Abstract
Renal cell carcinoma (RCC) is the most common cancer arising from the kidney in adults, with clear cell carcinoma (ccRCC) representing the majority of all RCCs. Expression of a human HIF1a triple mutant (P402A, P564A and N803A) construct in the proximal tubule cells of C57BL/6 mice (TRAnsgenic model of Cancer of the Kidney (TRACK) (1)) mimics the histological changes found in early stage human ccRCC. To better understand the genomic landscape, a high throughput sequence analysis was performed with cDNA libraries (RNAseq) derived from TRACK transgenic positive (TG+) kidney cortex along with human ccRCC transcripts from the Oncomine and TCGA databases. Importantly, the expression profiles of TRACK TG+ kidneys show significant similarities with those observed in human ccRCC, including increased expression of genes involved in glycolysis and the tricarboxylic acid cycle (TCA cycle). Some of the transcripts overexpressed in both the TRACK mouse model and human ccRCC include: ANKRD37, CA9, EGLN3, HK2, NDUFA4L2, and SLC16A3. These data suggest that constitutive activation of HIF1a in kidney proximal tubule cells transcriptionally re-programs the regulation of metabolic pathways in the kidney and that HIF1a is a major contributor to the altered metabolism observed in human ccRCC.
Implications
TRACK (GGT-HIF1αM3) kidney mRNA profiles show similarities to human ccRCC transcriptome and phenotypes associated with the Warburg effect.
Keywords: clear cell renal cell carcinoma, kidney cancer, RNAseq, HIF1α
Introduction
Renal cell carcinoma (RCC) is the most common primary cancer arising from the kidney in adults, with clear cell carcinoma (ccRCC) representing ∼75% of all RCCs (2, 3). Histologically, ccRCC cells are characterized by a transparent cytoplasm caused by deposition of glycogen, phospholipids, and neutral lipids, including cholesterol esters (4, 5). This phenotype suggests that there are metabolic changes in ccRCC cells resulting in abnormal deposition of glycogen and lipids. Seven genes commonly mutated in human kidney cancer, including VHL (NCBI Gene ID: 7428), MET (4233), FLCN (201163), TSC1 (7248), TSC2 (7249), FH (2271), and SDH (6390, 6391 and 6392), have been identified to date (6). Interestingly, all seven genes are involved in the regulation of metabolic pathways (6). These data support the theory that kidney cancer is a metabolic disease (6, 7).
Loss of expression or mutation of the von Hippel-Lindau (VHL) tumor suppressor gene is found in hereditary and most sporadic ccRCCs (2, 8). This suggests an etiological role for VHL gene loss in renal carcinogenesis. However, the exact pathway by which loss of VHL leads to ccRCC has not been definitively elucidated. The best studied and likely most important effect of VHL loss is the resulting increase in expression of the alpha subunits of hypoxia inducible factors 1 (HIF1α) and 2 (HIF2α) (9-11). Increased expression of these two transcription factors has been proposed as a key step in ccRCC carcinogenesis (9, 10) [for review, see (12)]. We previously generated the murine TRAnsgenic model of Cancer of the Kidney (TRACK) that expresses a triple mutant (P402A, P564A and N803A) human HIF1α construct in murine proximal tubule cells (PTCs) and showed that this model mimics the histological alterations found in early stage human ccRCC (1). The cellular histologies displayed in TRACK mice are also similar to those observed in the kidneys of individuals with VHL disease, including areas of distorted tubular structure, cells with clear cytoplasm and increased glycogen and lipid deposition, multiple renal cysts, and areas of multifocal ccRCC (1).
To delineate the gene expression pattern in TRACK TG+ kidneys, we sequenced the entire transcriptome of the TRACK TG+ kidney cortex by high throughput sequencing of cDNA libraries (RNAseq) and compared it with both the gene expression profile in WT/TG- kidneys and the gene expression profile observed in sporadic human ccRCC using the Oncomine database (Compendia Bioscience, Ann Arbor, MI) and the TCGA database. We report that the pattern of gene expression in TRACK TG+ kidneys is similar to that of human ccRCC, including expression of transcripts associated with glycolysis/gluconeogenesis, the pentose phosphate pathway, and the lipid metabolism pathway. These data provide evidence that constitutive activation of HIF1α in kidney proximal tubule cells transcriptionally re-programs the regulation of metabolic pathways in a manner similar to that observed in human ccRCC.
Material and methods
Animals
TRACK transgenic positive (TG+) and transgenic negative (TG-) mice were housed five per cage in a 12 hour light/dark cycle in the Research Animal Resource Center of Weill Cornell Medical College (WCMC). The care and use of animals in this study were approved by the Institutional Animal Care and Use Committee (IACUC) of WCMC.
Whole Transcriptome RNA Sequencing
Total RNA from one thin, outer slice of kidney cortex per kidney was used for whole transcriptome sequencing. Total RNA was extracted using mini-RNAeasy columns (Qiagen, Valencia, CA). The complete transcriptomes of kidney cortices from three γ-HIF1αM3 TRACK (#43 line) TG+ and three γ-HIF1αM3 TG- male C57BL/6 mice (about 13 months old) were sequenced (51-bp single-end reads) on an Illumina HiSeq2000 Sequencer following standard protocols in the Genomics Resources Core Facility at Weill Cornell Medical College. Three lanes were used to sequence all 12 samples (4 samples/lane, 2 kidney samples/mouse). The TRACK RNAseq data has been deposited in the GEO database (accession no. GSE54390, embargoed until publication.).
Data Analysis
Data analysis was mainly performed with the Tuxedo tools software (13). In brief, the RNAseq reads were first aligned to the Mus musculus genome (UCSC version mm10) using Tophat version 2.0.6 (13, 14). The aligned reads were assembled into transcripts, their abundance was estimated, and they were tested for differential expression using Cufflinks version 2.1.1 (13, 15). CummeRbund version 2.0.0 (13) was used to analyze the differential expression analysis results. To identify pathways changed in the TRACK TG+ vs TG- kidneys, functional enrichment analysis was performed using the goseq package in R software (16). A stringent threshold in selecting DE genes (FC>3, q <0.01) was used to reduce the false positive ratio. Heatmaps of log2 transformed RPKM values were created in R using the heatmap.2 command of the gplots package.
