Abstract
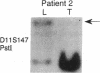
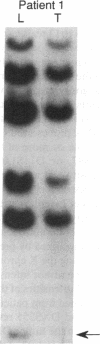
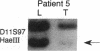
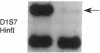
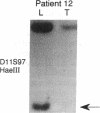
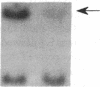
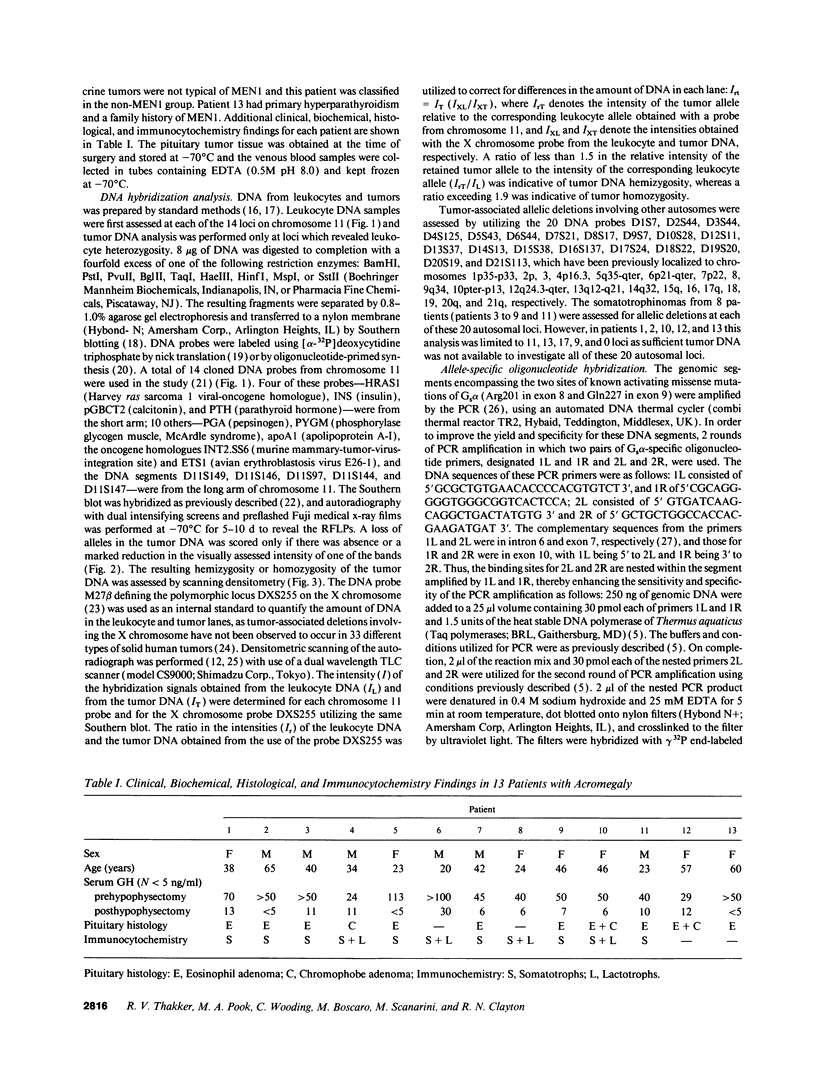
The molecular pathology of somatotrophinomas has been investigated by a combined search for dominant mutations of the gene encoding the Gs alpha protein and for recessive mutations involving chromosome 11q13, which contains the gene causing multiple endocrine neoplasia type 1 (MEN1). Somatotrophinomas and peripheral leukocytes were obtained from thirteen patients with acromegaly; one patient also suffered from MEN1. Five DNA probes identifying restriction fragment length polymorphisms from 11q revealed allele loss in pituitary tumors from five (four non-MEN1 and one MEN1) patients. Deletion mapping revealed that the region of allele loss common to the somatotrophinomas involved 11q13. An analysis for similar allelic deletions at 12 other loci from chromosomes 1-5, 7-9, 12-14, and 17 did not reveal generalized allele loss in the somatotrophinomas. These results, which represent the first report of chromosome 11 allele loss occurring in non-MEN1 somatotrophinomas, indicate that a recessive oncogene on 11q13 is specifically involved in the monoclonal development of somatotrophinomas. In addition Gs alpha mutations were detected in two non-MEN1 somatotrophinomas, one of which also revealed allele loss of chromosome 11. Thus, our results reveal that the development of somatotrophinomas is associated with alterations in both dominant and recessive oncogenes and further characterization of these genetic abnormalities will help to elucidate the multistep etiology and progression of somatotrophinomas.