Abstract
We have shown previously that atherosclerotic lesions can be induced in normocholesterolemic rabbits by immunization with mycobacterial heat shock protein 65 (hsp65), which has a high degree of sequence homology with mammalian hsp60. To investigate a possible relationship between hsp60 expression and the antigenic specificities of infiltrating T cells in the lesion, 38 New Zealand White rabbits were treated either by immunization with recombinant mycobacterial hsp65 or by administration of a 0.2% cholesterol diet. Atherosclerotic lesions were observed after 16 wk, particularly in the aortic arch and arterial bifurcations of rabbits immunized with hsp65 or fed with a cholesterol-rich diet. Hsp65 staining of aortas showed a heterogeneous distribution, and significantly increased staining intensity in atherosclerotic lesions compared to aortic media or adventitia. This abundantly expressed hsp65 was observed in atherosclerotic lesions induced by hsp65 immunization as well as those induced by cholesterol-rich diet alone. Interestingly, a population of the T lymphocytes isolated from all forms of atherosclerotic lesions specifically responded to hsp65 in vitro. IL-2-expanded T cell lines derived from atherosclerotic lesions showed a significantly higher hsp65 reactivity than those developed from peripheral blood of the same donor. Furthermore, levels of circulating antibodies and numbers of spleen cells specifically reacting against hsp65 were elevated in all experimental animals. Flow cytometric analysis of spleen cells showed elevated immune response-associated antigen expression in treated animals. In conclusion, increased hsp65 expression in intimal cells and the presence of hsp65-specific T cells in blood and in atherosclerotic lesions may be important in initiating the development of atherosclerosis and perpetuating the lesions.
Full text
PDF


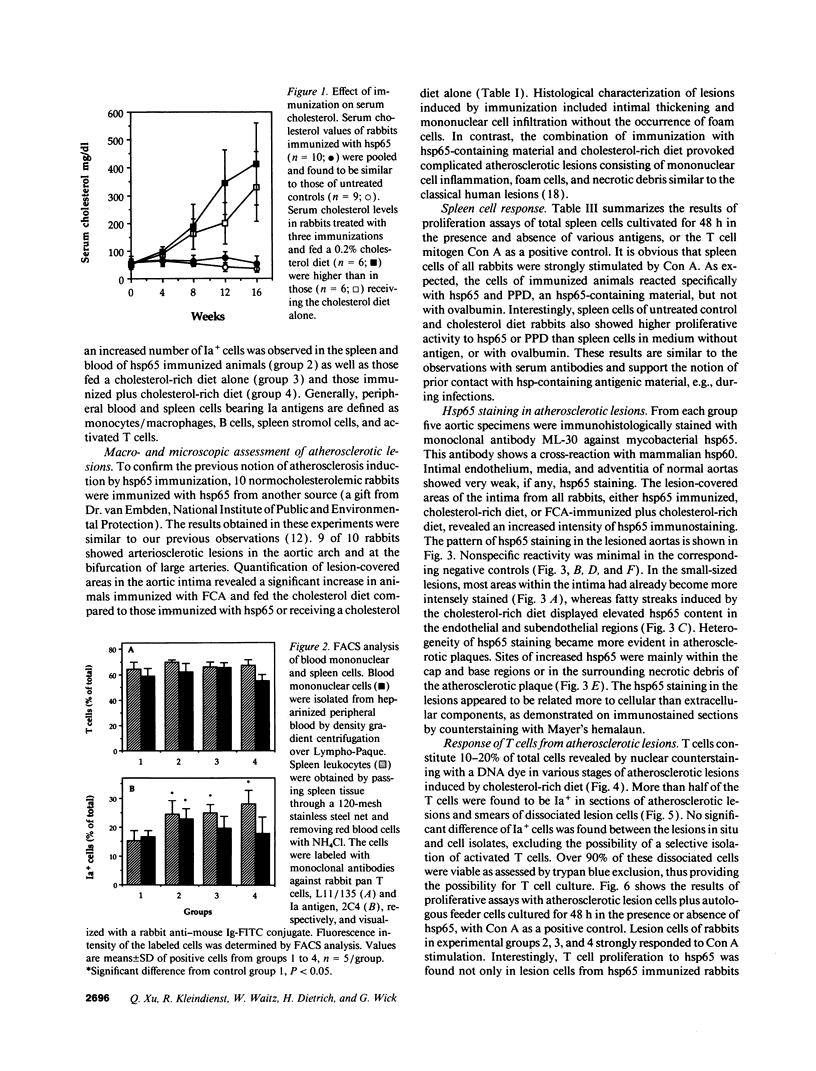

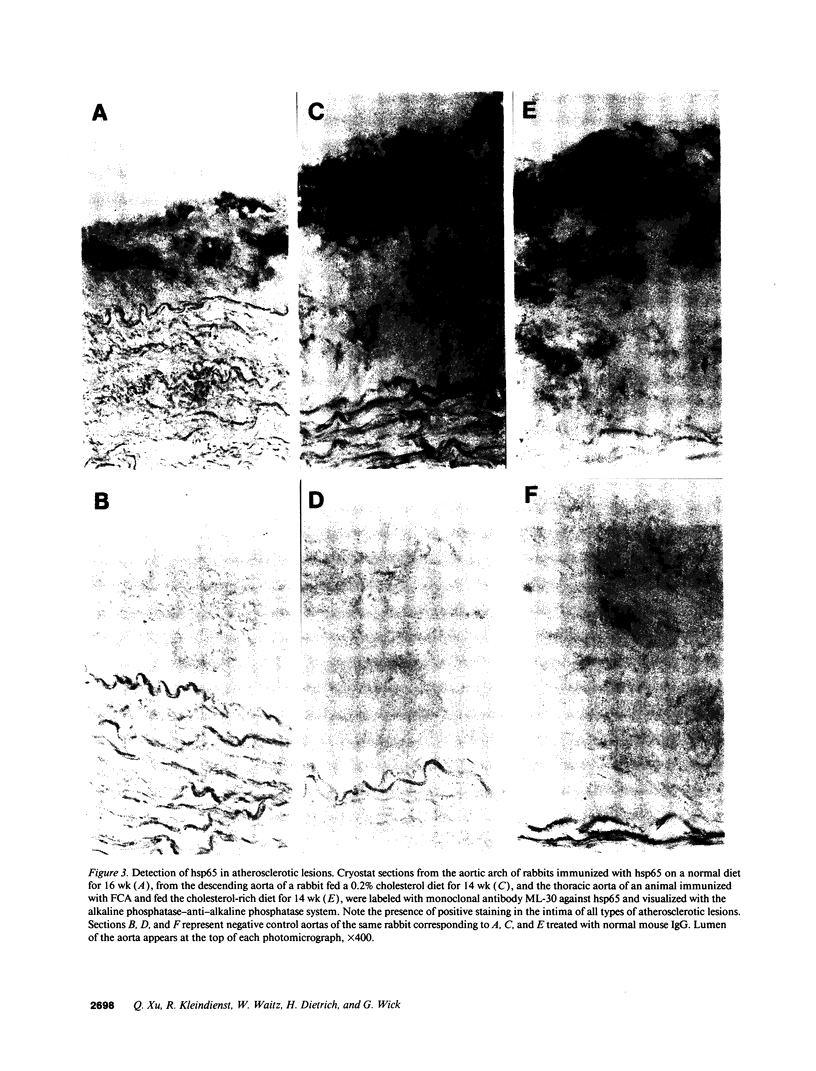



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berberian P. A., Myers W., Tytell M., Challa V., Bond M. G. Immunohistochemical localization of heat shock protein-70 in normal-appearing and atherosclerotic specimens of human arteries. Am J Pathol. 1990 Jan;136(1):71–80. [PMC free article] [PubMed] [Google Scholar]
- Butcher E. C. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991 Dec 20;67(6):1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- Clinton S. K., Underwood R., Hayes L., Sherman M. L., Kufe D. W., Libby P. Macrophage colony-stimulating factor gene expression in vascular cells and in experimental and human atherosclerosis. Am J Pathol. 1992 Feb;140(2):301–316. [PMC free article] [PubMed] [Google Scholar]
- Cybulsky M. I., Gimbrone M. A., Jr Endothelial expression of a mononuclear leukocyte adhesion molecule during atherogenesis. Science. 1991 Feb 15;251(4995):788–791. doi: 10.1126/science.1990440. [DOI] [PubMed] [Google Scholar]