Human ccRCC data retrieval
Human ccRCC gene expression changes were retrieved from Oncomine (Compendia Bioscience, Ann Arbor, MI) by combining five different datasets of human ccRCC patient samples (17-20). The same five Oncomine datasets of Cancer vs. Normal Analysis of ccRCC that we used in the γ-HIF2αM3 TG+ RNAseq analysis (21) were used in this analysis.
Human ccRCC mRNA data was downloaded from The Cancer Genome Atlas (TCGA, http://cancergenome.nih.gov/). Only tumor patient data with matched normal and normal patient data with matched tumor were downloaded. All data satisfying this requirement were downloaded, including a total of 470 tumor samples and 68 normal samples. Differential expression between ccRCC and normal kidneys was calculated using the downloaded RPKM (Reads Per Kilobase per Million mapped reads) values. Statistical analyses were performed by student's t-test followed by false discovery rate (FDR)-adjustment (q-value). Statistical significance was defined as q<0.05.
Results
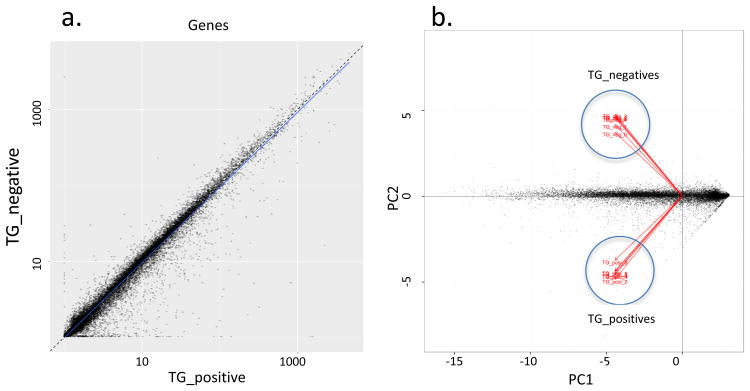
Expression of a mutated, constitutively active HIF1α in the proximal tubule (PT) cells of the γ-HIF1αM3 TRACK mice results in early stage tumors morphologically similar to human ccRCC (1). To identify changes in gene expression associated with ccRCC carcinogenesis, we examined the whole transcriptome from cells in TRACK kidney cortex slices compared with transgenic negative (TG-) controls. At the time of sacrifice (∼13 months old), about 50% of the proximal tubules in TRACK mice show clear cell abnormalities. However, further abnormalities, e.g. carcinoma in situ, are not ubiquitously seen in the kidney cortex. The average number of reads per sample was ∼45.6 million, and ∼96% of reads mapped to the Mus musculus genome (Supplemental Table 1). A scatter plot of the RPKM values of TRACK TG+ vs TG- kidneys shows that the majority of transcripts evaluated display no change between these two samples (Figure 1a), but there are transcripts that show increased or decreased levels in the TRACK TG+ vs TG- kidneys (Figure 1a). Changes in some of these transcripts have been confirmed by semi-quantitative RT-PCR ((1) and Supplemental Figure 1). Principal component analysis (PCA) shows that there is a clear distinction between TRACK TG+ and TG- kidneys (Figure 1b).
Figure 1. Global plotting of TRACK TG+ vs TG- kidney RNAseq result.
A scatter plot of the RPKM values between TRACK TG+ vs. TG- kidneys (a) and a principal component analysis result (b) are shown. The majority of transcripts show no differences in levels between TRACK TG+ vs. TG-. There are transcripts that show increased (dots in the bottom right part of A) or decreased (dots in the top left part of A) levels in the TRACK TG+ vs TG- kidneys. Principal component analysis (PCA) shows that there is a clear distinction between TRACK TG+ and TG- kidneys (b).
We have shown that the high expression of CA-IX, Glut1, and VEGF proteins in the TRACK kidneys is mainly localized in the clear cell proximal tubules (1). Here we also used immunohistochemistry to examine the protein levels of NDUFA4L2, SLC16A3, and HK2, three of the top genes overexpressed in TRACK kidneys by RNAseq. We detected high expression of NDUFA4L2, SLC16A3, and HK2 primarily in the clear cell proximal tubules (Supplemental Figure 2). These transcripts are also highly expressed in human ccRCC (see next section).
Certain metabolic pathways are over-represented among differentially expressed (DE) genes in TRACK TG+ kidneys
We first examined the over-representation of 274 DE genes (259 overexpressed and 15 underexpressed) in KEGG (Kyoto Encyclopedia of Genes and Genomes). KEGG is a database resource for understanding high-level functions and utilities of biological systems (22, 23). The KEGG PATHWAY is a collection of manually drawn pathway maps of molecular interactions and reaction networks. The 274 DE genes are over-represented in 5 KEGG pathways (q<0.05, top 10 pathways are shown in Table 1). Interestingly, seven of the 10 KEGG pathways are related to metabolism and five of these seven KEGG pathways have the lowest p-values in the list (Table 1), indicating that metabolic changes are the most prominent changes in TRACK TG+ kidneys. The most significant KEGG pathway is mmu00010: Glycolysis/Gluconeogenesis (p=3.87E-06). The pentose phosphate and peroxisome proliferator-activated receptor (PPAR) signaling pathways are also emphasized.
Table 1. Ten most significant KEGG pathways that are over-represented among the differentially expressed (DE) genes.
| p_value | q_value | KEGG_ID | KEGG_pathway |
|---|---|---|---|
| 3.87E-06 | 8.70E-04 | mmu00010 | Glycolysis / Gluconeogenesis |
| 2.27E-05 | 2.55E-03 | mmu00051 | Fructose and mannose metabolism |
| 2.36E-04 | 1.77E-02 | mmu03320 | PPAR signaling pathway |
| 6.70E-04 | 3.77E-02 | mmu00052 | Galactose metabolism |
| 8.91E-04 | 4.01E-02 | mmu00030 | Pentose phosphate pathway |
| 1.40E-03 | 5.24E-02 | mmu04060 | Cytokine-cytokine receptor interaction |
| 2.59E-03 | 8.32E-02 | mmu00670 | One carbon pool by folate |
| 3.50E-03 | 8.97E-02 | mmu01100 | Metabolic pathways |
| 3.59E-03 | 8.97E-02 | mmu00750 | Vitamin B6 metabolism |
| 4.27E-03 | 9.60E-02 | mmu04672 | Intestinal immune network for IgA production |
We also examined the over-representation of DE genes in the Gene Ontology (GO) consortium (24). The GO covers three domains: cellular component, molecular function, and biological process, and we focused on the biological process domain. The 274 DE genes are over-represented in 98 GO biological process ontologies (q<0.05, Supplemental Table 2). Similar to the KEGG pathway analysis, seven of the top ten over-represented GO ontologies are metabolism related, e.g. glucose metabolic process (p=3.57E-10), glucose catabolic process (p=2.36E-08).
