neural development by powerfully coordinating synapse formation and function, neuronal survival, and axon guidance. While astrocytes help to shape neural circuits in the developing brain, the mechanisms underlying their own development may play an equally crucial role in nervous system function. The onset of astrogenesis is a temporally regulated phenomenon that relies upon exogenously secreted cues and intrinsic chromatin changes. Defects in the mechanisms underlying astrogenesis or in astrocyte function during early development may contribute to the progression of a variety of neurodevelopmental disorders.
Introduction
The development of the nervous system is no small feat. For decades, neurobiologists have worked to understand how billions of neurons are born, migrate throughout the brain to their respective destinations, and proceed to establish intricate functional circuits. But this is only half the story. As new neurons populate the developing brain they are soon joined by a group of cells known as astrocytes. Astrocytes are not merely passive bystanders of nervous system development; beyond playing critical roles in energy metabolism, K+ buffering, and neurotransmitter recycling, astrocytes actively contribute to the formation and maintenance of neural circuits by powerfully controlling synapse formation, function, and elimination [1,2••]. In this light, astrocytes may be seen as choreographers of neural circuit formation. Defects in the carefully orchestrated steps required to form functional neural networks are believed to result in a cluster of diseases known as neurodevelopmental disorders. Naturally, this raises the question whether some of these conditions, long assumed to result from neuronal pathogenesis, may share an underlying etiology of astrocyte dysfunction.
Some conjecture that the cognitive abilities observed in humans stem from an increase in the relative size of the cerebral cortex, but the human brain is not the evolutionary outlier that is so commonly assumed. In fact, the human cortex contains precisely the number of neurons that would be expected from a scaled-up primate brain [3]. Human astrocytes, however, are quite unique when compared to other animals; they are over 20-times larger by volume and contact up to 10-times the number of synapses as individual rodent astrocytes [4]. Furthermore, transcriptome data of human brains demonstrate significantly increased expression of astrocyte-derived synaptogenic proteins [5]. These data raise the possibility that the non-neuronal dimension of information processing and circuit organization in the human brain may contribute to improved human cognitive ability and complexity. Conversely, the arrival of human neurodevelopmental disorders may therefore represent the end-product of disrupted astrocyte development.
Though we have begun to better understand the contributions of astrocytes to the nervous system throughout various stages of maturation, our insight into how these cells initially develop has been enigmatic. In this review we will focus on astrocyte development through the lens of neurodevelopmental disorders. We will begin with a brief overview of the mechanisms involved in astrogenesis (for comprehensive reviews, see [6–8]) followed by a discussion of emerging evidence suggesting that the pathogenesis of many neurodevelopmental disorders may be attributable to astrocyte dysfunction during development.
Temporal separation of neurogenesis and gliogenesis
Neurons, astrocytes, and oligodendrocytes share a common neuroepithelial origin and are born throughout embryogenesis in a temporally defined manner. In the mammalian system the first divisions of neural stem cells (NSCs) are exclusively neurogenic, either giving rise to neural-restricted intermediate progenitors or directly to young neurons. Once the bulk of neurogenesis has occurred, NSCs become primarily gliogenic. This phenomenon whereby NSCs switch from a largely neurogenic to gliogenic phenotype is also recapitulated in embryonic stem cell (ES) and induced pluripotent stem cell (iPSC) models, indicating that there are inherent temporal mechanisms that underlie the production of neurons versus glia.
Astrocytes arise from a number of various sources in the developing nervous system; they may be produced directly from radial glia [9], from a yet-to-be-identified astrocyte-restricted progenitor population, or from the proliferation of newly born astrocytes. In fact, recent reports indicate that the majority of mouse cortical glia arise from clonal divisions of early differentiated astrocytes [10•] and that these clones may specify domains of distinct astrocyte classes [11•]. These data also complement recent findings in the mouse spinal cord that astrocytes are allocated to spatial domains in accordance with their embryonic sites of origin in the ventricular zone [12••].
Activators of gliogenesis
A combination of extrinsic cues and epigenetic factors contribute to fate specification of NSCs during development. The idea that extrinsic signals are required for astrogenesis first arose from the observation that embryonic cortical precursors produce neurons when cultured on embryonic cortical slices, but astrocytes when cultured on postnatal cortical slices [13]. The question then arose as to the identity of these secreted cues and several key experiments soon converged on the IL-6 family of cytokines. The IL-6 subfamily includes ciliary neurotrophic factor (CNTF), leukemia inhibitor factor (LIF), and cardiotriphin-1 (CT-1). Each of these molecules act through the heterodimerization of the signal-transducing coreceptors LIFRb and gp130, which act upstream of the JAK-STAT pathway. Mice lacking either LIFRb or gp130 have pronounced deficits in astrogenesis, and gp130−/− or LIFRb−/− NSCs exhibit significantly impaired astrogenesis in vitro [14]. Though both CNTF and LIF are commonly used now for the induction of astrocytes in iPSC and ES models of astrocyte differentiation [15•,16], the relevant in vivo ligand of LIFRb and gp130 during development appears to be CT-1 that is secreted from newly born cortical neurons [17]. This creates an inherent timing mechanism whereby the extrinsic cues that are required for astrocyte formation are provided by the neurons whose development initially precedes gliogenesis. Interestingly, IL-6 is a highly versatile cytokine with pleiotropic effects in the nervous system. In addition to its roll in promoting astrogenesis, IL-6 is involved in controlling the intrinsic growth state of neurons. For example, IL-6 levels accumulate following brain injury or inflammation and promote neuronal survival and axonal regeneration [18,19]. Thus, the molecular cues that direct astrogenesis are not necessarily restricted to this purpose. Indeed, it is the intrinsic state of a given cell that dictates how an extrinsic cue is perceived, explaining how identical signals could act as inducers of astrogenesis in NSCs, or conversely as survival signals for distressed neurons.