- Damle N. K., Doyle L. V. Ability of human T lymphocytes to adhere to vascular endothelial cells and to augment endothelial permeability to macromolecules is linked to their state of post-thymic maturation. J Immunol. 1990 Feb 15;144(4):1233–1240. [PubMed] [Google Scholar]
- Delcayre C., Samuel J. L., Marotte F., Best-Belpomme M., Mercadier J. J., Rappaport L. Synthesis of stress proteins in rat cardiac myocytes 2-4 days after imposition of hemodynamic overload. J Clin Invest. 1988 Aug;82(2):460–468. doi: 10.1172/JCI113619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donati Y. R., Slosman D. O., Polla B. S. Oxidative injury and the heat shock response. Biochem Pharmacol. 1990 Dec 15;40(12):2571–2577. doi: 10.1016/0006-2952(90)90573-4. [DOI] [PubMed] [Google Scholar]
- Elias D., Markovits D., Reshef T., van der Zee R., Cohen I. R. Induction and therapy of autoimmune diabetes in the non-obese diabetic (NOD/Lt) mouse by a 65-kDa heat shock protein. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1576–1580. doi: 10.1073/pnas.87.4.1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emeson E. E., Robertson A. L., Jr T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol. 1988 Feb;130(2):369–376. [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Norton P., Ivanyi J. Distribution in tissue sections of the human groEL stress-protein homologue. APMIS. 1990 May;98(5):437–441. doi: 10.1111/j.1699-0463.1990.tb01055.x. [DOI] [PubMed] [Google Scholar]
- Faggiotto A., Ross R., Harker L. Studies of hypercholesterolemia in the nonhuman primate. I. Changes that lead to fatty streak formation. Arteriosclerosis. 1984 Jul-Aug;4(4):323–340. doi: 10.1161/01.atv.4.4.323. [DOI] [PubMed] [Google Scholar]
- Feldman D. L., Mogelesky T. C., Liptak B. F., Gerrity R. G. Leukocytosis in rabbits with diet-induced atherosclerosis. Arterioscler Thromb. 1991 Jul-Aug;11(4):985–994. doi: 10.1161/01.atv.11.4.985. [DOI] [PubMed] [Google Scholar]
- Fincato G., Polentarutti N., Sica A., Mantovani A., Colotta F. Expression of a heat-inducible gene of the HSP70 family in human myelomonocytic cells: regulation by bacterial products and cytokines. Blood. 1991 Feb 1;77(3):579–586. [PubMed] [Google Scholar]
- Frostegård J., Wu R., Giscombe R., Holm G., Lefvert A. K., Nilsson J. Induction of T-cell activation by oxidized low density lipoprotein. Arterioscler Thromb. 1992 Apr;12(4):461–467. doi: 10.1161/01.atv.12.4.461. [DOI] [PubMed] [Google Scholar]
- Garry R. F., Ulug E. T., Bose H. R., Jr Induction of stress proteins in Sindbis virus- and vesicular stomatitis virus-infected cells. Virology. 1983 Sep;129(2):319–332. doi: 10.1016/0042-6822(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Gerrity R. G., Naito H. K., Richardson M., Schwartz C. J. Dietary induced atherogenesis in swine. Morphology of the intima in prelesion stages. Am J Pathol. 1979 Jun;95(3):775–792. [PMC free article] [PubMed] [Google Scholar]
