Abstract
Background
Growth factors, energy sources, and mitochondrial function strongly affect embryo growth and development in vitro. The biological role and prospective significance of the mitofusin gene Mfn2 in the development of preimplantation embryos remain poorly understood. Our goal is to profile the role of Mfn2 in mouse embryos and determine the underlying mechanism of Mfn2 function in embryo development.
Methods
We transfected Mfn2-siRNA into 2-cell fertilized eggs and then examined the expression of Mfn2, the anti-apoptotic protein Bcl-2, and the apoptosis-promoting protein Bax by Western blot. Additionally, we determined the blastocyst formation rate and measured ATP levels, mtDNA levels, mitochondrial membrane potential (ΔΨm), and apoptosis in all of the embryos.
Results
The results indicate that the Mfn2 and Bcl-2 levels were markedly decreased, whereas Bax levels were increased in the T group (embryos transfected with Mfn2-siRNA) compared with the C group (embryos transfected with control-siRNA). The blastocyst formation rate was significantly decreased in the T group. The ATP content and the relative amounts of mtDNA and cDNA in the T group were significantly reduced compared with the C group. In the T group, ΔΨm and Ca2+ levels were reduced, and the number of apoptotic cells was increased.
Conclusion
Low in vitro expression of Mfn2 attenuates the blastocyst formation rate and cleavage speed in mouse zygotes and causes mitochondrial dysfunction, as confirmed by the ATP and mtDNA levels and mitochondrial membrane potential. Mfn2 deficiency induced apoptosis through the Bcl-2/Bax and Ca2+ pathways. These findings indicate that Mfn2 could affect preimplantation embryo development through mitochondrial function and cellular apoptosis.
Introduction
Successful embryonic development requires a series of coordinated molecular and cellular events that culminate in blastocyst formation [1], and correct gene expression regulation is important in embryo development [2, 3]. The development of fertilized oocytes, including cleavage, blastocyst formation, and implantation, requires normal levels of specific gene expression and organelle activity [4]. Mitochondria occupy a key role in the management of many cellular functions, such as stress responses, cell metabolism, and cell death [5]. Mitochondria function as the energy source of cells, contribute to redox and Ca2+ homeostasis, provide intermediary metabolites, and store proapoptotic factors during embryonic development. In addition, mitochondria regulate Ca2+ homeostasis and modulate apoptosis through the release of a number of death inducing cell molecules [6, 7]. Moreover, mitochondria serve as a source of reactive oxygen species (ROS). Maintaining normal maternally derived mitochondrial function is critical for the early embryo. Mitochondrial dysfunction might disturb embryonic development and trigger apoptosis. This dual mitochondrial role might represent a control system that determines whether early embryo development proceeds normally or is quickly eliminated.
Mitofusin-2 (Mfn2) is a mitochondrial protein that controls mitochondria fusion and tethering [8, 9]; however, the latter study suggests that a small portion of Mfn2 is present in the endoplasmic reticulum (ER) [10, 11]. Mfn2, which is connected to altered mitochondrial energy supplies, is a signaling molecule that plays a vital role in cell activities [12]. Various studies have reported that Mfn2 plays a positive role in embryonic development [13–15]. If zygotes lack Mfn2, blastocyst formation is impaired [16]. However, the mechanism by which Mfn2 regulates embryo development remains unclear. The aim of this study was to further characterize the effects of Mfn2 and its mechanism of action during embryo development.
Materials and Methods
The Ethics Committee of the Center of Reproductive Medicine of Tongji Medical College of Huazhong University of Science and Technology in China approved this study (permit number: 2011–149). All of the reagents used in the mouse fertilized-egg cultures were purchased from Sigma (St. Louis, MO, USA).
Collection and Culture of Mouse Embryo Tissue Samples
The 5-week old Kunming white (KM) mice were primed with 10 IU of serum gonadotropin from pregnant mares (PMSG, The Bohn Pharmaceutical Co, Ltd., Chifeng, China) followed by 10 IU of human chorionic gonadotropin (hCG) 48 h later (Livzon Group Livzon Pharmaceutical Factory, Guangzhou, China) to obtain zygotes. The mice were mated with males of proven fertility, and plugs were verified the next morning. The 2-cell embryos were collected from oviduct at 48 h time point of post-hCG injections and cultured in M2 medium. The embryos were washed in IVF-30 thrice and transferred to 5% CO2-equilibrated IVF-30 (Vitrolife, Kungsbacka, Sweden). The embryos were cultured in a humidified incubator at 37°C and 5% CO2.
siRNA-Mediated Mfn2 Knockdown
Dose dependent and time dependent experiments of siRNA transfected fertilized eggs were done and optimized methods was obtained. The 2-cell fertilized eggs were cultured in IVF-30 and transfected with siRNA (5 nM) for 6 h using Lipofectamine 2000 (Invitrogen) according to our optimized transfection methods. ON-TARGET plus siRNA-Mouse Mfn2 (T group) and non-targeting control siRNA (C group) were from Ruibo (Guangzhou, China). The transfection efficiencies were determined by qPCR. Fertilized eggs transfected were cultured and harvested for detecting at 4-cell (C1 and T1), 8-cell (C2 and T2) and blastocyst (C3 and T3) stages.