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Alexander J. M., Biller B. M., Bikkal H., Zervas N. T., Arnold A., Klibanski A. Clinically nonfunctioning pituitary tumors are monoclonal in origin. J Clin Invest. 1990 Jul;86(1):336–340. doi: 10.1172/JCI114705. [DOI] [PMC free article] [PubMed] [Google Scholar]
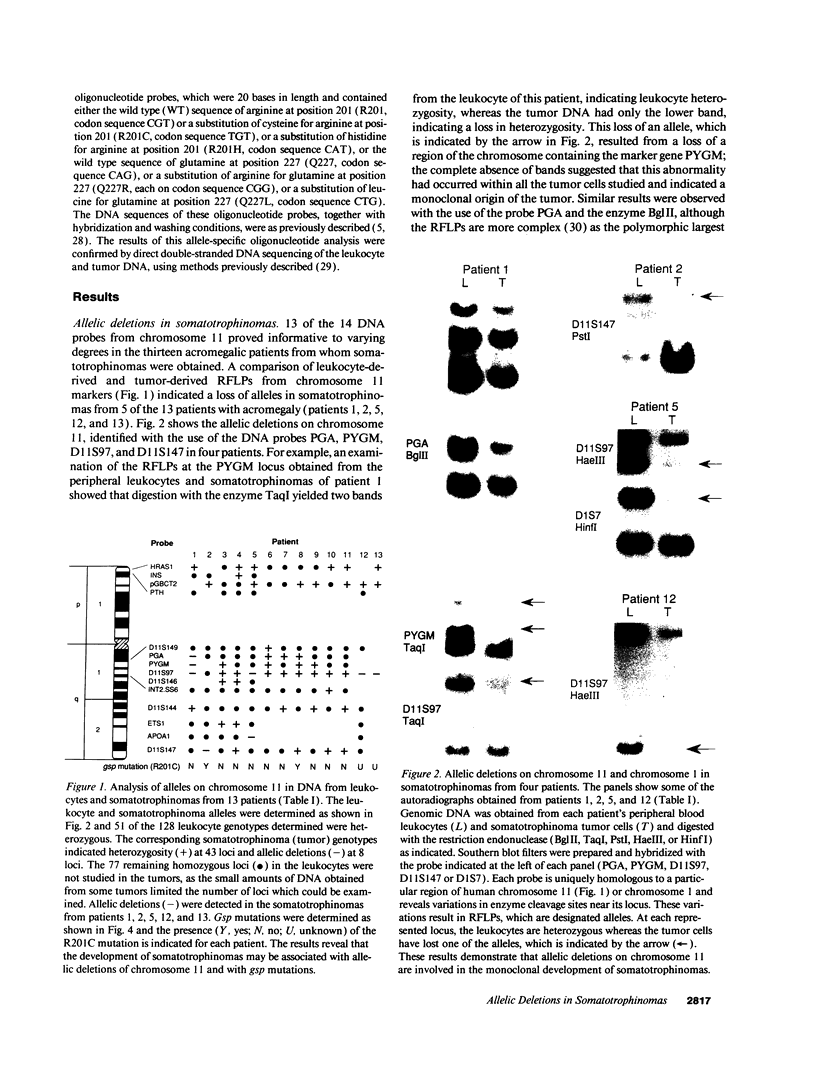
- Bale A. E., Norton J. A., Wong E. L., Fryburg J. S., Maton P. N., Oldfield E. H., Streeten E., Aurbach G. D., Brandi M. L., Friedman E. Allelic loss on chromosome 11 in hereditary and sporadic tumors related to familial multiple endocrine neoplasia type 1. Cancer Res. 1991 Feb 15;51(4):1154–1157. [PubMed] [Google Scholar]
- Blatt C., Eversole-Cire P., Cohn V. H., Zollman S., Fournier R. E., Mohandas L. T., Nesbitt M., Lugo T., Jones D. T., Reed R. R. Chromosomal localization of genes encoding guanine nucleotide-binding protein subunits in mouse and human. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7642–7646. doi: 10.1073/pnas.85.20.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudi F. H., Lothe R. A., Taggart R. T. Human pepsinogen A (PGA): an informative gene complex located at 11q13. Hum Genet. 1990 Feb;84(3):293–295. doi: 10.1007/BF00200579. [DOI] [PubMed] [Google Scholar]