Metabolic changes in ccRCC can be explained by gene expression changes observed in TRACK TG+ kidneys
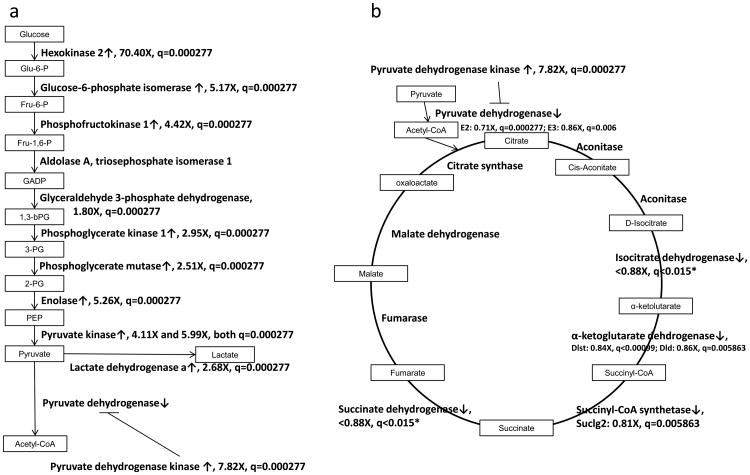
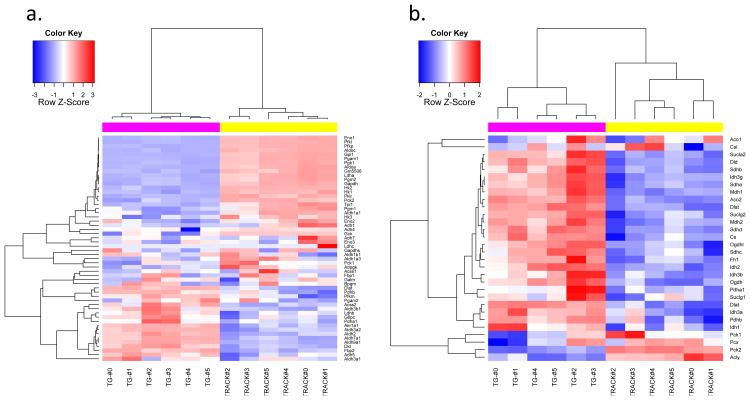
The most significant metabolic change that occurs in ccRCC, and probably in most cancers, is the shift from oxidative phosphorylation of glucose to glycolysis under normoxic conditions, known as the Warburg effect (25). We examined the transcript levels of key genes involved in glycolysis and the TCA cycle in the TRACK TG+ kidneys vs. TG- kidneys (Figure 2). Most glycolysis gene transcripts are increased in the TRACK TG+ kidneys compared to the TG- kidneys, e.g. hexokinase, phosphofructokinase, and pyruvate kinase. TCA cycle genes do not show large decreases in transcript levels in TRACK TG+ kidneys compared to TG- kidneys (FC between 0.8 and 0.9), presumably as a result of the presence of non-transformed cells in the cortex samples. Transcripts encoding pyruvate dehydrogenase kinase, the enzyme that inactivates pyruvate dehydrogenase through phosphorylation (26), are increased in the TRACK TG+ kidneys compared to TG- kidneys (fold change (FC) =7.8). Inactivation of pyruvate dehydrogenase by pyruvate dehydrogenase kinase prevents the conversion of pyruvate to acetyl-CoA (26), which is oxidized in mitochondria in the TCA cycle. The lower level of acetyl-CoA thereby inhibits the TCA cycle. Consistent with this, the transcript for lactate dehydrogenase, which converts pyruvate to lactate, is increased in the TRACK TG+ relative to TG- kidneys. All of these changes indicate an important role for HIF1α in changing the metabolism of kidney cells to glycolysis under normoxic conditions. Heatmaps created from genes involved in glycolysis and in the TCA cycle also confirmed these changes (Figure 3).
Figure 2. Transcript changes for glycolysis and TCA cycle transcripts in TRACK+/TG- kidney cells.
Simplified pathway maps of glycolysis (a) and the TCA cycle (b) with mRNA changes shown. Un-boxed inputs are the gene names. Genes that are over-expressed (FC>2) in the TRACK TG+ kidneys are indicated by ↑, while genes that are under-expressed (FC<0.9) in the TRACK TG+ kidneys are indicated by ↓. Since the under-expressed genes are potentially under-estimated in the RNAseq results, the fold change threshold for under-expressed genes is set at <0.90. * Both isocitrate dehydrogenase (IDH) and succinate dehydrogenase (SDH) complexes contain more than 2 transcripts that are significantly decreased in TRACK TG+ kidneys. The fold change and q-value shown are the least significant ones in these two complexes.
Figure 3. Heatmaps of transcripts encoded by genes involved in glycolysis and the TCA cycle, TRACK+/TG-.
Heatmaps of genes involved in glycolysis (a) and the TCA cycle (b). Log2 transformed RPKM values of 61 and 31 genes involved in glycolysis and TCA cycle pathways were used to create these heatmaps. The animal identity is indicated by the colored rows on top of the heatmap matrix. The magenta color indicates TRACK TG+ mice. The yellow color indicates TRACK TG- mice. The blue color in the heatmap matrix indicates relatively decreased transcript levels and the red color indicates relatively increased transcript levels compared to the mean transcript level for each gene. Brighter blue or red color indicates a greater fold change. The glycolysis genes listed in Figure 2 show increased transcript levels in the TRACK TG+ kidneys (red color) compared to TG- kidneys (a). The TCA cycle genes generally show decreased transcript levels in the TRACK TG+ kidneys (blue colored) compared to TG- kidneys (b).
The TRACK/HIF1α kidney transcriptome shows similarities with the human ccRCC transcriptome
Using a fold-expression change of >2 or < 0.5 and q <0.05, 655 genes showed increased mRNA levels and 55 genes showed decreased mRNA levels in TRACK TG+ kidney samples compared with the TG- control kidney cortex samples. As expected, several HIF1α target genes were highly expressed at the transcript level in TRACK kidney samples, including hexokinase 2 (HK2, 70X), carbonic anhydrase IX (CA-IX, 9.3X), glucose transporter 1 (Glut-1 or Slc2a1, 4.8X) etc, which were confirmed by semi-quantitative RT-PCR ((1) and Supplemental Figure 1).
We first compared the expression profile of human ccRCC, as reported in the Oncomine database (27), with our TRACK+ expression profile. We identified the 20 genes most highly overexpressed and 20 genes most highly underexpressed at the RNA level in human ccRCC by combining five different datasets of human ccRCC patient samples (17-20). The total number of ccRCC patients in all five data sets is 175. Five of the 20 genes most highly overexpressed in human ccRCC were similarly expressed in TRACK TG+ kidneys (FC>2) (Table 2): NDUFA4L2, C7ORF68, EGLN3, SLC16A3, and CA-IX. None of the 20 genes highly underexpressed in human ccRCC shows significant downregulation in our TRACK TG+ kidneys (FC<0.5) (Table 2). However, the numbers of genes that show reduced expression in TRACK TG+ vs TG- kidneys is most likely an underestimate because only 30-50% of PTs in TRACK TG+ kidneys demonstrate morphologic changes (1).
Table 2. Twenty genes that show the highest fold increases in transcript levels in human ccRCC compared to normal human kidney from the Oncomine database.
| Oncomine Median Rank | Oncomine p-Value | Oncomine Median Fold Change | Gene | fold-change TRACK TG+/TG- | p-value | q-value |
|---|---|---|---|---|---|---|
| Increased in ccRCC | ||||||
| 7 | 5.55E-23 | 53.9 | NDUFA4L2 | 67.92514 | 5e-05 | 2.77e-04 |
| 14 | 3.91E-13 | 16.5 | C7ORF68 | 2.824433 | 5e-05 | 2.77e-04 |
| 14 | 2.82E-11 | 12.5 | ENO2 | 1.668066 | 1e-04 | 5.27e-04 |
| 16 | 9.50E-19 | 12.2 | EGLN3 | 30.98842 | 5e-05 | 2.77e-04 |
| 21 | 1.73E-14 | 11.0 | IGFBP3 | 1.572941 | 5e-05 | 2.77e-04 |
| 25 | 1.23E-10 | 12.2 | SPAG4 | 1.174346 | 1 | 1 |
| 28 | 2.83E-17 | 15.0 | AHNAK2 | Not found | ||
| 30 | 3.79E-7 | 8.4 | TMCC1 | 1.04368 | 0.60145 | 0.754576 |
| 36 | 2.19E-16 | 8.6 | RNASET2 | 1.048042 | 0.62605 | 0.774746 |
| 38 | 1.93E-13 | 8.2 | CAV1 | 1.385522 | 5e-05 | 2.77e-04 |
| 41 | 2.54E-13 | 7.1 | SLC16A3 | 114.6301 | 5e-05 | 2.77e-04 |
| 46 | 6.8E-10 | 4.6 | LCP2 | 1.774849 | 5e-05 | 2.77e-04 |
| 47 | 6.92E-16 | 18.0 | CA-IX | 9.340484 | 5e-05 | 2.77e-04 |
| 48 | 7.84E-10 | 15.4 | NETO2 | 1.513053 | 1 | 1 |
| 49 | 8.25E-10 | 2.6 | IFNGR2 | 1.242553 | 1e-04 | 5.27e-04 |
| 57 | 3.12E-15 | 4.9 | ABCG1 | 1.500543 | 5e-05 | 2.77e-04 |
| 57 | 1.37E-12 | 2.3 | UBE2L6 | 1.62286 | 5e-05 | 2.77e-04 |
| 59 | 1.59E-10 | 3.2 | DIAPH2 | 1.001564 | 0.97995 | 0.988178 |
| 59 | 1.50E-9 | 2.4 | ALDOA | 1.840662 | 5e-05 | 2.77e-04 |
| 59 | 1.69E-8 | 3.4 | SLC15A4 | 1.107461 | 0.28295 | 0.453989 |
| Decreased in ccRCC | ||||||
| 4 | 1.41E-019 | -283.867 | UMOD | 1.038859 | 0.5042 | 0.675799 |
| 4 | 2.63E-019 | -44.184 | SFRP1 | 1.029511 | 0.5378 | 0.705209 |
| 22 | 1.38E-012 | -6.616 | TFCP2L1 | 1.343029 | 5e-05 | 2.77e-04 |
| 36 | 1.40E-006 | -72.014 | SLC12A1 | 1.020973 | 0.9791 | 0.987744 |
| 41 | 9.48E-011 | -14.893 | GATA3 | 1.078646 | 0.2454 | 0.410806 |
| 44 | 3.25E-012 | -51.115 | CLDN8 | 1.038964 | 0.44125 | 0.618216 |
| 44 | 6.68E-011 | -20.454 | LPPR1 | No mouse homolog | ||
| 45 | 2.75E-013 | -10.533 | ALDH6A1 | 0.7895559 | 5e-05 | 2.77e-04 |
| 45 | 3.32E-012 | -223.376 | CALB1 | 1.019128 | 0.69485 | 0.821341 |
| 47 | 4.80E-013 | -8.431 | DCN | 2.206112 | 5e-05 | 2.77e-04 |
| 51 | 3.67E-010 | -47.197 | ATP6V0A4 | 0.7976494 | 1e-04 | 5.27e-04 |
| 51 | 2.65E-006 | -172.954 | KNG1 | 1.283858 | 0.10715 | 0.217875 |
| 54 | 1.82E-012 | -34.705 | ALDOB | HIDATA | 1 | 1 |
| 56 | 2.08E-012 | -7.697 | TCF21 | 1.3445 | 4e-04 | 1.86e-03 |
| 57 | 3.71E-006 | -17.206 | MAL | 1.11156 | 0.02155 | 0.0601907 |
| 61 | 7.17E-010 | -16.262 | PTH1R | 0.7618173 | 5e-05 | 2.77e-04 |
| 65 | 1.17E-009 | -3.402 | GABARAPL3 | No mouse homolog | ||
| 68 | 1.45E-009 | -3.413 | PROSAPIP1 | 0.6268898 | 5e-05 | 2.77e-04 |
| 77 | 2.44E-009 | -50.953 | KCNJ1 | 1.030991 | 0.51935 | 0.689311 |
| 79 | 1.87E-011 | -17.438 | ERBB4 | 1.001307 | 0.9846 | 0.990418 |
The list of the top 20 genes that show elevated or decreased transcript levels in human ccRCC was retrieved from Oncomine by combining five different datasets of human ccRCC patient samples, totaling 175 patients. These five datasets are the same as those discussed in (21). The fold changes in mRNA levels in these genes in TRACK TG+ mice versus TG- kidneys are listed.
We also compared the expression profile of human ccRCC mRNA downloaded from the TCGA database with our TRACK+ expression profile. We identified the 20 genes most highly overexpressed and the 20 genes most highly underexpressed at the RNA level in human ccRCC from TCGA data. Five of the 20 genes most highly overexpressed in human ccRCC were similarly expressed in TRACK TG+ kidneys (FC>2) (Table 3). None of the 20 genes highly underexpressed in human ccRCC shows significant downregulation in our TRACK TG+ kidneys (FC<0.5) (Table 3).
Table 3. Twenty genes that show the highest fold increases or decreases in transcript levels in human ccRCC compared to normal human kidney from TCGA.
| TCGA q-value | TCGA Fold Change | Gene | fold-change TRACK TG+/TG- | p-value | q-value |
|---|---|---|---|---|---|
| Increased in ccRCC | |||||
| 2.85E-02 | 140.62 | HP | 4.47 | 5.00E-05 | 2.77E-04 |
| 2.59E-10 | 81.87 | GUCA2B | 0.88 | 7.30E-03 | 2.41E-02 |
| 1.74E-122 | 54.30 | CA9 | 9.34 | 5.00E-05 | 2.77E-04 |
| 6.09E-28 | 51.35 | FABP7 | 0.82 | 1.47E-02 | 4.36E-02 |
| 1.21E-23 | 47.63 | MCHR1 | 0.69 | 2.35E-03 | 9.01E-03 |
| 3.19E-02 | 45.56 | HHATL | 0.88 | 8.02E-02 | 1.73E-01 |
| 3.06E-105 | 44.88 | NDUFA4L2 | 67.93 | 5.00E-05 | 2.77E-04 |
| 6.80E-48 | 44.07 | HSF4 | 1.78 | 5.00E-05 | 2.77E-04 |
| 1.82E-45 | 34.72 | KISS1R | 1.06 | 9.80E-01 | 9.88E-01 |
| 9.17E-61 | 34.31 | CYP2J2 | 0.93 | 8.85E-01 | 9.38E-01 |
| 2.65E-41 | 31.87 | PNCK | 1.71 | 8.10E-03 | 2.63E-02 |
| 2.15E-33 | 31.45 | APOC1 | 1.62 | 4.45E-02 | 1.08E-01 |
| 5.89E-17 | 29.99 | CD5L | 0.39 | 5.00E-05 | 2.77E-04 |
| 2.20E-110 | 29.31 | ANGPTL4 | 0.76 | 5.00E-05 | 2.77E-04 |
| 1.34E-94 | 28.73 | COL23A1 | 1.34 | 2.23E-02 | 6.19E-02 |
| 2.92E-06 | 24.59 | LBP | 1.73 | 5.00E-05 | 2.77E-04 |
| 1.39E-91 | 21.66 | ENPP3 | 0.99 | 9.10E-01 | 9.52E-01 |
| 7.13E-94 | 20.73 | C7orf68 | 2.82 | 5.00E-05 | 2.77E-04 |
| 2.51E-26 | 19.66 | KCNN1 | 0.78 | 1.07E-02 | 3.34E-02 |
| 5.94E-44 | 19.58 | NXPH4 | 10.02 | 5.00E-05 | 2.77E-04 |
| Decreased in ccRCC | |||||
| 9.67E-16 | 0.002 | AQP2 | 1.260 | 5.00E-05 | 2.77E-04 |
| 3.20E-14 | 0.002 | UMOD | 1.039 | 5.04E-01 | 6.76E-01 |
| 5.23E-16 | 0.003 | SLC12A1 | 1.021 | 9.79E-01 | 9.88E-01 |
| 5.73E-13 | 0.004 | TMEM207 | 0.888 | 2.22E-01 | 3.80E-01 |
| 3.09E-11 | 0.005 | FXYD4 | 1.055 | 4.58E-01 | 6.34E-01 |
| 6.23E-12 | 0.006 | CALB1 | 1.019 | 6.95E-01 | 8.21E-01 |
| 2.95E-12 | 0.008 | NPHS2 | 1.249 | 5.00E-05 | 2.77E-04 |
| 1.70E-18 | 0.009 | DUSP9 | 1.043 | 5.90E-01 | 7.47E-01 |
| 1.18E-11 | 0.009 | SOST | 1.091 | 4.90E-01 | 6.64E-01 |
| 1.07E-20 | 0.013 | KNG1 | 1.284 | 1.07E-01 | 2.18E-01 |
| 5.63E-21 | 0.013 | HS6ST2 | 1.724 | 5.00E-05 | 2.77E-04 |
| 5.46E-12 | 0.014 | DDN | 1.071 | 3.69E-01 | 5.48E-01 |
| 3.20E-17 | 0.014 | CLDN16 | 1.068 | 4.52E-01 | 6.29E-01 |
| 1.07E-24 | 0.015 | KCNJ1 | 1.066 | 8.46E-01 | 9.18E-01 |
| 1.07E-24 | 0.015 | KCNJ1 | 1.031 | 5.19E-01 | 6.89E-01 |
| 1.05E-13 | 0.015 | ESRRB | 0.884 | 4.01E-02 | 9.96E-02 |
| 6.04E-24 | 0.016 | KCNJ10 | 1.113 | 2.36E-02 | 6.49E-02 |
| 1.22E-09 | 0.016 | SLC22A8 | 0.549 | 5.00E-05 | 2.77E-04 |
| 4.49E-24 | 0.016 | TFAP2B | 1.055 | 3.23E-01 | 4.99E-01 |
| 3.08E-21 | 0.017 | RALYL | 1.488 | 2.81E-02 | 7.49E-02 |
The list of the top 20 genes that show elevated or decreased transcript levels in human ccRCC was retrieved from TCGA. The fold changes in mRNA levels in these genes in TRACK TG+ mice versus TG- kidneys are listed.
We then performed the reverse comparison by identifying the top genes over- or under-expressed at the RNA level in the TRACK TG+ vs. TG- WT kidneys and comparing these transcripts to those in the TCGA database and the combined Oncomine human ccRCC datasets. Eleven of the 20 genes highly overexpressed in TRACK TG+ kidneys show overexpression (FC>2) in the TCGA data (Table 4). Ten of the 20 genes highly underexpressed in TRACK TG+ kidneys show underexpression (FC<0.5) in the TCGA dataset (Table 4). Four of the 20 genes highly overexpressed in TRACK TG+ kidneys show overexpression (FC>2) in the combined Oncomine datasets (Table 4). Eight of the 20 genes highly underexpressed in TRACK TG+ kidneys show underexpression (FC<-2) in the combined Oncomine datasets (Table 4). We conclude from analysis of these data that expression of a mutant, constitutively active HIF1α protein in kidney PTs results in a transcriptome that partially resembles those of human ccRCCs.
Table 4. Twenty genes that show the greatest increases or decreases in transcript levels in the TRACK TG+ vs. WT kidneys by RNAseq.
| fold-change TRACK TG+/TG- | p-value | q-value | Gene | TCGA Human ccRCC/normal kidney fold change | Oncomine Human ccRCC/normal kidney median fold change |
|---|---|---|---|---|---|
| Increased in TRACK | |||||
| 260.2342364 | 5e-05 | 2.77e-04 | F2 | 11.76 | 1.008 |
| 241.0075905 | 5e-05 | 2.77e-04 | IGFBP2 | 0.29 | -5.309 |
| 212.4951738 | 5e-05 | 2.77e-04 | GRIN1 | 2.67 | 1.342 |
| 208.5679355 | 5e-05 | 2.77e-04 | AIRE | 2.06 | -1.075 |
| 196.9580885 | 5e-05 | 2.77e-04 | APOA4 | 244.29 | -1.227 |
| 158.8470346 | 5e-05 | 2.77e-04 | RBP4 | 1.11 | -1.13 |
| 148.1545998 | 5e-05 | 2.77e-04 | APOC3 | 3.61 | -1.815 |
| 127.8628806 | 5e-05 | 2.77e-04 | A2M | 1.16 | 1.809 |
| 114.6301281 | 5e-05 | 2.77e-04 | SLC16A3 | 8.15 | 7.132 |
| 97.86820107 | 5e-05 | 2.77e-04 | ADAM7 | 7.79 | 1.07 |
| 86.29550828 | 5e-05 | 2.77e-04 | ZFP92 | 0.74 | -0.0815 |
| 78.7336936 | 6e-04 | 0.00266966 | CRISP1 | 0.21 | -1.003 |
| 77.00377581 | 5e-05 | 2.77e-04 | AREG | 1.43 | 1.312 |
| 70.96599132 | 5e-05 | 2.77e-04 | RASGRF2 | 1.10 | 1.568 |
| 70.39792583 | 5e-05 | 2.77e-04 | NLRP10 | 0.29 | -1.286 |
| 70.39041145 | 5e-05 | 2.77e-04 | HK2 | 10.17 | 13.568 |
| 67.9251413 | 5e-05 | 2.77e-04 | NDUFA4L2 | 44.88 | 53.935 |
| 50.96140222 | 0.00015 | 0.000762465 | CPB2 | 8.97 | 1.035 |
| 42.27652553 | 5e-05 | 2.77e-04 | ANKRD37 | 2.15 | 3.846 |
| 38.17346108 | 5e-05 | 2.77e-04 | DKK1 | 1.51 | -1.058 |
| Decreased in TRACK | |||||
| 0.03993031 | 0.0145 | 0.0431824 | DIO3OS | 5.18 | -1.025 |
| 0.042320553 | 5e-05 | 2.77e-04 | SOX2OT | 0.38 | -2.8495 |
| 0.112181952 | 0.0017 | 0.0067467 | KRT13 | 0.37 | -1.020 |
| 0.122714679 | 5e-05 | 2.77e-04 | HPD | 0.04 | -79.57 |
| 0.196070105 | 5e-05 | 2.77e-04 | IDO2 | 1.58 | 1.019 |
| 0.215859171 | 5e-05 | 2.77e-04 | C4A | 3.40 | 2.176 |
| 0.218647669 | 5e-05 | 2.77e-04 | HRG | 0.24 | -14.081 |
| 0.251872656 | 5e-05 | 2.77e-04 | AZGP1 | 0.29 | -12.585 |
| 0.312454798 | 5e-05 | 2.77e-04 | NLRC4 | 3.32 | -1.0665 |
| 0.31642082 | 5e-05 | 2.77e-04 | PZP | 1.14 | 1.012 |
| 0.327772061 | 5e-05 | 2.77e-04 | CTXN3 | 0.10 | -7.343 |
| 0.331900011 | 5e-05 | 2.77e-04 | ANXA13 | 3.27 | 1.971 |
| 0.361353874 | 5e-05 | 2.77e-04 | UPP2 | 0.04 | -5.804 |
| 0.378206047 | 5e-05 | 2.77e-04 | APOH | 1.23 | -6.871 |
| 0.39051491 | 5e-05 | 2.77e-04 | CD5L | 29.99 | -1.017 |
| 0.409784094 | 5e-05 | 2.77e-04 | TMEM28 | 0.60 | -1.095 |
| 0.426331824 | 5e-05 | 2.77e-04 | CNDP2 | 1.59 | 1.185 |
| 0.42792761 | 5e-05 | 2.77e-04 | FCAMR | 0.37 | -2.2115 |
| 0.429718771 | 0.0156 | 0.0459592 | ANGPTL7 | 0.34 | -1.049 |
| 0.436898506 | 0.0156 | 0.0459592 | GPT | 0.71 | -1.344 |
The list of the top 20 genes that show elevated or decreased transcript levels in the TRACK TG+ vs. TG- kidneys was compiled from the RNAseq results. The fold changes in mRNA levels of these genes in human ccRCC were compiled from Oncomine (median fold changed are used here) by combining five different sets of human ccRCC patient samples (see Table 1 for more information) or from TCGA. Genes that have no measurements in all five datasets were excluded from this list.