RNA Isolation, Reverse Transcription, and PCR
Total RNA was extracted from 50 embryos using TRIzol according to the manufacturer’s instructions. Equal aliquots of total RNA from each group were quantified using a spectrophotometer at 260 nm and processed to synthesize the complimentary DNA (cDNA). cDNA was synthesized using 1 μg of RNA, oligo dT primers (Qiagen, Valencia, CA), and the Revert Aid First Strand cDNA Synthesis Kit (Fermentas, Rockford, USA) according to the manufacturer’s protocol. Amplification and SYBR Green II (Gene Copoeia, Maryland, USA) detection were performed using an MX3000P Real-Time PCR Detection System (Stratagene). The samples were run in triplicate, and the gene expression level for each sample was normalized to β-actin mRNA using the comparative threshold cycle (Ct) method.
Western Blot
The protein samples (40 μg) were separated by SDS-PAGE and transferred to nitrocellulose membranes. Nonspecific binding was blocked with 0.01% TBS-Tween containing 5% nonfat milk for 1 h at room temperature. The membranes were hybridized with rabbit anti-Mfn2 (Abcam, Cambridge, MA), rabbit anti-Bax, rabbit anti-Bcl-2, and rabbit anti-β-actin primary antibodies (Santa Cruz Biotech, CA) in Tris-buffered saline with Tween (TBS-T) containing 1% nonfat milk at 4°C overnight. The membranes were washed three times with TBS-T, and horseradish peroxidase-conjugated secondary antibodies were used in a standard enhanced chemiluminescence reaction according to the manufacturer’s instructions (Pierce, Rockford, IL.).
Determination of Mitochondrial Membrane Potential (ΔΨm)
Mitochondrial stability was assessed by fluorescence microscopy after incubation with JC-1 (5,59,6,69-tetrachloro-1,19,3,39-tetraethylben zimidazolylcarbocyanine iodide; Molecular Probes, Eugene, OR). The mouse embryos were grown in 35-mm Petri dishes in 40 μl of IVF-30. The fertilized eggs were incubated with 0.1 μM JC-1 (Cayman Chemical Company, USA) fluorescent dye for 30 min in the CO2 incubator and then slowly washed with PBS several times. The mitochondrial membrane potential was evaluated under a fluorescence microscope using a 540/570 nm filter. The green JC-1 signals were measured at 485/535 nm, whereas the red signals were measured at 590/610 nm.
Detection of Apoptosis
The embryos were analyzed for apoptosis with annexin V-FITC using a fluorescence microscope. The blastocysts were fixed to allow for annexin V-FITC labeling of cells with an intact plasma membrane, whereas fertilized eggs with disrupted or otherwise damaged plasma membranes were stained with propidium iodide (PI). Following three washes with PBS at 37°C, the blastocysts were incubated in a binding buffer that contained 1 μl/100 μl annexin V-FITC and 1 μl/100 μl PI in a Petri dish for 10 min at room temperature while avoiding light exposure. After the incubation, the fertilized eggs were washed in PBS, and then immediately observed under an inverted fluorescence microscope with the appropriate bandwidth filters for green and red fluorescence.
Determination of ATP Levels
To evaluate mitochondrial function, we determined ATP levels in the embryos. The reagent was dissolved in PBS on ice, and the ATP standard solution (The Shenyang Branch Liang Horse Biological Engineering Co, Ltd., China) was diluted with double distilled water to a suitable concentration gradient. Thirty blastocysts from each group were collected in PBS. After three washes, the blastocysts were suspended in calcium and magnesium ion-free PBS. Then, 100 μl of ATP-detection working solution was added to each well of a 96-well plate, and a standard curve was plotted using multifunctional microplate fluorescence emission values. We added ATP detection lysates and ATP detection reagent to each of the detection holes and measured the luminescence using an automatic microplate reader (BIO-TEK).
Detection of Free Cytoplasmic Ca2+
Fluo-3 imaging reveals spatial dynamics in Ca2+ signaling, and fluo-3 absorbs the compatible spectrum with excitation at 488 nm by argon ion laser sources. A significant increase in fluorescence intensity in response to Ca2+ binding is observed. The zygotes were washed three times in PBS without Ca2+ and then incubated in fluo-3 solution (1 mM) for 15–30 min at 37°C and 5% CO2. After an additional PBS wash, the zygotes were observed under a fluorescence microscope.