- Byström C., Larsson C., Blomberg C., Sandelin K., Falkmer U., Skogseid B., Oberg K., Werner S., Nordenskjöld M. Localization of the MEN1 gene to a small region within chromosome 11q13 by deletion mapping in tumors. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1968–1972. doi: 10.1073/pnas.87.5.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi E., Malgaretti N., Meldolesi J., Taramelli R. A new constitutively activating mutation of the Gs protein alpha subunit-gsp oncogene is found in human pituitary tumours. Oncogene. 1990 Jul;5(7):1059–1061. [PubMed] [Google Scholar]
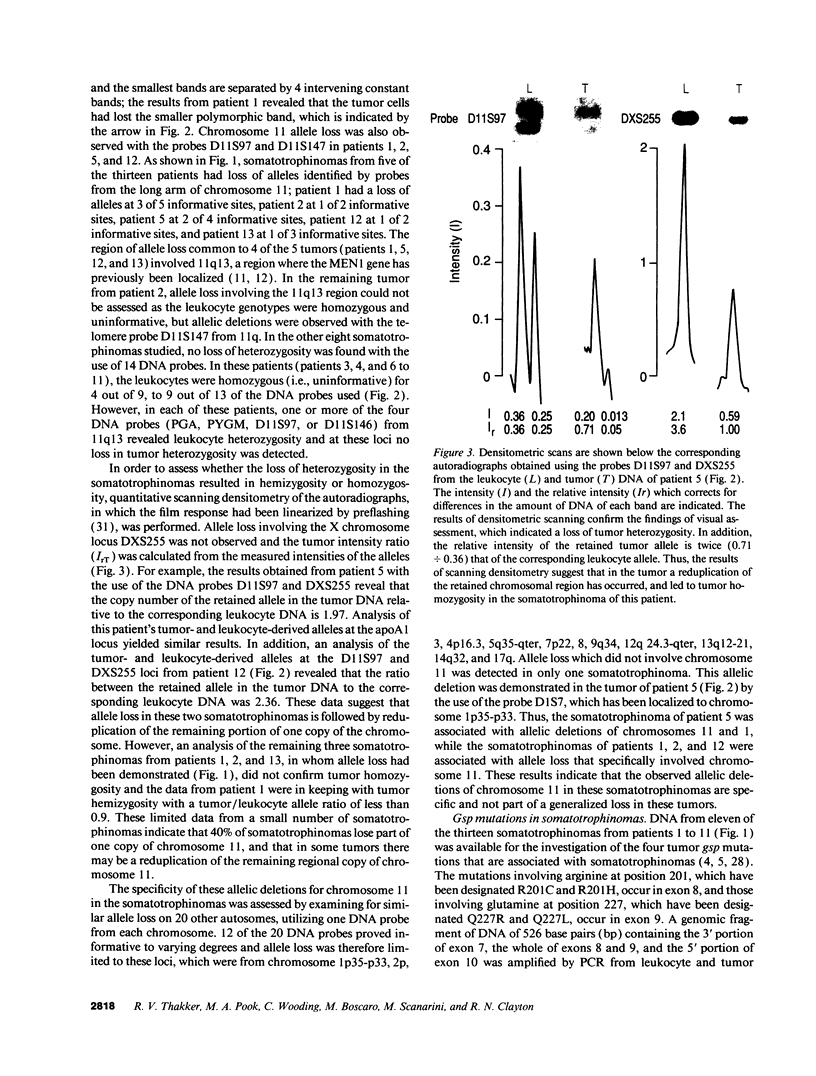
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B., Feinberg A. P. Somatic deletion and duplication of genes on chromosome 11 in Wilms' tumours. Nature. 1984 May 10;309(5964):176–178. doi: 10.1038/309176a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fraser N. J., Boyd Y., Brownlee G. G., Craig I. W. Multi-allelic RFLP for M27 beta, an anonymous single copy genomic clone at Xp11.3-Xcen [HGM9 provisional no. DXS255]. Nucleic Acids Res. 1987 Nov 25;15(22):9616–9616. doi: 10.1093/nar/15.22.9616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman E., Sakaguchi K., Bale A. E., Falchetti A., Streeten E., Zimering M. B., Weinstein L. S., McBride W. O., Nakamura Y., Brandi M. L. Clonality of parathyroid tumors in familial multiple endocrine neoplasia type 1. N Engl J Med. 1989 Jul 27;321(4):213–218. doi: 10.1056/NEJM198907273210402. [DOI] [PubMed] [Google Scholar]
- Friend S. H., Dryja T. P., Weinberg R. A. Oncogenes and tumor-suppressing genes. N Engl J Med. 1988 Mar 10;318(10):618–622. doi: 10.1056/NEJM198803103181007. [DOI] [PubMed] [Google Scholar]