In addition to IL-6 cytokines, BMPs and Notch signaling molecules are also potent activators of astrogenesis. How do these seemingly independent pathways synergize into a convergent picture of astrocyte development? The common denominator is their association with JAK/STAT activation, the canonical pathway regulating astrocyte gene expression. STAT3 activity is crucial for astrogenesis to occur [20] and is a direct transcriptional activator of the astrocytic genes, GFAP and S100β [21]. Most significantly, STAT3 does not act alone; it interacts with the p300/CBP co-activator complex to initiate astrocyte gene expression. Furthermore, BMP signaling via downstream Smad effector proteins also synergizes with the STAT3:p300/CBP pathway to activate gliogenesis through the formation of a larger Smad1:p300/CBP:STAT3 complex [22]. Not surprisingly, STAT3 has also been implicated as an important player in the astrogliosis that accompanies nervous system damage. Studies focusing primarily in the spinal cord demonstrate that STAT3 knockdown leads to a failure of astrocyte hypertrophy, and pronounced disruption of astroglial scar formation after spinal cord injury [23,24].
Regardless of the inciting source, STAT3 activation alone is insufficient to initiate astrogenesis. During early embryogenesis, chromatin modifications at gliogenic promoters restrict the competence of NSCs by preventing extrinsic cues from triggering astrocyte fate specification. The most striking of these chromatin modification is methylation of the GFAP promoter, which prevents its expression even when STAT3 is activated [25]. This is a transient methylation state; as development continues the Notch effector protein, NFIA, binds to the GFAP promoter and induces the dissociation of the DNA methylating enzyme, DNA methyltransferase 1 (DNMT1) [26]. The lack of methylation then relaxes the chromatin state and allows the STAT3:p300/CBP complex to initiate transcription.
Notch signaling is another potent regulator of cell fate during embryogenesis with temporally regulated effects. Early in development, Notch activation promotes the maintenance of NSCs and prevents premature neurogenesis, while at later stages it robustly stimulates gliogenesis. RBP-Jκ, the downstream effector of Notch activation, directly regulates expression of the Hes family of bHLH genes. Hes genes inhibit neurogenesis during the neurogenic period and are capable of promoting astrogenesis when ectopically expressed during the gliogenic period [27]. Hes1 itself is capable of inducing phosphorylation and subsequent activation of STAT3 by facilitating interactions between STAT3 and its kinase JAK2 [28]. The Notch target NFIA is another powerful regulator of astrogenesis that functions by repressing excessive Notch activation through the inhibition of Hes1 [29•] while simultaneously encouraging astrogenesis by promoting demethylation at the GFAP promoter [30]. A final Notch target, the HMG box transcription factor Sox9, has also long been known to activate astrogenesis. Now, recent evidence suggests that Sox9 drives NFIA expression and that these two transcription factors complex with each other in order to coregulate genes that are integral to the initiation of gliogenesis [31•]. Overall, Notch signaling is a powerful inducer of astrogenesis both as an extrinsic source of STAT3 activation and by triggering the gliogenic competence of NSCs.