Quantification of mtDNA Relative to Nuclear DNA (Mt/N)
One hundred blastocysts from each group were collected to prepare total DNA using the DNeasy Blood & Tissue Kit (Qiagen, Germany). The total DNA was dissolved in 10 μl of solution, and the mtDNA content was assessed by quantification of CoxII relative to the nuclear gene β-actin. The CoxII primer sequences were as follows: forward 5′-GAGCAGTCCCCTCCCTAGGA-3′ and reverse 5′-GTCG GTTTGATGTTACTGTTGCTT-3′. Nuclear and mitochondrial DNA contents were quantified by real-time PCR. For each gene, PCR quantification was performed in triplicate, and the amplified transcripts were quantified using the comparative Ct method. Briefly, the Ct values were calculated according to the following equation: ΔCt = CtCoxII—Ctβ-actin, where ΔCt is the difference in the Ct values between CoxII and β-actin. The relative quantity of DNA expression in the silent gene groups was compared with the control groups. Xn is calculated by the formula Xn = 2-ΔCt.
Statistical Analysis
The data were analyzed using the Statistical Program for Social Science (SPSS, Inc., Chicago, IL, USA) software. Each experiment was performed at least three separate times, and the data were presented as the mean ± standard error (x±s). Significance was established at the 95% confidence level (P<0.05).
Results
Mfn2 Expression in Fertilized Mouse Eggs after Transfection
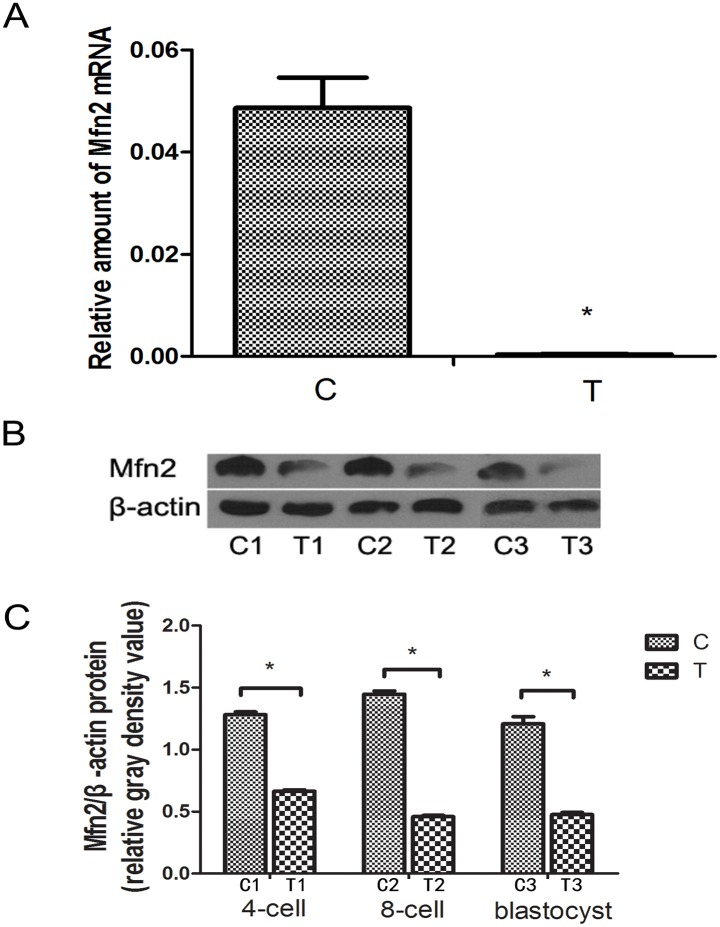
We measured Mfn2 expression by PCR and Western blotting and found that the mRNA levels were decreased in the fertilized eggs treated with Mfn2-siRNA compared with eggs transfected with the control-siRNA (Fig 1A). Western blot analysis confirmed these findings (Fig 1B). At the 4-cell stage, the Mfn2 expression level was significantly reduced, and this low expression continued until the blastocyst stage (Fig 1C).
Fig 1. Mfn2 expression in mouse fertilized eggs after transfection.
Mfn2 mRNA expression was detected by qPCR and its level is significant lower in T group than that in C group (A). Mfn2 protein levels were determined by Western blotting (B). The quantitation of Mfn2 protein levels. C1 and T1: 4 cells fertilized eggs, C2 and T2: 8 cells fertilized eggs, C3 and T3: blastocyst. *P<0.05. Data are means ± SD of 3 separate experiments (C).
Low Mfn2 Expression Attenuates the Blastocyst Formation Rate and Cleavage Speed
To examine the effects of Mfn2 on embryo development, we determined the blastocyst formation rate and cleavage speed of the embryos in two groups. The blastocyst formation rate was significantly reduced in the T group (embryos transfected with Mfn2-siRNA) compared with the C group (embryos transfected with control-siRNA). When the 2-cell fertilized eggs were removed at 72 h, 72.95% blastocyst formation was noted in the C group, whereas 23.19% was observed in the T group (p<0.01, Table 1). In the T group, the cleavage speed was reduced compared with the control group. After 24 h, the 2-cell fertilized eggs were removed and examined. Approximately 80% of the zygotes developed into the 4-cell stage (Fig 2C1 and 2T1). At 36 h, approximately 80% of the cells entered the 8-cell stage in the C group (Fig 2C2), whereas only a limited number had entered this stage in the T group (Fig 2T2). After 72 h, 80% of the cells were blastocysts in the C group (Fig 2C3); the cells in the Mfn2-deficient group were dead (Fig 2T3). These results suggest that normal Mfn2 expression is essential for the development of preimplantation embryos.
Table 1. Blastocyst formation rate.
| Groups | Control-siRNA transfected | Mfn2-siRNA transfected |
|---|---|---|
| The number of 2-cell fertilized eggs | 122 | 138* |
| The number of developed blastocysts | 89 | 32** |
| Blastocyst formation rate (%) | 72.95 | 23.19** |
The blastocyst formation rate of the fertilized eggs collected from 5-week-old Kunming mice. The table presents the number of fertilized eggs collected from five mice. The values represent the rate of successful blastocyst development.
* P>0.05 vs control-siRNA transfected,
** P<0.01 vs control-siRNA transfected.
Fig 2. Low Mfn2 expression attenuates the blastocyst formation rate and cleavage speed.
In T group, the cleavage speed was reduced compared with that in control group, the blastocyst formation rate was significantly reduced in the T group compared with the C group. C1 and T1: 4-cell fertilized eggs, C2 and T2: 8-cell fertilized eggs. C3 and T3: Blastocyst. Data are means ± SD of 3 separate experiments.
Low Mfn2 Expression causes Mitochondrial Dysfunction in Mouse Embryos
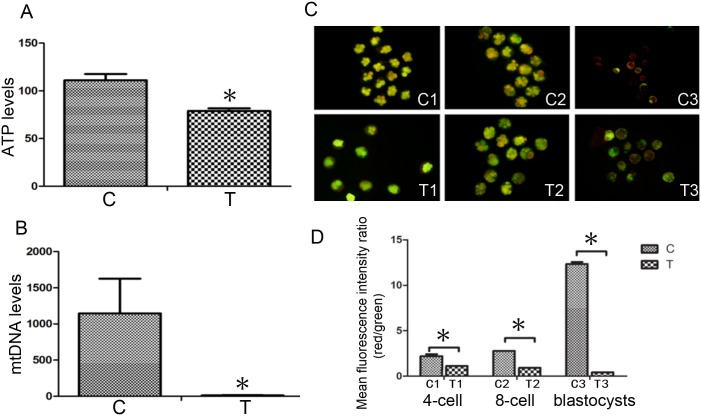
To further determine the possible effects of Mfn2 on embryo development via mitochondrial function, we measured ATP levels and mtDNA production in fertilized eggs treated with control-siRNA or Mfn2-siRNA. The results revealed that the ATP content of the 30 mouse blastocysts in the T group was significantly reduced compared with the C group (P<0.05; Fig 3A). COXII and β-actin served as markers for mitochondrial DNA (mtDNA) and nuclear DNA (cDNA), respectively. The relative ratio of mtDNA to cDNA (Fig 3B) was determined showing reduction in the T group compared with the C group.
Fig 3.
A) The ATP content of the mouse blastocysts was detected via firefly luciferase analysis. B) The relative expression levels of mtDNA and cDNA. Values are presented as multiples of COXII DNA relative to the β-actin DNA. C) Mitochondrial membrane potential was assessed by JC-1. D) Statistical analyses of the red and green mean fluorescence intensity ratios revealed that the mitochondrial membrane potential of the T group was significantly reduced compared with the C group, *P<0.05. Data are means ± SD of 3 separate experiments.
We assessed ΔΨm using the JC-1. At the 4-cell (Fig 3C, 3C1 and 3T1) and 8-cell stages (Fig 3C, 3C2 and 3T2), ΔΨm levels were low in the C and T-groups. However, a difference in these levels was noted between the two groups; ΔΨm was significantly increased (red) in the C group compared with the T group (green; Fig 3C, 3C3 and 3T3; Fig 3D). These results suggest that Mfn2 participates in early mouse embryonic development by affecting mitochondrial function through energy metabolism and the quantity of mtDNA.