Discussion
We previously established the TRACK model, which mimics early stage human ccRCC through expression of a mutated, constitutively active human HIF1α construct in the PT cells (1). Here we present genome-wide transcriptome analysis of the TRACK TG+ kidney cortex cells, which are mainly PT cells. We identified 655 up-regulated genes and 55 down-regulated genes that are differentially expressed in the TRACK TG+ kidneys compared to the TG- kidneys (FC>2). Some of these genes also show increased or decreased transcript levels in human ccRCC specimens compared to normal kidneys, respectively (Table 4). For example, NDUFA4L2 is the gene overexpressed to the greatest extent in the human ccRCC datasets in Oncomine. The median fold change in human ccRCC compared to normal kidney from all 5 datasets is 53.9 (Table 2). Similarly, NDUFA4L2 transcript levels are about 68 fold higher in TRACK TG+ kidneys compared to TG- kidneys (Table 2). Similarities can also be seen in genes that are down-regulated in TRACK TG+ vs. TG- and human ccRCC vs. normal kidneys, e.g. 4-hydroxyphenylpyruvic acid dioxygenase (HPD) (Table 4). These results suggest that the transcriptome of the TRACK TG+ kidneys partially resembles that of human ccRCC cells. Furthermore, we have examined the expression patterns of some of these top genes overexpressed in the TRACK kidneys by immunohistochemistry ((1) and Supplemental Figure 2). The high expression of these proteins occurs primarily in the clear cell proximal tubules. These data support our contention that the changes in the transcriptome of TRACK kidneys that we have seen by RNAseq are mainly, if not all, caused by changes in these clear cell proximal tubules.
New evidence suggests that ccRCC is a metabolic disease (6, 7). All known kidney cancer susceptible genes are involved in the regulation of metabolic pathways (6). ccRCC cells contain increased amounts of glycogen and lipid in their cytoplasm (4, 5). Increased glycogen and lipid are also seen in the TRACK TG+ kidney cells (1). Our analysis of the entire transcriptome of the TRACK TG+ kidney PT cells identified some altered metabolic pathways, including increased transcript levels of genes involved in glycolysis/gluconeogenesis (Figure 2A) and decreased transcript levels of genes involved in the TCA cycle (Figure 2B). Furthermore, increased mRNA levels of the protein pyruvate dehydrogenase kinase can inactivate pyruvate dehydrogenase, which converts pyruvate to acetyl-CoA (26). Decreased levels of acetyl-CoA, together with decreased transcript levels of genes involved in the TCA cycle, should result in decreased activity of the TCA cycle. The products of the TCA cycle, NADH and succinate, are used in the oxidative phosphorylation pathway. Decreased TCA cycle activity and decreased levels of NADH and succinate should result in lower levels of substrates, and as a result, decreased oxidative phosphorylation. This phenotype recapitulates much of what is described for the Warburg effect in tumor cells (25). HIF1α, rather than HIF2α, is the main regulator of these pathways (28, 29), further emphasizing the importance of HIF1α in modulating the metabolic alterations in ccRCC.
The histone deacetylase, Sirtuin-6 (SIRT6), can suppress the transcription of the HIF1α gene (30). Increased glucose uptake and increased HIF1α activity were shown in SIRT6-deficient cells and mice and this increase in HIF1α activity was sufficient to cause tumorigenesis (30). SIRT6 has been reported to be a potential tumor suppressor gene (31, 32). Thus, either the absence of VHL or the lack of SIRT6 can results in an increase in HIF1α activity and tumorigenesis. In summary, our results suggest that constitutive activation of HIF1α in kidney PT cells in our TRACK model induces a phenotype similar to the tumor phenotype associated with the Warburg effect (25).
In addition to inactivating mutations in the VHL gene, inactivating mutations in other genes, e.g. PBRM1 (33), SETD2 (34), etc., are commonly seen in patients with advanced ccRCC (35-38). Mutations and/or changes in the expression of these genes probably play important roles in the development of advanced ccRCC. This might explain why TRACK mice only mimic early stage ccRCC. This hypothesis is being tested in the TRACK mouse model by mutation/knockout of additional genes, such as PBRM1 and SETD2.
Importantly, the transgenic mice we generated that overexpress a constitutively active HIF2α do not show major transcript changes that reflect those in human ccRCC (21). Thus our data reported here and those of many other researchers (reviewed in 12) indicate that the activity of HIF1α is more closely linked to ccRCC tumorigenesis than the activity of HIF2α.
Supplementary Material
Acknowledgments
This research was supported by WCMC, the Genitourinary Oncology Research Fund, and the Turobiner Kidney Cancer Research Fund. LF held the Robert H. McCooey Genitourinary Oncology Research Fellowship and is supported by National Cancer Institute (NCI) Grant NIH T32 CA062948. DM is supported by an NIH T32 Training Grant (5T32CA062948). The results published here are in part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/. We thank Daniel Stummer for editorial assistance and the Gudas and Nanus labs for thoughtful discussions of data.
Footnotes
All authors declare no conflicts of interests.