The inhibition of gliogenesis
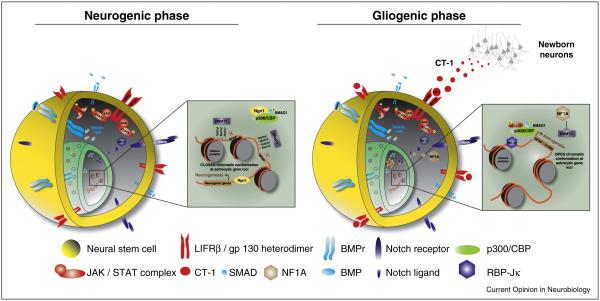
Gliogenic cytokines are present in the embryonic cortex throughout the neurogenic phase. This raises the question of how astrocytic fates are inhibited during early phases of embryogenesis. The answer is largely due to epigenetic silencing of astrocytic genes (discussed above) accompanied by inhibition of gliogenic transcription factor complexes. Throughout neurogenesis, the proneuronal factor Ngn1 binds and sequesters the p300/CBP complex to prevent its interaction with STAT3 while simultaneously promoting the expression of neuronal genes [32]. Neurotrophins like BDNF are also capable of interfering with JAK/STAT signaling by activating the neurogenic SHP2-Ras-Raf-MEK-ERK pathway. SHP2 has dual effects; it dephosphorylates STAT3 to inhibit JAK/STAT signaling in non-neuronal cells [33] and its expression triggers pro-neural MEK-ERK signaling. Constitutively activating mutations in SHP2 are found in the human neurodevelopmental disorder Noonan syndrome (NS) [34]. NS is a relatively common but complex congenital disorder that presents with an assortment of cardiac, gastrointestinal, musculoskeletal, and hematological dysfunction. Furthermore, most NS children suffer from considerable learning disabilities, a phenotype that new studies are attempting to reconcile with abnormal MEK-ERK signaling during neurodevelopment. In support of this hypothesis and consistent with the neurogenic role of SHP2, mouse models of NS that express mutant constitutively active forms of human NS-SHP2 exhibit excessive neurogenesis and decreased astrogenesis [35•]. This imbalance in the neuron to glial ratio may disrupt normal neural circuit formation and lead to behavioral phenotypes like learning disabilities. A generalized schematic of the mechanisms involved in the activation and inhibition of gliogenesis are summarized in Figure 1.
Figure 1.
Molecular mechanisms that govern neurogenic and gliogenic fates in neural stem cells. (a) During the neurogenic state, Ngn1 binds and sequesters the p300/CBP complex to prevent interactions with the gliogenic binding partner STAT3. Concomitant DNMT1 expression leads to methylation and subsequent repression of glial gene transcription. (b) Later in embryogenesis, proglial signals like BMPs, Notch, and CT-1 lead to the activation of the STAT3:p300/CBP complex. NFIA-mediated repression of DNMT1 helps to eliminate methylation at the GFAP promoter and results in a relaxed DNA confirmation where STAT3:p300/CBP can bind and initiate gliogenic gene transcription.
Neurodevelopmental diseases resulting from abnormal timing of astrogenesis
Disruptions in any of the mechanisms described above that affect the timing or efficiency of neurogenesis and astrogenesis may lead to perturbations in the relative ratios of these two cell types. A prominent example of this phenomenon is a class of syndromes known as ‘RASopathies’ that result from alterations in the Ras-Raf-MEK-ERK signaling pathway. As seen in NS, these syndromes have a variety of clinical manifestations, but nearly all share a degree of mental impairment [36]. Recent mouse models have demonstrated abnormal astrocyte development and proliferation in several of these disorders including Noonan syndrome [34], Neurofibromatosis-1 [37], Costello syndrome [38], and Cardiofaciocutaneous syndrome [39]. While RASopathy disorders are rare, they represent an excellent example of how precocious astrocyte development can lead to serious neurodevelopmental dysfunction. New data suggest that this phenomenon may also contribute to the pathogenesis of Down syndrome (trisomy 21), a neurodevelopmental disorder with significantly broader prevalence. Histologic findings in Down syndrome patients have long demonstrated reduced neuronal numbers, alteration of synaptic spines, delayed myelination, and increased astrocytes [40]. Now, new evidence is providing some of the first mechanistic clues into what may underlie precocious gliogenesis in these patients. A study from Lu et al. suggests that premature exit from neurogenesis may result from increased olig2 expression, a gliogenic transcription factor residing on the trisomic chromosome 21 [41]. Though olig2 is largely recognized for its role in oligodendrocyte formation, its expression is also critical for the formation of white matter astrocytes [42] and may act to drive premature gliogenesis in Down syndrome patients.
Astrocytes driving neuronal pathology in autism
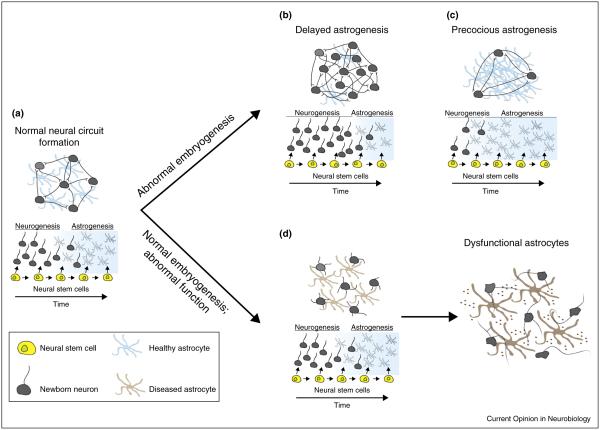
Adequate neurogenesis and gliogenesis represent only the initial steps of nervous system development. Proper neural circuit formation also requires choreographed patterns of neuronal migration, dendritic growth, axon target guidance, and synapse formation. Each of these stages requires appropriate astrocyte-derived factors and raises the question of whether astrocyte dysfunction during these critical steps could contribute to the pathogenesis of neurodevelopmental disorders (Figure 2). Recent evidence of astrocyte involvement in these processes has emerged for a broad class of syndromes known as autism spectrum disorders (ASDs). Observational studies in human ASD patient samples demonstrate elevated expression of GFAP in the superior frontal, parietal, and cerebellar cortices [43] as well as the cerebrospinal fluid [44]. In addition to GFAP, the astrocytic markers AQP4 and CX43 also demonstrate abnormal expression patterns in ASD patient samples [45]. More recent reports indicate that astrocytes of autistic patients exhibit reduced branching processes, branching length, and cell body sizes, which may be attributable to decreased Wnt/β-catenin signaling in these individuals [46].