- Hansen M. F., Cavenee W. K. Retinoblastoma and the progression of tumor genetics. Trends Genet. 1988 May;4(5):125–128. doi: 10.1016/0168-9525(88)90134-5. [DOI] [PubMed] [Google Scholar]
- Herman V., Fagin J., Gonsky R., Kovacs K., Melmed S. Clonal origin of pituitary adenomas. J Clin Endocrinol Metab. 1990 Dec;71(6):1427–1433. doi: 10.1210/jcem-71-6-1427. [DOI] [PubMed] [Google Scholar]
- Khosla S., Patel V. M., Hay I. D., Schaid D. J., Grant C. S., van Heerden J. A., Thibodeau S. N. Loss of heterozygosity suggests multiple genetic alterations in pheochromocytomas and medullary thyroid carcinomas. J Clin Invest. 1991 May;87(5):1691–1699. doi: 10.1172/JCI115186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knudson A. G., Jr, Strong L. C., Anderson D. E. Heredity and cancer in man. Prog Med Genet. 1973;9:113–158. [PubMed] [Google Scholar]
- Kozasa T., Itoh H., Tsukamoto T., Kaziro Y. Isolation and characterization of the human Gs alpha gene. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2081–2085. doi: 10.1073/pnas.85.7.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis C. A., Masters S. B., Spada A., Pace A. M., Bourne H. R., Vallar L. GTPase inhibiting mutations activate the alpha chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature. 1989 Aug 31;340(6236):692–696. doi: 10.1038/340692a0. [DOI] [PubMed] [Google Scholar]
- Larsson C., Shepherd J., Nakamura Y., Blomberg C., Weber G., Werelius B., Hayward N., Teh B., Tokino T., Seizinger B. Predictive testing for multiple endocrine neoplasia type 1 using DNA polymorphisms. J Clin Invest. 1992 Apr;89(4):1344–1349. doi: 10.1172/JCI115720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson C., