Abstract
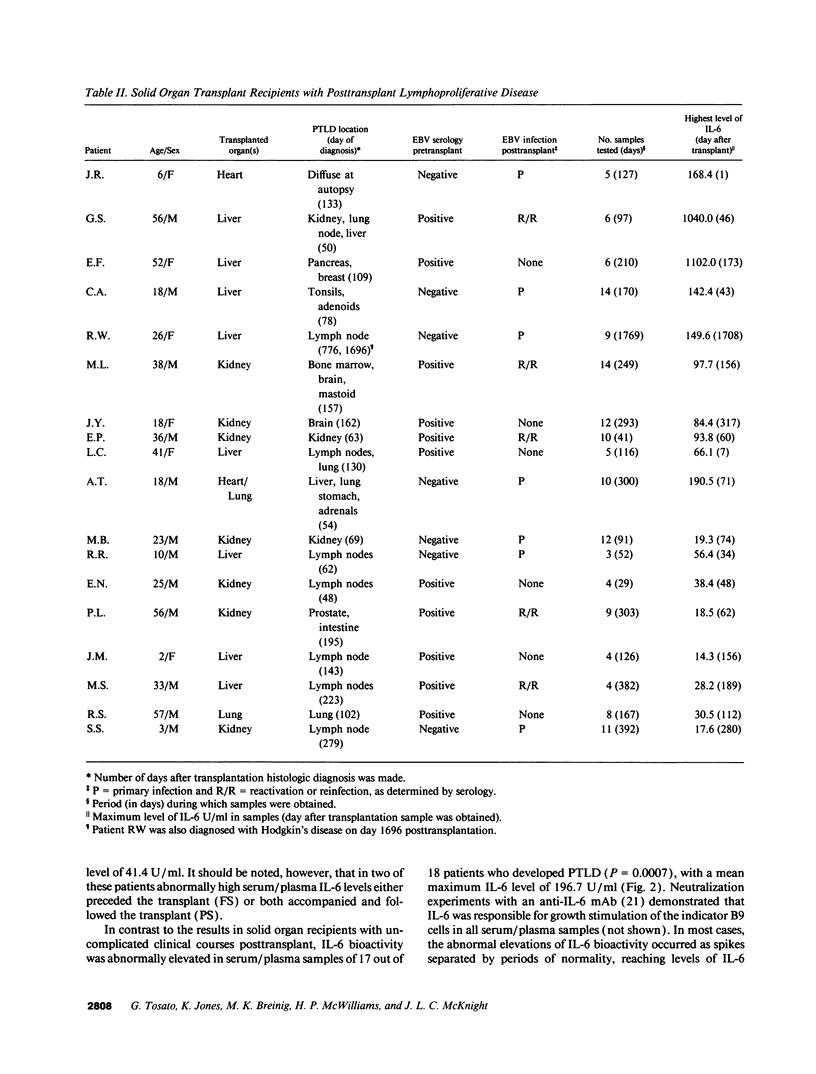
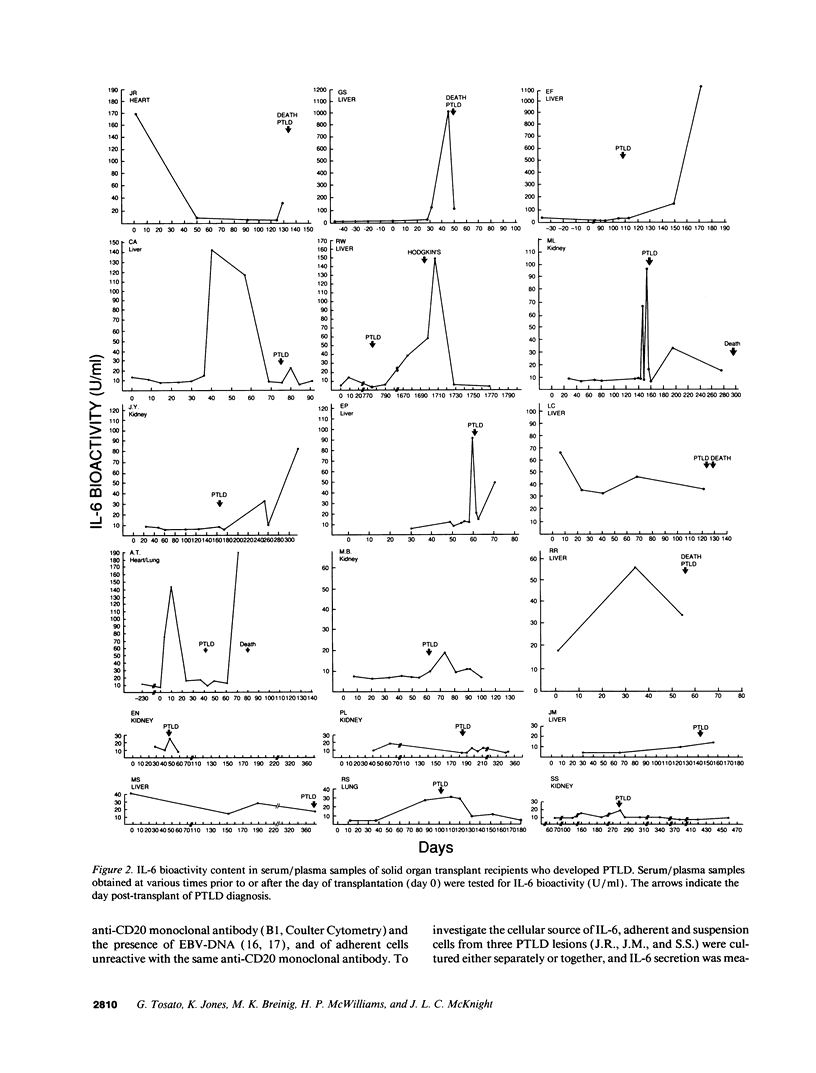
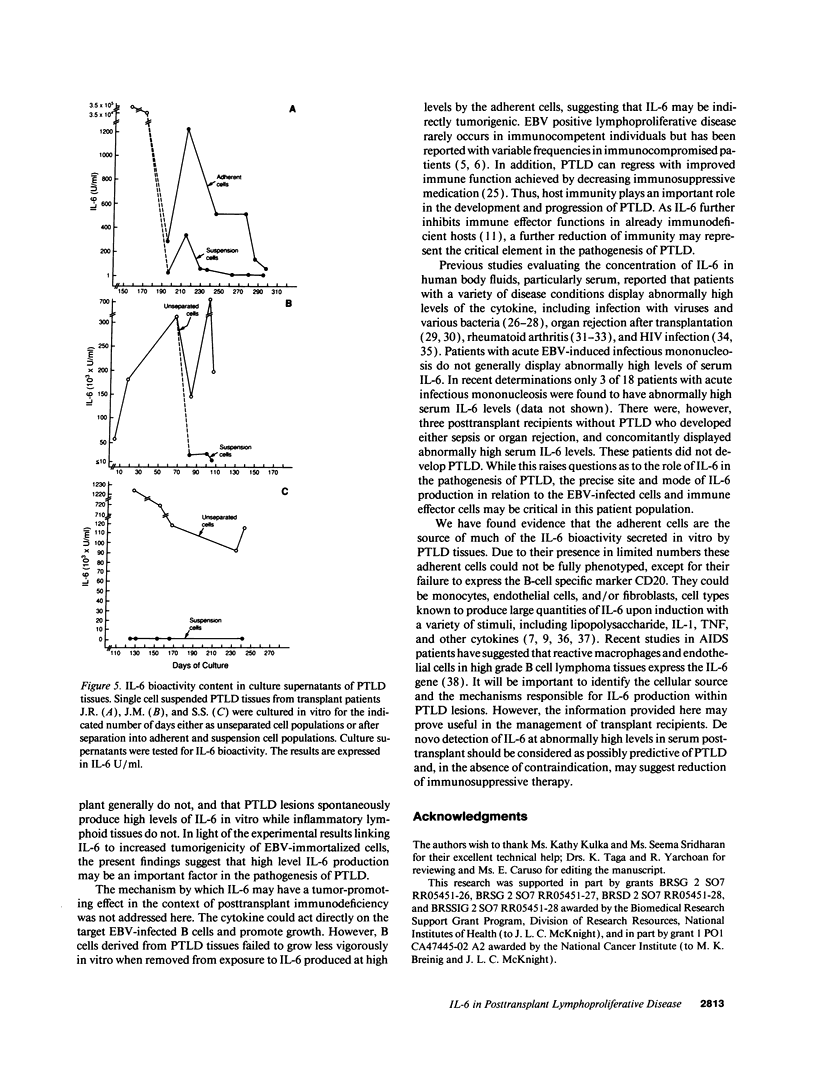
IL-6, a multifunctional cytokine produced by monocytes, fibroblasts, and endothelial cells, promotes the growth of EBV-immortalized B cells in vitro and renders these cells tumorigenic in athymic mice. In the present study, serum/plasma IL-6 bioactivity was found to be abnormally elevated, albeit transiently, in 17 of 18 solid organ transplant recipients with posttransplant lymphoproliferative disease (PTLD), with a mean maximal level of 196.7 U/ml. This represents a 16.4 increase above the normal mean (11.3 U/ml). In contrast, only 3 of 10 solid organ transplant recipients with uncomplicated courses posttransplant had abnormally elevated serum/plasma IL-6 bioactivity (mean maximal level 41.4 U/ml, P = 0.0007). When transferred to single cell culture, the 11 PTLD tissues produced 640 to 1.25 x 10(6) IL-6 U/ml in the culture supernatant, with a mean maximal level of 35,025 IL-6 U/ml. Cell separation experiments demonstrated that the adherent cells, identified as non-B cells, were the principal source of IL-6 production in vitro by PTLD tissue. Control cultures of inflammatory lymphoid tissue negative for lymphoproliferative disease as well as of PBL from patients with acute EBV-induced infectious mononucleosis consistently produced < 10 IL-6 U/ml. Thus, IL-6 is produced at high levels by PTLD tissues and may play a critical role in the pathogenesis of PTLD.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarden L. A., De Groot E. R., Schaap O. L., Lansdorp P. M. Production of hybridoma growth factor by human monocytes. Eur J Immunol. 1987 Oct;17(10):1411–1416. doi: 10.1002/eji.1830171004. [DOI] [PubMed] [Google Scholar]
- Astaldi G. C., Janssen M. C., Lansdorp P., Willems C., Zeijlemaker W. P., Oosterhof F. Human endothelial culture supernatant (HECS): a growth factor for hybridomas. J Immunol. 1980 Oct;125(4):1411–1414. [PubMed] [Google Scholar]
- Bhardwaj N., Santhanam U., Lau L. L., Tatter S. B., Ghrayeb J., Rivelis M., Steinman R. M., Sehgal P. B., May L. T. IL-6/IFN-beta 2 in synovial effusions of patients with rheumatoid arthritis and other arthritides. Identification of several isoforms and studies of cellular sources. J Immunol. 1989 Oct 1;143(7):2153–2159. [PubMed] [Google Scholar]
- Birx D. L., Redfield R. R., Tencer K., Fowler A., Burke D. S., Tosato G. Induction of interleukin-6 during human immunodeficiency virus infection. Blood. 1990 Dec 1;76(11):2303–2310. [PubMed] [Google Scholar]
- Breen E. C., Rezai A. R., Nakajima K., Beall G. N., Mitsuyasu R. T., Hirano T., Kishimoto T., Martinez-Maza O. Infection with HIV is associated with elevated IL-6 levels and production. J Immunol. 1990 Jan 15;144(2):480–484. [PubMed] [Google Scholar]
- Cen H., Breinig M. C., Atchison R. W., Ho M., McKnight J. L. Epstein-Barr virus transmission via the donor organs in solid organ transplantation: polymerase chain reaction and restriction fragment length polymorphism analysis of IR2, IR3, and IR4. J Virol. 1991 Feb;65(2):976–980. doi: 10.1128/jvi.65.2.976-980.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. I. Epstein-Barr virus lymphoproliferative disease associated with acquired immunodeficiency. Medicine (Baltimore) 1991 Mar;70(2):137–160. doi: 10.1097/00005792-199103000-00005. [DOI] [PubMed] [Google Scholar]
- Emilie D., Coumbaras J., Raphael M., Devergne O., Delecluse H. J., Gisselbrecht C., Michiels J. F., Van Damme J., Taga T., Kishimoto T. Interleukin-6 production in high-grade B lymphomas: correlation with the presence of malignant immunoblasts in acquired immunodeficiency syndrome and in human immunodeficiency virus-seronegative patients. Blood. 1992 Jul 15;80(2):498–504. [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Frei K., Leist T. P., Meager A., Gallo P., Leppert D., Zinkernagel R. M., Fontana A. Production of B cell stimulatory factor-2 and interferon gamma in the central nervous system during viral meningitis and encephalitis. Evaluation in a murine model infection and in patients. J Exp Med. 1988 Jul 1;168(1):449–453. doi: 10.1084/jem.168.1.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerper P., Whang-Peng J., Monroe J. H. Transformation and chromosome changes induced by Epstein-Barr virus in normal human leukocyte cultures. Proc Natl Acad Sci U S A. 1969 Jul;63(3):740–747. doi: 10.1073/pnas.63.3.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfgott D. C., Tatter S. B., Santhanam U., Clarick R. H., Bhardwaj N., May L. T., Sehgal P. B. Multiple forms of IFN-beta 2/IL-6 in serum and body fluids during acute bacterial infection. J Immunol. 1989 Feb 1;142(3):948–953. [PubMed] [Google Scholar]
- Helle M., Boeije L., de Groot E., de Vos A., Aarden L. Sensitive ELISA for interleukin-6. Detection of IL-6 in biological fluids: synovial fluids and sera. J Immunol Methods. 1991 Apr 8;138(1):47–56. doi: 10.1016/0022-1759(91)90063-l. [DOI] [PubMed] [Google Scholar]
- Henle W., Henle G. E., Horwitz C. A. Epstein-Barr virus specific diagnostic tests in infectious mononucleosis. Hum Pathol. 1974 Sep;5(5):551–565. doi: 10.1016/s0046-8177(74)80006-7. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Ho M., Jaffe R., Miller G., Breinig M. K., Dummer J. S., Makowka L., Atchison R. W., Karrer F., Nalesnik M. A., Starzl T. E. The frequency of Epstein-Barr virus infection and associated lymphoproliferative syndrome after transplantation and its manifestations in children. Transplantation. 1988 Apr;45(4):719–727. doi: 10.1097/00007890-198804000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho M., Miller G., Atchison R. W., Breinig M. K., Dummer J. S., Andiman W., Starzl T. E., Eastman R., Griffith B. P., Hardesty R. L. Epstein-Barr virus infections and DNA hybridization studies in posttransplantation lymphoma and lymphoproliferative lesions: the role of primary infection. J Infect Dis. 1985 Nov;152(5):876–886. doi: 10.1093/infdis/152.5.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Katz B. Z., Raab-Traub N., Miller G. Latent and replicating forms of Epstein-Barr virus DNA in lymphomas and lymphoproliferative diseases. J Infect Dis. 1989 Oct;160(4):589–598. doi: 10.1093/infdis/160.4.589. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Nalesnik M. A., Jaffe R., Starzl T. E., Demetris A. J., Porter K., Burnham J. A., Makowka L., Ho M., Locker J. The pathology of posttransplant lymphoproliferative disorders occurring in the setting of cyclosporine A-prednisone immunosuppression. Am J Pathol. 1988 Oct;133(1):173–192. [PMC free article] [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Scala G., Quinto I., Ruocco M. R., Arcucci A., Mallardo M., Caretto P., Forni G., Venuta S. Expression of an exogenous interleukin 6 gene in human Epstein Barr virus B cells confers growth advantage and in vivo tumorigenicity. J Exp Med. 1990 Jul 1;172(1):61–68. doi: 10.1084/jem.172.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner J., Tosato G. Impairment of natural killer functions by interleukin 6 increases lymphoblastoid cell tumorigenicity in athymic mice. J Clin Invest. 1991 Jul;88(1):239–247. doi: 10.1172/JCI115283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. A., Allday M. J., Crawford D. H. Epstein-Barr virus-associated lymphoproliferative disorders in immunocompromised individuals. Adv Cancer Res. 1991;57:329–380. doi: 10.1016/s0065-230x(08)61003-9. [DOI] [PubMed] [Google Scholar]
- Tosato G., Blaese R. M., Yarchoan R. Relationship between immunoglobulin production and immortalization by Epstein Barr virus. J Immunol. 1985 Aug;135(2):959–964. [PubMed] [Google Scholar]
- Tosato G., Magrath I. T., Blaese R. M. T cell-mediated immunoregulation of Epstein Barr virus- (EBV) induced B lymphocyte activation in EBV-seropositive and EBV-seronegative individuals. J Immunol. 1982 Feb;128(2):575–579. [PubMed] [Google Scholar]
- Tosato G., Seamon K. B., Goldman N. D., Sehgal P. B., May L. T., Washington G. C., Jones K. D., Pike S. E. Monocyte-derived human B-cell growth factor identified as interferon-beta 2 (BSF-2, IL-6). Science. 1988 Jan 29;239(4839):502–504. doi: 10.1126/science.2829354. [DOI] [PubMed] [Google Scholar]
- Tosato G., Tanner J., Jones K. D., Revel M., Pike S. E. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990 Jun;64(6):3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Oers M. H., Van der Heyden A. A., Aarden L. A. Interleukin 6 (IL-6) in serum and urine of renal transplant recipients. Clin Exp Immunol. 1988 Feb;71(2):314–319. [PMC free article] [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousem S. A., Randhawa P., Locker J., Paradis I. L., Dauber J. A., Griffith B. P., Nalesnik M. A. Posttransplant lymphoproliferative disorders in heart-lung transplant recipients: primary presentation in the allograft. Hum Pathol. 1989 Apr;20(4):361–369. doi: 10.1016/0046-8177(89)90046-4. [DOI] [PubMed] [Google Scholar]