References
- 1.Fu L, Wang G, Shevchuk MM, Nanus DM, Gudas LJ. Generation of a Mouse Model of Von Hippel-Lindau Kidney Disease Leading to Renal Cancers by Expression of a Constitutively Active Mutant of HIF1alpha. Cancer research. 2011;71:6848–56. doi: 10.1158/0008-5472.CAN-11-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maher ER, Kaelin WG., Jr von Hippel-Lindau disease. Medicine (Baltimore) 1997;76:381–91. doi: 10.1097/00005792-199711000-00001. [DOI] [PubMed] [Google Scholar]
- 3.Patard JJ, Leray E, Rioux-Leclercq N, Cindolo L, Ficarra V, Zisman A, et al. Prognostic value of histologic subtypes in renal cell carcinoma: a multicenter experience. J Clin Oncol. 2005;23:2763–71. doi: 10.1200/JCO.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 4.Gebhard RL, Clayman RV, Prigge WF, Figenshau R, Staley NA, Reesey C, et al. Abnormal cholesterol metabolism in renal clear cell carcinoma. J Lipid Res. 1987;28:1177–84. [PubMed] [Google Scholar]
- 5.Krishnan B, Truong LD. Renal epithelial neoplasms: the diagnostic implications of electron microscopic study in 55 cases. Hum Pathol. 2002;33:68–79. doi: 10.1053/hupa.2002.30210. [DOI] [PubMed] [Google Scholar]
- 6.Linehan WM, Srinivasan R, Schmidt LS. The genetic basis of kidney cancer: a metabolic disease. Nat Rev Urol. 2010;7:277–85. doi: 10.1038/nrurol.2010.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinthus JH, Whelan KF, Gallino D, Lu JP, Rothschild N. Metabolic features of clear-cell renal cell carcinoma: mechanisms and clinical implications. Can Urol Assoc J. 2011;5:274–82. doi: 10.5489/cuaj.10196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Linehan WM, Pinto PA, Srinivasan R, Merino M, Choyke P, Choyke L, et al. Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res. 2007;13:671s–9s. doi: 10.1158/1078-0432.CCR-06-1870. [DOI] [PubMed] [Google Scholar]
- 9.Kaelin WG., Jr The von Hippel-Lindau tumor suppressor protein and clear cell renal carcinoma. Clin Cancer Res. 2007;13:680s–4s. doi: 10.1158/1078-0432.CCR-06-1865. [DOI] [PubMed] [Google Scholar]
- 10.Semenza GL. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene. 2010;29:625–34. doi: 10.1038/onc.2009.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordan JD, Simon MC. Hypoxia-inducible factors: central regulators of the tumor phenotype. Current opinion in genetics & development. 2007;17:71–7. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gudas LJ, Fu L, Minton DR, Mongan NP, Nanus DM. The role of HIF1α in renal cell carcinoma tumorigenesis. J Mol Med (Berl) 2014 doi: 10.1007/s00109-014-1180-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trapnell C, Roberts A, Goff L, Pertea G, Kim D, Kelley DR, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–78. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trapnell C, Williams BA, Pertea G, Mortazavi A, Kwan G, van Baren MJ, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–5. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Young MD, Wakefield MJ, Smyth GK, Oshlack A. Gene ontology analysis for RNA-seq: accounting for selection bias. Genome Biol. 2010;11:R14. doi: 10.1186/gb-2010-11-2-r14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beroukhim R, Brunet JP, Di Napoli A, Mertz KD, Seeley A, Pires MM, et al. Patterns of gene expression and copy-number alterations in von-hippel lindau disease-associated and sporadic clear cell carcinoma of the kidney. Cancer research. 2009;69:4674–81. doi: 10.1158/0008-5472.CAN-09-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gumz ML, Zou H, Kreinest PA, Childs AC, Belmonte LS, LeGrand SN, et al. Secreted frizzled-related protein 1 loss contributes to tumor phenotype of clear cell renal cell carcinoma. Clin Cancer Res. 2007;13:4740–9. doi: 10.1158/1078-0432.CCR-07-0143. [DOI] [PubMed] [Google Scholar]
- 19.Lenburg ME, Liou LS, Gerry NP, Frampton GM, Cohen HT, Christman MF. Previously unidentified changes in renal cell carcinoma gene expression identified by parametric analysis of microarray data. BMC Cancer. 2003;3:31. doi: 10.1186/1471-2407-3-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yusenko MV, Kuiper RP, Boethe T, Ljungberg B, van Kessel AG, Kovacs G. High-resolution DNA copy number and gene expression analyses distinguish chromophobe renal cell carcinomas and renal oncocytomas. BMC Cancer. 2009;9:152. doi: 10.1186/1471-2407-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fu L, Wang G, Shevchuk MM, Nanus DM, Gudas LJ. Activation of HIF2α in kidney proximal tubule cells causes abnormal glycogen deposition but not tumorigenesis. Cancer Res. 2013;73:2916–25. doi: 10.1158/0008-5472.CAN-12-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanehisa M, Goto S. KEGG: kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M, Goto S, Sato Y, Furumichi M, Tanabe M. KEGG for integration and interpretation of large-scale molecular data sets. Nucleic Acids Res. 2012;40:D109–14. doi: 10.1093/nar/gkr988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–9. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–70. [PubMed] [Google Scholar]
- 26.Yeaman SJ, Hutcheson ET, Roche TE, Pettit FH, Brown JR, Reed LJ, et al. Sites of phosphorylation on pyruvate dehydrogenase from bovine kidney and heart. Biochemistry. 1978;17:2364–70. doi: 10.1021/bi00605a017. [DOI] [PubMed] [Google Scholar]
- 27.Rhodes DR, Yu J, Shanker K, Deshpande N, Varambally R, Ghosh D, et al. ONCOMINE: a cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14:1983–91. [PubMed] [Google Scholar]
- 29.Kim JW, Tchernyshyov I, Semenza GL, Dang CV. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–85. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140:280–93. doi: 10.1016/j.cell.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sebastián C, Zwaans BM, Silberman DM, Gymrek M, Goren A, Zhong L, et al. The histone deacetylase SIRT6 is a tumor suppressor that controls cancer metabolism. Cell. 2012;151:1185–99. doi: 10.1016/j.cell.2012.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhong L, Mostoslavsky R. SIRT6: a master epigenetic gatekeeper of glucose metabolism. Transcription. 2010;1:17–21. doi: 10.4161/trns.1.1.12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature. 2011;469:539–42. doi: 10.1038/nature09639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duns G, van den Berg E, van Duivenbode I, Osinga J, Hollema H, Hofstra RM, et al. Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res. 2010;70:4287–91. doi: 10.1158/0008-5472.CAN-10-0120. [DOI] [PubMed] [Google Scholar]
- 35.Gerlinger M, Horswell S, Larkin J, Rowan AJ, Salm MP, Varela I, et al. Genomic architecture and evolution of clear cell renal cell carcinomas defined by multiregion sequencing. Nat Genet. 2014;46:225–33. doi: 10.1038/ng.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Creighton CJ, Morgan M, Gunaratne PH, Wheeler DA, Gibbs RA, Gordon Robertson A, et al. Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013 doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sato Y, Yoshizato T, Shiraishi Y, Maekawa S, Okuno Y, Kamura T, et al. Integrated molecular analysis of clear-cell renal cell carcinoma. Nat Genet. 2013;45:860–7. doi: 10.1038/ng.2699. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.