Figure 2.
Mechanisms of astrocyte dysfunction that affect neural circuit development. (a) The establishment of appropriate neural circuits requires interplay between both neurons and astrocytes during development. Variations in the precise timing of the neurogenic to gliogenic switch can lead to a dearth (b) or surplus (c) of astrocytes. Too few astrocytes may eliminate essential cues required for axon guidance, neuronal survival, or synapse formation, while precocious astrogenesis may limit the number of neurons available to contribute to specific circuits. Alternately, neurogenesis and astrogenesis may proceed normally with circuit dysfunction arising from primary astrocyte pathology instead. (d) The secretion of toxic factors or the absence of survival and/or synaptogenic signals may contribute to the pathogenesis of aberrant neural circuits.
Beyond correlative evidence, what proof exists that astrocytes are capable of driving neuronal aberrations in models of autism? Rodent studies of monogenetic models of autism have provided the best avenues for studying this question. Most significantly, a role for astrocytes has been demonstrated in the progression of Rett syndrome, an X-linked ASD caused by mutations in the transcription factor methyl-CpG-binding protein 2 (MECP2). Ballas et al. demonstrated that mutant astrocytes from an RTT mouse fail to support normal dendritic morphology in healthy neurons [47••]. Furthermore, astrocyte-specific re-expression of MECP2 in global MECP2−/− mice was capable of rescuing a subset of Rett symptoms [48•]. Now, ChIP-seq and gene expression analyses of MECP2−/− astrocytes are beginning to identify aberrantly expressed target genes that may play a direct role in astrocyte-mediated disease pathogenesis [49]. Mouse models of another monogenetic form of autism, Fragile-X syndrome, have also demonstrated non-cell-autonomous deleterious astrocyte effects. Co-culture experiments with mutant Fragile X astrocytes and healthy neurons recapitulate in vivo observations of delayed dendritic maturation and abnormal synaptic protein expression [50]. Taken together, these studies in monogenetic models of autism provide the most direct evidence that primary astrocyte dysfunction is capable of driving the complex behavioral phenotypes observed in neurodevelopmental disorders.
Many autism-linked genes are also expressed in astrocytes
Most ASDs do not result from monogenetic disorders, but rather from a complex genetic landscape that we do not yet fully understand. As sequencing capabilities have improved, genome wide association studies (GWAS) have provided a nonbiased approach to identify genes that may be implicated in autism. The initial targets that arose from this approach included a number of genes with obvious roles in synaptic function. Considering the integral roles that astrocytes play in synaptic formation and function, we wondered whether any of these autism-associated genes were also expressed in astrocytes. We addressed this question with RNA-Seq mouse expression data from populations of acutely purified cell types (data in preparation). Of the top 46 most significantly autism-linked genes identified in recent GWAS, 30 (65%) are expressed in astrocytes. This only slightly lags behind the 77% found in neurons. Furthermore, a subset of these highly implicated autism genes are predominantly enriched in astrocytes, including the well-known K+ channel Kir4.1 as well as less studied astrocyte genes like SYNE1 [51•] and TSPAN7 [52].
Astrocyte dysfunction underlying neurodevelopmental origins of psychiatric disease
A relatively new avenue of discussion has begun to question whether astrocyte dysfunction may play a role in the neurodevelopmental origins of psychiatric disorders. Like the ASDs, initial evidence has been largely observational in conditions like schizophrenia and major depressive disorder (MDD). Laser capture microdissection of astrocytes in schizophrenic patients reveals alterations of some (DIO2, AQP4, S100β, EAAT2, and TSP), but not all astrocyte markers (ALDH1L1 and VIM) in the deep layers of the anterior cingulate gyrus [53]. Furthermore, reduced phosphorylated GFAP expression has been reported in the frontal cortices of patients with schizophrenia [54] and reduced numbers of glia are well-documented in the anterior cingulate of patients suffering from MDD and bipolar disorder [55]. How could altered astrocyte number and function contribute to the neurobiological foundations of schizophrenia? Recent evidence suggests that decreased astrocyte numbers in deeper cortical layers are accompanied by diminished expression of the astrocytic glutamate transporter, EAAT2 [56]. This fits well with the popular hypothesis that glutamatergic dyshomeostasis contributes to the pathogenesis of schizophrenia [57].
Concluding remarks
The breadth of neurologic disorders that have already been associated with some amount of astrocyte dysfunction is remarkably diverse and easily expands beyond those examples mentioned above. Several profound questions remain before we can understand how astrocytes might contribute to the pathogenesis of each of these conditions. How developmentally and functionally heterogeneous are astrocyte populations? In the setting of complex disorders like autism, how similar are human and rodent astrocytes? What qualities of astrocyte dysfunction actually contribute to aberrant neural function during development? Numerous new tools are required to answer these questions. New markers of astrocyte maturation and diversity will help to address heterogeneity in the brain. Human iPSC-derived astrocytes from affected patients could help to confirm observations made in rodent models and lead to potential therapeutic screens. We are entering an exciting era of astrocyte biology, and their consideration as drivers of neurodevelopmental dysfunction is of immediate relevance.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Clarke LE, Barres BA. Emerging roles of astrocytes in neural circuit development. Nat Rev Neurosci. 2013;14:311–321. doi: 10.1038/nrn3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chung W-S, Clarke LE, Wang GX, Stafford BK, Sher A, Chakraborty C, Joung J, Foo LC, Thompson A, Chen C, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 2013;504:394–400. doi: 10.1038/nature12776. The first study in the mammalian system to demonstrate that astrocytes actively phagocytose CNS synapses during development and into adulthood. The MEGF10 and MERTK pathways are critical players in astrocyte mediated synapse elimination. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herculano-Houzel S. The remarkable, yet not extraordinary, human brain as a scaled-up primate brain and its associated cost. Proc Natl Acad Sci USA. 2012;109(Suppl):10661–10668. doi: 10.1073/pnas.1201895109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberheim NA, Takano T, Han X, He W, Lin JHC, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, et al. Uniquely hominid features of adult human astrocytes. J Neurosci. 2009;29:3276–3287. doi: 10.1523/JNEUROSCI.4707-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cáceres M, Suwyn C, Maddox M, Thomas JW, Preuss TM. Increased cortical expression of two synaptogenic thrombospondins in human brain evolution. Cereb Cortex. 2007;17:2312–2321. doi: 10.1093/cercor/bhl140. [DOI] [PubMed] [Google Scholar]
- 6.Freeman MR, Rowitch DH. Evolving concepts of gliogenesis: a look way back and ahead to the next 25 years. Neuron. 2013;80:613–623. doi: 10.1016/j.neuron.2013.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanski R, van Strien ME, van Tijn P, Hol EM. A star is born: new insights into the mechanism of astrogenesis. Cell Mol Life Sci. 2013;71:433–447. doi: 10.1007/s00018-013-1435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Molofsky AV, Krencik R, Krenick R, Ullian EM, Ullian E, Tsai H, Deneen B, Richardson WD, Barres Ba, Rowitch DH. Astrocytes and disease: a neurodevelopmental perspective. Genes Dev. 2012;26:891–907. doi: 10.1101/gad.188326.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinto L, Götz M. Radial glial cell heterogeneity – the source of diverse progeny in the CNS. Prog Neurobiol. 2007;83:2–23. doi: 10.1016/j.pneurobio.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 10.Ge W-P, Miyawaki A, Gage FH, Jan YN, Jan LY. Local generation of glia is a major astrocyte source in postnatal cortex. Nature. 2012;484:376–380. doi: 10.1038/nature10959. This study demonstrated that local proliferation of differentiated cortical astrocytes provides the major source of glia in the postnatal cortex of mice. The authors used lineage tracing techniques to label dividing cells and their progeny and found that differentiated cortical astrocytes undergo several rounds of symmetric divisions in the first three weeks of postnatal development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.García-Marqués J, López-Mascaraque L. Clonal identity determines astrocyte cortical heterogeneity. Cereb Cortex. 2013;23:1463–1472. doi: 10.1093/cercor/bhs134. The authors demonstrate that the positional and morphological identities of cortical astrocytes are carefully specified during brain development. The authors utilized in utero electroporation to drive stochastic expression of fluorescent proteins in developing cortical astrocytes. This brainbow-like technique identified highly specific clonal arrangements of astrocytes distributed throughout the cortex. [DOI] [PubMed] [Google Scholar]
- 12.Tsai H-H, Li H, Fuentealba LC, Molofsky AV, Taveira-Marques R, Zhuang H, Tenney A, Murnen AT, Fancy SPJ, Merkle F, et al. Regional astrocyte allocation regulates CNS synaptogenesis and repair. Science. 2012 doi: 10.1126/science.1222381. http://dx.doi.org/10.1126/science.1222381 This was the first study to demonstrate that astrocytes are allocated to spatial domains in the spinal cord depending on their embryonic sites of origin. Furthermore, the authors demonstrated that domain-specific depletion of mature astrocytes is not accompanied by immigration of astrocytes from adjoining regions. [DOI] [PMC free article] [PubMed]
- 13.Morrow T, Song MR, Ghosh A. Sequential specification of neurons and glia by developmentally regulated extracellular factors. Development. 2001;128:3585–3594. doi: 10.1242/dev.128.18.3585. [DOI] [PubMed] [Google Scholar]
- 14.Nakashima K, Wiese S, Yanagisawa M, Arakawa H, Kimura N, Hisatsune T, Yoshida K, Kishimoto T, Sendtner M, Taga T. Developmental requirement of gp130 signaling in neuronal survival and astrocyte differentiation. J Neurosci. 1999;19:5429–5434. doi: 10.1523/JNEUROSCI.19-13-05429.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krencik R, Zhang S-C. Directed differentiation of functional astroglial subtypes from human pluripotent stem cells. Nat Protoc. 2011;6:1710–1717. doi: 10.1038/nprot.2011.405. One of the first published methods for the efficient generation of iPSC-derived astrocytes. The protocol consists of regular dissociation of neuroepithelial progenitors followed by administration of CNTF over the course of three to four months. Following this publication a number of studies [13] have since attempted to improve upon these conditions in order to generate astrocytes that best resemble in vivo human astrocytes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Serio A, Bilican B, Barmada SJ, Ando DM, Zhao C, Siller R, Burr K, Haghi G, Story D, Nishimura AL, et al. Astrocyte pathology and the absence of non-cell autonomy in an induced pluripotent stem cell model of TDP-43 proteinopathy. Proc Natl Acad Sci USA. 2013;110:4697–4702. doi: 10.1073/pnas.1300398110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnabé-Heider F, Wasylnka Ja, Fernandes KJL, Porsche C, Sendtner M, Kaplan DR, Miller FD. Evidence that embryonic neurons regulate the onset of cortical gliogenesis via cardiotrophin-1. Neuron. 2005;48:253–265. doi: 10.1016/j.neuron.2005.08.037. [DOI] [PubMed] [Google Scholar]
- 18.Yang P, Wen H, Ou S, Cui J, Fan D. IL-6 promotes regeneration and functional recovery after cortical spinal tract injury by reactivating intrinsic growth program of neurons and enhancing synapse formation. Exp Neurol. 2012;236:19–27. doi: 10.1016/j.expneurol.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Cafferty WBJ, Gardiner NJ, Das P, Qiu J, McMahon SB. Thompson SWN: conditioning injury-induced spinal axon regeneration fails in interleukin-6 knock-out mice. J Neurosci. 2004;24:4432–4443. doi: 10.1523/JNEUROSCI.2245-02.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He F, Ge W, Martinowich K, Becker-Catania S, Coskun V, Zhu W, Wu H, Castro D, Guillemot F, Fan G, et al. A positive autoregulatory loop of Jak-STAT signaling controls the onset of astrogliogenesis. Nat Neurosci. 2005;8:616–625. doi: 10.1038/nn1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Urayama S, Semi K, Sanosaka T, Hori Y, Namihira M, Kohyama J, Takizawa T, Nakashima K. Chromatin accessibility at a STAT3 target site is altered prior to astrocyte differentiation. Cell Struct Funct. 2013;38:55–66. doi: 10.1247/csf.12034. [DOI] [PubMed] [Google Scholar]
- 22.Nakashima K. Synergistic signaling in fetal brain by STAT3-Smad1 complex bridged by p300. Science. 1999;284:479–482. doi: 10.1126/science.284.5413.479. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann JE, Imura T, Song B, Qi J, Ao Y, Nguyen TK, Korsak RA, Takeda K, Akira S, Sofroniew MV. STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci. 2008;28:7231–7243. doi: 10.1523/JNEUROSCI.1709-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wanner IB, Anderson MA, Song B, Levine J, Fernandez A, Gray-Thompson Z, Ao Y, Sofroniew MV. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. J Neurosci. 2013;33:12870–12886. doi: 10.1523/JNEUROSCI.2121-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takizawa T, Nakashima K, Namihira M, Ochiai W, Uemura a, Yanagisawa M, Fujita N, Nakao M, Taga T. DNA methylation is a critical cell-intrinsic determinant of astrocyte differentiation in the fetal brain. Dev Cell. 2001;1:749–758. doi: 10.1016/s1534-5807(01)00101-0. [DOI] [PubMed] [Google Scholar]
- 26.Hutnick LK, Golshani P, Namihira M, Xue Z, Matynia A, Yang XW, Silva AJ, Schweizer FE, Fan G. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum Mol Genet. 2009;18:2875–2888. doi: 10.1093/hmg/ddp222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kageyama R, Ohtsuka T, Hatakeyama J, Ohsawa R. Roles of bHLH genes in neural stem cell differentiation. Exp Cell Res. 2005;306:343–348. doi: 10.1016/j.yexcr.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 28.Kamakura S, Oishi K, Yoshimatsu T, Nakafuku M, Masuyama N, Gotoh Y. Hes binding to STAT3 mediates crosstalk between Notch and JAK-STAT signalling. Nat Cell Biol. 2004;6:547–554. doi: 10.1038/ncb1138. [DOI] [PubMed] [Google Scholar]
- 29.Piper M, Barry G, Hawkins J, Mason S, Lindwall C, Little E, Sarkar A, Smith AG, Moldrich RX, Boyle GM, et al. NFIA controls telencephalic progenitor cell differentiation through repression of the Notch effector Hes1. J Neurosci. 2010;30:9127–9139. doi: 10.1523/JNEUROSCI.6167-09.2010. This paper provides evidence for the dual roles of NFIA to both activate astrocyte-specific genes while simultaneously downregulating the Notch signaling pathway through the repression of the key Notch effector Hes1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Namihira M, Kohyama J, Semi K, Sanosaka T, Deneen B, Taga T, Nakashima K. Committed neuronal precursors confer astrocytic potential on residual neural precursor cells. Dev Cell. 2009;16:245–255. doi: 10.1016/j.devcel.2008.12.014. [DOI] [PubMed] [Google Scholar]
- 31.Kang P, Lee HK, Glasgow SM, Finley M, Donti T, Gaber ZB, Graham BH, Foster AE, Novitch BG, Gronostajski RM, et al. Sox9 and NFIA coordinate a transcriptional regulatory cascade during the initiation of gliogenesis. Neuron. 2012;74:79–94. doi: 10.1016/j.neuron.2012.01.024. This study demonstrated that NFIA is induced by Sox9 expression. Following its induction, NFIA then complexes with Sox9 in order to coregulate various genes that contribute to the gliogenic switch. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun Y, Nadal-Vicens M, Misono S, Lin MZ, Zubiaga a, Hua X, Fan G, Greenberg ME. Neurogenin promotes neurogenesis and inhibits glial differentiation by independent mechanisms. Cell. 2001;104:365–376. doi: 10.1016/s0092-8674(01)00224-0. [DOI] [PubMed] [Google Scholar]
- 33.Xu D, Qu C-K. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–4932. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tartaglia M, Mehler EL, Goldberg R, Zampino G, Brunner HG, Kremer H, van der Burgt I, Crosby AH, Ion A, Jeffery S, et al. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 35.Gauthier AS, Furstoss O, Araki T, Chan R, Neel BG, Kaplan DR, Miller FD. Control of CNS cell-fate decisions by SHP-2 and its dysregulation in Noonan syndrome. Neuron. 2007;54:245–262. doi: 10.1016/j.neuron.2007.03.027. The authors used cultured cortical precursors to demonstrate that SHP-2 knockdown results in precocious astrocyte formation. Conversely, the authors observed impaired astrogenesis when they expressed the constitutively active form of SHP-2 that is found in patients with Noonan Syndrome. Reduced astrocyte numbers were also confirmed in a mouse knockin model expressing the human constitutively active form of SHP-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tidyman WE, Rauen Ka. The RASopathies: developmental syndromes of Ras/MAPK pathway dysregulation. Curr Opin Genet Dev. 2009;19:230–236. doi: 10.1016/j.gde.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegedus B, Dasgupta B, Shin JE, Emnett RJ, Hart-Mahon EK, Elghazi L, Bernal-Mizrachi E, Gutmann DH. Neurofibromatosis-1 regulates neuronal and glial cell differentiation from neuroglial progenitors in vivo by both cAMP- and Ras-dependent mechanisms. Cell Stem Cell. 2007;1:443–457. doi: 10.1016/j.stem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 38.Paquin A, Hordo C, Kaplan DR, Miller FD. Costello syndrome H-Ras alleles regulate cortical development. Dev Biol. 2009;330:440–451. doi: 10.1016/j.ydbio.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 39.Urosevic J, Sauzeau V, Soto-Montenegro ML, Reig S, Desco M, Wright EMB, Cañamero M, Mulero F, Ortega S, Bustelo XR, et al. Constitutive activation of B-Raf in the mouse germ line provides a model for human cardio-facio-cutaneous syndrome. Proc Natl Acad Sci USA. 2011;108:5015–5020. doi: 10.1073/pnas.1016933108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zdaniuk G, Wierzba-bobrowicz T, Szpak GM, Stępień T. Astroglia disturbances during development of the central nervous system in fetuses with Down’s syndrome. Folia Neuropathol. 2011 no volume. [PubMed] [Google Scholar]
- 41.Lu J, Lian G, Zhou H, Esposito G, Steardo L, Delli-Bovi LC, Hecht JL, Lu QR, Sheen V. OLIG2 over-expression impairs proliferation of human Down syndrome neural progenitors. Hum Mol Genet. 2012;21:2330–2340. doi: 10.1093/hmg/dds052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai J, Chen Y, Cai W-H, Hurlock EC, Wu H, Kernie SG, Parada LF, Lu QR. A crucial role for Olig2 in white matter astrocyte development. Development. 2007;134:1887–1899. doi: 10.1242/dev.02847. [DOI] [PubMed] [Google Scholar]
- 43.Laurence Ja, Fatemi SH. Glial fibrillary acidic protein is elevated in superior frontal, parietal and cerebellar cortices of autistic subjects. Cerebellum. 2005;4:206–210. doi: 10.1080/14734220500208846. [DOI] [PubMed] [Google Scholar]
- 44.Ahlsén G, Rosengren L, Belfrage M, Palm a, Haglid K, Hamberger a, Gillberg C. Glial fibrillary acidic protein in the cerebrospinal fluid of children with autism and other neuropsychiatric disorders. Biol Psychiatry. 1993;33:734–743. doi: 10.1016/0006-3223(93)90124-v. [DOI] [PubMed] [Google Scholar]
- 45.Fatemi SH, Folsom TD, Reutiman TJ, Lee S. Expression of astrocytic markers aquaporin 4 and connexin 43 is altered in brains of subjects with autism. Synapse. 2008;62:501–507. doi: 10.1002/syn.20519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cao F, Yin A, Wen G, Sheikh AM, Tauqeer Z, Malik M, Nagori A, Schirripa M, Schirripa F, Merz G, et al. Alteration of astrocytes and Wnt/β-catenin signaling in the frontal cortex of autistic subjects. J Neuroinflamm. 2012;9:223. doi: 10.1186/1742-2094-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Ballas N, Lioy DT, Grunseich C, Mandel G. Non-cell autonomous influence of MeCP2-deficient glia on neuronal dendritic morphology. Nat Neurosci. 2009;12:311–317. doi: 10.1038/nn.2275. The first study to demonstrate that the lack of functional MeCP2 in astrocytes is capable of non-cell autonomous detrimental effects on cultured neurons. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lioy DT, Garg SK, Monaghan CE, Raber J, Foust KD, Kaspar BK, Hirrlinger PG, Kirchhoff F, Bissonnette JM, Ballas N, et al. A role for glia in the progression of Rett’s syndrome. Nature. 2011;475:497–500. doi: 10.1038/nature10214. The influential follow-up study from [40] where the authors demonstrate that re-xpression of MeCP2 specifically in astrocytes in a global MeCP2−/− mouse is capable of rescuing Rett symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasui DH, Xu H, Dunaway KW, Lasalle JM, Jin L-W, Maezawa I. MeCP2 modulates gene expression pathways in astrocytes. Mol Autism. 2013;4:3. doi: 10.1186/2040-2392-4-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jacobs S, Nathwani M, Doering LC. Fragile X astrocytes induce developmental delays in dendrite maturation and synaptic protein expression. BMC Neurosci. 2010;11:132. doi: 10.1186/1471-2202-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu TW, Chahrour MH, Coulter ME, Jiralerspong S, Okamura-Ikeda K, Ataman B, Schmitz-Abe K, Harmin DA, Adli M, Malik AN, et al. Using whole-exome sequencing to identify inherited causes of autism. Neuron. 2013;77:259–273. doi: 10.1016/j.neuron.2012.11.002. This group used whole exome sequencing in order to identify novel ASD-associated genes that have less penetrance than typical GWAS targets. As opposed to traditional studies of primarily simplex families where the bulk of the known de novo point mutations and copy number changes have been identified, this group sequenced ASD families enriched for inheritance due to consanguinity. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piton A, Gauthier J, Hamdan FF, Lafrenière RG, Yang Y, Henrion E, Laurent S, Noreau A, Thibodeau P, Karemera L, et al. Systematic resequencing of X-chromosome synaptic genes in autism spectrum disorder and schizophrenia. Mol Psychiatry. 2011;16:867–880. doi: 10.1038/mp.2010.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Katsel P, Davis KL, Gorman JM, Haroutunian V. Variations in differential gene expression patterns across multiple brain regions in schizophrenia. Schizophr Res. 2005;77:241–252. doi: 10.1016/j.schres.2005.03.020. [DOI] [PubMed] [Google Scholar]
- 54.Webster MJ, Knable MB, Johnston-Wilson N, Nagata K, Inagaki M, Yolken RH. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav Immun. 2001;15:388–400. doi: 10.1006/brbi.2001.0646. [DOI] [PubMed] [Google Scholar]
- 55.Gittins RA, Harrison PJ. A morphometric study of glia and neurons in the anterior cingulate cortex in mood disorder. J Affect Disord. 2011;133:328–332. doi: 10.1016/j.jad.2011.03.042. [DOI] [PubMed] [Google Scholar]
- 56.Bauer D, Haroutunian V, Meador-Woodruff JH, McCullumsmith RE. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr Res. 2010;117:92–98. doi: 10.1016/j.schres.2009.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Moghaddam B, Javitt D. From revolution to evolution: the glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology. 2012;37:4–15. doi: 10.1038/npp.2011.181. [DOI] [PMC free article] [PubMed] [Google Scholar]