- Gown A. M., Tsukada T., Ross R. Human atherosclerosis. II. Immunocytochemical analysis of the cellular composition of human atherosclerotic lesions. Am J Pathol. 1986 Oct;125(1):191–207. [PMC free article] [PubMed] [Google Scholar]
- Hajjar D. P. Warner-Lambert/Parke-Davis Award Lecture. Viral pathogenesis of atherosclerosis. Impact of molecular mimicry and viral genes. Am J Pathol. 1991 Dec;139(6):1195–1211. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Holm J., Jonasson L. Detection of activated T lymphocytes in the human atherosclerotic plaque. Am J Pathol. 1989 Jul;135(1):169–175. [PMC free article] [PubMed] [Google Scholar]
- Hansson G. K., Seifert P. S., Olsson G., Bondjers G. Immunohistochemical detection of macrophages and T lymphocytes in atherosclerotic lesions of cholesterol-fed rabbits. Arterioscler Thromb. 1991 May-Jun;11(3):745–750. doi: 10.1161/01.atv.11.3.745. [DOI] [PubMed] [Google Scholar]
- Heitzmann H., Richards F. M. Use of the avidin-biotin complex for specific staining of biological membranes in electron microscopy. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3537–3541. doi: 10.1073/pnas.71.9.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holoshitz J., Koning F., Coligan J. E., De Bruyn J., Strober S. Isolation of CD4- CD8- mycobacteria-reactive T lymphocyte clones from rheumatoid arthritis synovial fluid. Nature. 1989 May 18;339(6221):226–229. doi: 10.1038/339226a0. [DOI] [PubMed] [Google Scholar]
- Ivanyi L., Ivanyi J. Elevated antibody levels to mycobacterial 65-kDa stress protein in patients with superficial candidiasis. J Infect Dis. 1990 Aug;162(2):519–522. doi: 10.1093/infdis/162.2.519. [DOI] [PubMed] [Google Scholar]
- Jonasson L., Holm J., Skalli O., Bondjers G., Hansson G. K. Regional accumulations of T cells, macrophages, and smooth muscle cells in the human atherosclerotic plaque. Arteriosclerosis. 1986 Mar-Apr;6(2):131–138. doi: 10.1161/01.atv.6.2.131. [DOI] [PubMed] [Google Scholar]
- Jürgens G., Xu Q. B., Huber L. A., Böck G., Howanietz H., Wick G., Traill K. N. Promotion of lymphocyte growth by high density lipoproteins (HDL). Physiological significance of the HDL binding site. J Biol Chem. 1989 May 25;264(15):8549–8556. [PubMed] [Google Scholar]
- Kaufmann S. H. Heat shock proteins and the immune response. Immunol Today. 1990 Apr;11(4):129–136. doi: 10.1016/0167-5699(90)90050-j. [DOI] [PubMed] [Google Scholar]
- Knowlton A. A., Brecher P., Apstein C. S. Rapid expression of heat shock protein in the rabbit after brief cardiac ischemia. J Clin Invest. 1991 Jan;87(1):139–147. doi: 10.1172/JCI114963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krömer G., Schauenstein K., Wick G. Avian lymphokines: an improved method for chicken IL-2 production and assay. A Con A-erythrocyte complex induces higher T cell proliferation and IL-2 production than does free mitogen. J Immunol Methods. 1984 Oct 26;73(2):273–281. doi: 10.1016/0022-1759(84)90402-2. [DOI] [PubMed] [Google Scholar]
- Libby P., Hansson G. K. Involvement of the immune system in human atherogenesis: current knowledge and unanswered questions. Lab Invest. 1991 Jan;64(1):5–15. [PubMed] [Google Scholar]
- Miyajima A., Miyatake S., Schreurs J., De Vries J., Arai N., Yokota T., Arai K. Coordinate regulation of immune and inflammatory responses by T cell-derived lymphokines. FASEB J. 1988 Jun;2(9):2462–2473. doi: 10.1096/fasebj.2.9.2836253. [DOI] [PubMed] [Google Scholar]
- Munk M. E., Schoel B., Kaufmann S. H. T cell responses of normal individuals towards recombinant protein antigens of Mycobacterium tuberculosis. Eur J Immunol. 1988 Nov;18(11):1835–1838. doi: 10.1002/eji.1830181128. [DOI] [PubMed] [Google Scholar]
- Munro J. M., van der Walt J. D., Munro C. S., Chalmers J. A., Cox E. L. An immunohistochemical analysis of human aortic fatty streaks. Hum Pathol. 1987 Apr;18(4):375–380. doi: 10.1016/s0046-8177(87)80168-5. [DOI] [PubMed] [Google Scholar]
- Res P. C., Schaar C. G., Breedveld F. C., van Eden W., van Embden J. D., Cohen I. R., de Vries R. R. Synovial fluid T cell reactivity against 65 kD heat shock protein of mycobacteria in early chronic arthritis. Lancet. 1988 Aug 27;2(8609):478–480. doi: 10.1016/s0140-6736(88)90123-7. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. E., Khoo J. C., Miller E., Parthasarathy S., Palinski W., Witztum J. L. Macrophage-derived foam cells freshly isolated from rabbit atherosclerotic lesions degrade modified lipoproteins, promote oxidation of low-density lipoproteins, and contain oxidation-specific lipid-protein adducts. J Clin Invest. 1991 Jan;87(1):90–99. doi: 10.1172/JCI115006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld M. E., Ylä-Herttuala S., Lipton B. A., Ord V. A., Witztum J. L., Steinberg D. Macrophage colony-stimulating factor mRNA and protein in atherosclerotic lesions of rabbits and humans. Am J Pathol. 1992 Feb;140(2):291–300. [PMC free article] [PubMed] [Google Scholar]
- Salonen J. T., Ylä-Herttuala S., Yamamoto R., Butler S., Korpela H., Salonen R., Nyyssönen K., Palinski W., Witztum J. L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992 Apr 11;339(8798):883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- Steinberg D., Witztum J. L. Lipoproteins and atherogenesis. Current concepts. JAMA. 1990 Dec 19;264(23):3047–3052. [PubMed] [Google Scholar]
- Stemme S., Holm J., Hansson G. K. T lymphocytes in human atherosclerotic plaques are memory cells expressing CD45RO and the integrin VLA-1. Arterioscler Thromb. 1992 Feb;12(2):206–211. doi: 10.1161/01.atv.12.2.206. [DOI] [PubMed] [Google Scholar]
- Stemme S., Rymo L., Hansson G. K. Polyclonal origin of T lymphocytes in human atherosclerotic plaques. Lab Invest. 1991 Dec;65(6):654–660. [PubMed] [Google Scholar]
- Subjeck J. R., Shyy T. T. Stress protein systems of mammalian cells. Am J Physiol. 1986 Jan;250(1 Pt 1):C1–17. doi: 10.1152/ajpcell.1986.250.1.C1. [DOI] [PubMed] [Google Scholar]
- Traill K. N., Böck G., Winter U., Hilchenbach M., Jürgens G., Wick G. Simple method for comparing large numbers of flow cytometry histograms exemplified by analysis of the CD4 (T4) antigen and LDL receptor on human peripheral blood lymphocytes. J Histochem Cytochem. 1986 Sep;34(9):1217–1221. doi: 10.1177/34.9.2426348. [DOI] [PubMed] [Google Scholar]
- Udelsman R., Blake M. J., Holbrook N. J. Molecular response to surgical stress: specific and simultaneous heat shock protein induction in the adrenal cortex, aorta, and vena cava. Surgery. 1991 Dec;110(6):1125–1131. [PubMed] [Google Scholar]
- Xu Q. B., Oberhuber G., Gruschwitz M., Wick G. Immunology of atherosclerosis: cellular composition and major histocompatibility complex class II antigen expression in aortic intima, fatty streaks, and atherosclerotic plaques in young and aged human specimens. Clin Immunol Immunopathol. 1990 Sep;56(3):344–359. doi: 10.1016/0090-1229(90)90155-j. [DOI] [PubMed] [Google Scholar]
- Xu Q., Dietrich H., Steiner H. J., Gown A. M., Schoel B., Mikuz G., Kaufmann S. H., Wick G. Induction of arteriosclerosis in normocholesterolemic rabbits by immunization with heat shock protein 65. Arterioscler Thromb. 1992 Jul;12(7):789–799. doi: 10.1161/01.atv.12.7.789. [DOI] [PubMed] [Google Scholar]
- Xu Q., Willeit J., Marosi M., Kleindienst R., Oberhollenzer F., Kiechl S., Stulnig T., Luef G., Wick G. Association of serum antibodies to heat-shock protein 65 with carotid atherosclerosis. Lancet. 1993 Jan 30;341(8840):255–259. doi: 10.1016/0140-6736(93)92613-x. [DOI] [PubMed] [Google Scholar]
- Yang X. D., Gasser J., Riniker B., Feige U. Treatment of adjuvant arthritis in rats: vaccination potential of a synthetic nonapeptide from the 65 kDa heat shock protein of mycobacteria. J Autoimmun. 1990 Feb;3(1):11–23. doi: 10.1016/0896-8411(90)90003-b. [DOI] [PubMed] [Google Scholar]
- Young R. A., Elliott T. J. Stress proteins, infection, and immune surveillance. Cell. 1989 Oct 6;59(1):5–8. doi: 10.1016/0092-8674(89)90861-1. [DOI] [PubMed] [Google Scholar]
- Zimmerman L. H., Levine R. A., Farber H. W. Hypoxia induces a specific set of stress proteins in cultured endothelial cells. J Clin Invest. 1991 Mar;87(3):908–914. doi: 10.1172/JCI115097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Eden W., Thole J. E., van der Zee R., Noordzij A., van Embden J. D., Hensen E. J., Cohen I. R. Cloning of the mycobacterial epitope recognized by T lymphocytes in adjuvant arthritis. Nature. 1988 Jan 14;331(6152):171–173. doi: 10.1038/331171a0. [DOI] [PubMed] [Google Scholar]