Low Mfn2 Expression Induces Apoptosis in Mouse Embryos
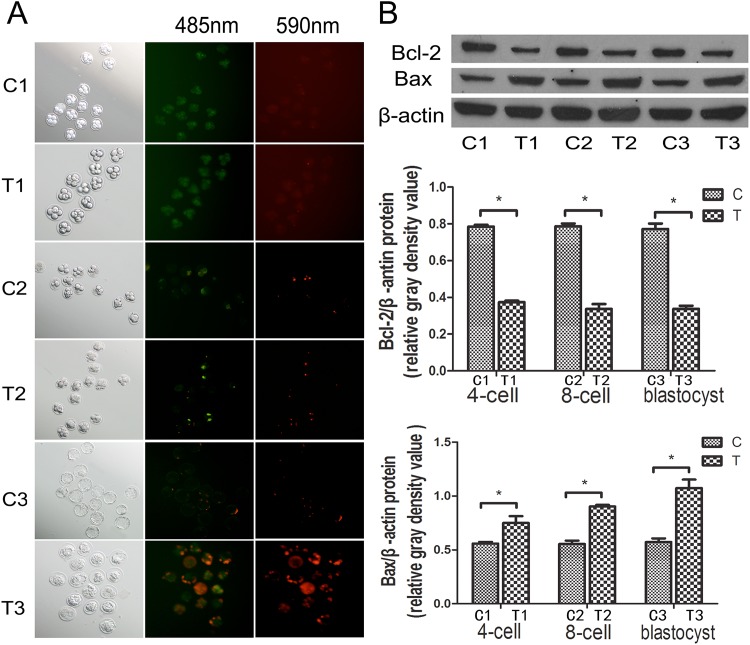
The fluorescence microscope images of the annexin V and PI staining indicated that only a small number of cells at the 4-cell stage in the two groups were undergoing apoptosis or death. At the 8-cell stage, few apoptotic or dead cells were observed in the T group (Fig 4AT2), and evidence of these cellular processes were not obvious in the C group (Fig 4AC2). At the blastocyst stage, the number or apoptotic and dead cells in the T group (Fig 4AT3) increased significantly (Fig 4AC3). We verified the occurrence of apoptosis through detection of the apoptosis-related proteins Bcl-2 and Bax by Western blot (Fig 4B). Using a gray scale analysis, we discovered that Bax expression increased gradually in the T group, whereas overall Bcl-2 expression decreased. A statistically significant difference in the gray value was observed in the 8-cell and the blastocyst stages (Fig 4B). These results indicate that low Mfn2 expression potentially leads to the development of a disordered preimplantation embryo by promoting apoptosis.
Fig 4.
A) Annexin V and PI stains were observed at 485 nm and 590 nm, respectively, using a fluorescence microscope and were used to visualize the cells undergoing apoptosis (green) and death (red), respectively. B) Bcl-2 and Bax protein levels were determined by Western blot and quantitation was showed that Bax expression increased significantly and Bcl-2 expression decreased in the T group compared with the C group. *P<0.05. Data are means ± SD of 3 separate experiments.
Low Mfn2 Expression causes Decreased Cytoplasmic Ca2+ Levels
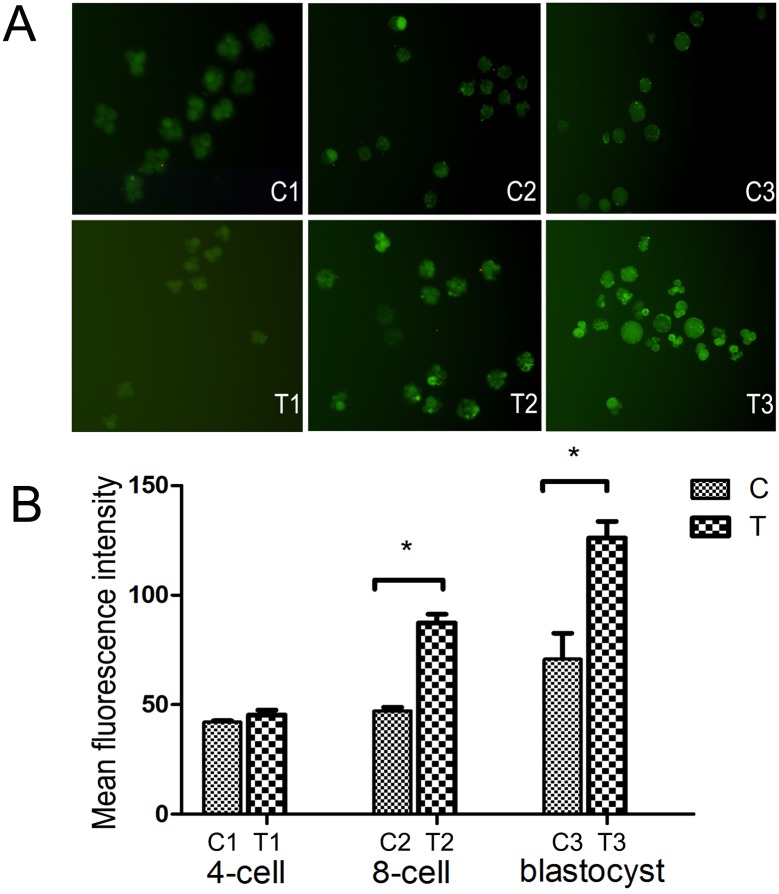
We used fluo-3 colored fluorescence to measure the Ca2+ levels. The free Ca2+ level gradually increased in the T group (Fig 5) during the observation period, whereas no obvious change was noted in the C group. The fluorescence intensity analysis indicated a statistically significant difference between the groups.
Fig 5.
A) Fluo-3 colored fluorescence to measure the Ca2+ levels. B) The Ca2+ level difference was shown between the C and T-groups in the fertilized eggs and blastocysts. *P<0.05. Data are means ± SD of 3 separate experiments.
Discussion
In this study, we observed that low Mfn2 expression affects the development of mouse preimplantation embryos by obstructing mitochondrial metabolism. Mitochondria, which are maternally inherited, have a well-established role in determining embryo quality [14], ΔΨm, ROS, and metabolic outputs. Mitochondrial content is an important indicator of cellular health, reflecting overall mitochondrial integrity and metabolic activity [17–19].
Our results indicate that ATP levels were significantly reduced in the Mfn2-deficient group compared with the control group, indicating that low Mfn2 expression potentially causes mitochondrial dysfunction. Mfn2 plays a central role in mitochondrial outer-membrane fusion and mitochondrial function regulation. The process of preimplantation embryo development requires large amounts of ATP to provide the necessary energy [20, 21]. Mfn2-deficiency resulted in a reduced rate of ATP production and did not provide sufficient energy for embryonic cell division. This effect could cause the reduced division speed in the T group compared with the C group.
In addition, ΔΨm is an indicator of mitochondrial function, which reflects the metabolic activity of the mitochondrial membrane [4]. Our results indicate that the ΔΨm in the Mfn2-deficient group was considerably reduced compared with the control-siRNA transfected group, indicating that Mfn2 deficiency likely results in mitochondrial dysfunction. Previous studies have demonstrated that pre-compaction stage embryos exhibit reduced levels of cellular activities, including low respiratory rates, O2 consumption, glucose utilization [22–27], and ΔΨm levels. As the embryo divides, the metabolic parameters change, and the metabolic activity of the reduced number of mitochondria per cell must increase to meet the increasing demands of cellular activity [28, 29], and the ΔΨm remains at a high level. Many mitochondrial functions, including ATP generation, Ca2+ homeostasis, and protein import, depend on the maintenance of ΔΨm [30]. Our results indicated that Mfn2 deficiency results in a low ΔΨm and failure to meet the demands of cellular activity, leading to disordered embryonic development. This finding demonstrates that normal Mfn2 expression is necessary to support metabolic activity.
Previous studies have demonstrated that mtDNA levels remain low after fertilization; however, levels begin to increase near the time of implantation [31]. The expression of mtDNA is upregulated, causing the reactivation of mtDNA replication at the blastocyst stage. Mitochondria begin to differentiate into elongated organelles with increased membrane potential and oxygen consumption; mitochondria exhibit increased oxidative phosphorylation activity, which is important for ATP production [32]. In our study, we observed that the mtDNA/cDNA ratio was increased at the blastocyst stage in the control-siRNA transfected group compared with the Mfn2-siRNA transfected group. This effect might contribute to the developmental disorders in Mfn2-siRNA transfected blastocysts.
Another possible mechanism involves the role of Mfn2 in the regulation of apoptosis; Mfn2 expression has an apoptotic effect in the mitochondrial apoptotic pathway [13]. Bcl-2 is a member of the human proto-oncogene Bcl-2 family [33], which includes pro-apoptotic and anti-apoptotic proteins [34]. Bax does not induce apoptosis until it is translocated to the mitochondria [35]. Bax and Bcl-2 activation prompts cytochrome c translocation into the cytoplasm via the outer mitochondrial membrane, which leads to the activation of the caspase cascade and subsequent apoptosis [36, 37]. In this study, Mfn2 deficiency profoundly increases the mitochondrial Bax/Bcl-2 ratio, indicating that Mfn2 deficiency potentially induces apoptosis in the mouse embryo through the Bcl-2 and Bax pathway.
In addition to apoptotic cells, we also observed numerous dead cells in the Mfn2 siRNA-transfected mouse embryos. The death of these cells potentially occurred through programmed necrosis, which is dominated by Ca2+-dependent permeabilization of the outer and inner mitochondrial membranes. Previous studies have demonstrated that mitochondria are central regulators of programmed cell death, apoptosis, and necrosis [38] that Ca2+ transported from the sarcoplasmic reticulum (SR) to mitochondria is dependent upon “Ca2+ hot-spots”. Because Mfn2 is an important protein that creates Ca2+ hot spots by tethering to the organelle, Mfn2 deficiency could promote cell programming. In our results, Mfn2 downregulation in mouse embryos caused increased Ca2+ concentrations and cell mortality and reduced energy metabolism; this finding suggests that a defect in Mfn2 expression could induce abnormal Ca2+ transportation, which is potentially involved in apoptosis.
Our results demonstrate that inadequate Mfn2 expression affects embryonic development and that one of the mechanisms involves mitochondrial dysfunction and apoptosis induced by low Mfn2 expression. These findings indicate that Mfn2 plays a decisive role in successful preimplantation development. Further investigation is needed to address the mechanism of Mfn2 regulation signaling.
Supporting Information
This figure is a certification that American Journal Experts (AJE) has edited our manuscript, including citations and references and AJE reminded us to pay attention the additional instructions.
(PDF)
This figure shows that our manuscript has been edited by American Journal Experts and AJE has changed our manuscript to meet PLOS guidelines and provided language editing, translation, manuscript formatting, and figure formatting to ensure our manuscript meets submission guidelines.
(PDF)
Acknowledgments
We are grateful to Dr. Xu Xiaoyan for her invaluable contributions to the sample collection and analysis.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by a grant (31201079) from the Natural Science Foundation of China (NSFC). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Shaw L, Sneddon SF, Brison DR, Kimber SJ (2012) Comparison of gene expression in fresh and frozen-thawed human preimplantation embryos. Reproduction 144: 569–582. 10.1530/REP-12-0047 [DOI] [PubMed] [Google Scholar]
- 2. Gasca S1, Pellestor F, Assou S, Loup V, Anahory T, Dechaud H, et al. (2007) Identifying new human oocyte marker genes: a microarray approach. Reprod Biomed Online 14: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kimber SJ, Sneddon SF, Bloor DJ, El-Bareg AM, Hawkhead JA, Metcalfe AD, et al. (2008) Expression of genes involved in early cell fate decisions in human embryos and their regulation by growth factors. Reproduction 135: 635–647. 10.1530/REP-07-0359 [DOI] [PubMed] [Google Scholar]
- 4. Acton BM, Jurisicova A, Jurisica I, Casper RF (2004) Alterations in mitochondrial membrane potential during preimplantation stages of mouse and human embryo development. Mol Hum Reprod 10: 23–32. [DOI] [PubMed] [Google Scholar]
- 5. Galluzzi L, Kepp O, Trojel-Hansen C, Kroemer G (2012) Mitochondrial control of cellular life, stress, and death. Circ Res 111: 1198–1207. 10.1161/CIRCRESAHA.112.268946 [DOI] [PubMed] [Google Scholar]
- 6. Lenaz G, Bovina C, D'Aurelio M, Fato R, Formiggini G, Genova ML, et al. (2002) Role of mitochondria in oxidative stress and aging. Ann N Y Acad Sci 959: 199–213. [DOI] [PubMed] [Google Scholar]
- 7. Ravagnan L, Roumier T, Kroemer G (2002) Kroemer, Mitochondria, the killer organelles and their weapons. J Cell Physiol 192: 131–137. [DOI] [PubMed] [Google Scholar]
- 8. Koshiba T, Detmer SA, Kaiser JT, Chen H, McCaffery JM, Chen DC (2004) Structural basis of mitochondrial tethering by mitofusin complexes. Science 305: 858–862. [DOI] [PubMed] [Google Scholar]
- 9. Santel A, Fuller MT (2001) Control of mitochondrial morphology by a human mitofusin. J Cell Sci 114: 867–874. [DOI] [PubMed] [Google Scholar]
- 10. de Brito OM, Scorrano L (2010) An intimate liaison: spatial organization of the endoplasmic reticulum-mitochondria relationship. EMBO J 29: 2715–2723. 10.1038/emboj.2010.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Brito OM, Scorrano L (2008) Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456: 605–610. 10.1038/nature07534 [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Jiang L, Hu W, Zheng Q, Xiang W (2011) Mitochondrial dysfunction during in vitro hepatocyte steatosis is reversed by omega-3 fatty acid induced upregulation of mitofusin 2. Metabolism 60: 767–775. 10.1016/j.metabol.2010.07.026 [DOI] [PubMed] [Google Scholar]
- 13. Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC (2003) Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Thouas GA, Trounson AO, Wolvetang EJ, Jones GM (2004) Mitochondrial dysfunction in mouse oocytes results in preimplantation embryo arrest in vitro . Biol Reprod 71: 1936–1942. [DOI] [PubMed] [Google Scholar]
- 15. Pang W, Zhang Y, Zhao N, Darwiche SS, Fu X, Xiang W (2013) Low expression of Mfn2 is associated with mitochondrial damage and apoptosis in the placental villi of early unexplained miscarriage. Placenta 34: 613–618. 10.1016/j.placenta.2013.03.013 [DOI] [PubMed] [Google Scholar]
- 16. Jiang GJ, Pan L, Huang XY, Han M, Wen JK, Sun FZ (2005) Expression of HSG is essential for mouse blastocyst formation. Biochem Biophys Res Commun 335: 351–355. [DOI] [PubMed] [Google Scholar]
- 17. May-Panloup P, Chrétien MF, Jacques C, Vasseur C, Malthièry Y,Reynier P (2005) Low oocyte mitochondrial DNA content in ovarian insufficiency. Hum Reprod 20: 593–597. [DOI] [PubMed] [Google Scholar]
- 18. Reers M, Smiley ST, Mottola-Hartshorn C, Chen A, Lin M, Chen LB (1995) Mitochondrial membrane potential monitored by JC-1 dye. Methods Enzymol 260: 406–417. [DOI] [PubMed] [Google Scholar]
- 19. Santos TA, El Shourbagy S, St John JC (2006) Mitochondrial content reflects oocyte variability and fertilization outcome. Fertil Steril 85: 584–591. [DOI] [PubMed] [Google Scholar]
- 20. Steuerwald N, Barritt JA, Adler R, Malter H, Schimmel T, Cohen J, et al. (2000) Quantification of mtDNA in single oocytes, polar bodies and subcellular components by real-time rapid cycle fluorescence monitored PCR. Zygote 8: 209–215. [DOI] [PubMed] [Google Scholar]
- 21. Guillet V, Gueguen N, Cartoni R, Chevrollier A, Desquiret V, Angebault C, et al. (2011) Bioenergetic defect associated with mKATP channel opening in a mouse model carrying a mitofusin 2 mutation. FASEB J 25: 1618–1627. 10.1096/fj.10-173609 [DOI] [PubMed] [Google Scholar]
- 22. Van Blerkom J, Davis PW, Lee J (1995) ATP content of human oocytes and developmental potential and outcome after in vitro fertilization and embryo transfer. Hum Reprod 10: 415–424. [DOI] [PubMed] [Google Scholar]
- 23. Houghton FD, Thompson JG, Kennedy CJ, Leese HJ (1996) Oxygen consumption and energy metabolism of the early mouse embryo. Mol Reprod Dev 44: 476–485. [DOI] [PubMed] [Google Scholar]
- 24. Thompson JG, Partridge RJ, Houghton FD, Cox CI, Leese HJ (1996) Oxygen uptake and carbohydrate metabolism by in vitro derived bovine embryos. J Reprod Fertil 106: 299–306. [DOI] [PubMed] [Google Scholar]
- 25. Trimarchi JR, Liu L, Porterfield DM, Smith PJ, Keefe DL (2000) Oxidative phosphorylation-dependent and-independent oxygen consumption by individual preimplantation mouse embryos. Biol Reprod 62: 1866–1874. [DOI] [PubMed] [Google Scholar]
- 26. Biggers JD, Whittingham DG, Donahue RP (1967) The pattern of energy metabolism in the mouse oocyte and zygote. Proc Natl Acad Sci U S A 58: 560–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leese HJ, Barton AM (1984) Pyruvate and glucose uptake by mouse ova and preimplantation embryos. J Reprod Fertil 72: 9–13. [DOI] [PubMed] [Google Scholar]
- 28. Gardner DK (1998) Changes in requirements and utilization of nutrients during mammalian preimplantation embryo development and their significance in embryo culture. Theriogenology 49: 83–102. [DOI] [PubMed] [Google Scholar]
- 29. Pikó L, Taylor KD (1987) Amounts of mitochondrial DNA and abundance of some mitochondrial gene transcripts in early mouse embryos. Dev Biol 123: 364–374. [DOI] [PubMed] [Google Scholar]
- 30. Voisine C, Craig EA, Zufall N, von Ahsen O, Pfanner N, Voos W (1999) The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell 97: 565–574. [DOI] [PubMed] [Google Scholar]
- 31. Thundathil J, Filion F, Smith LC (2005) Molecular control of mitochondrial function in preimplantation mouse embryos. Mol Reprod Dev 71: 405–413. [DOI] [PubMed] [Google Scholar]
- 32. Houghton FD (2006) Energy metabolism of the inner cell mass and trophectoderm of the mouse blastocyst. Differentiation 74: 11–8. [DOI] [PubMed] [Google Scholar]
- 33. Tsujimoto Y, Cossman J, Jaffe E, Croce CM (1985) Involvement of the bcl-2 gene in human follicular lymphoma. Science 228: 1440–1443. [DOI] [PubMed] [Google Scholar]
- 34. Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122: 437–441. 10.1242/jcs.031682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, et al. (1993) Bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74: 597–608. [DOI] [PubMed] [Google Scholar]
- 36. Hsu YT, Wolter KG, Youle RJ (1997) Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci U S A 94: 3668–3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang W, Lu J, Zhu F, Wei J, Jia C, Zhang Y, et al. (2012) Pro-apoptotic and anti-proliferative effects of mitofusin-2 via Bax signaling in hepatocellular carcinoma cells. Med Oncol 29: 70–76. 10.1007/s12032-010-9779-6 [DOI] [PubMed] [Google Scholar]
- 38. Dimmer KS, Scorrano L (2006) (De) constructing mitochondria: what for? Physiology (Bethesda) 21: 233–241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This figure is a certification that American Journal Experts (AJE) has edited our manuscript, including citations and references and AJE reminded us to pay attention the additional instructions.
(PDF)
This figure shows that our manuscript has been edited by American Journal Experts and AJE has changed our manuscript to meet PLOS guidelines and provided language editing, translation, manuscript formatting, and figure formatting to ensure our manuscript meets submission guidelines.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.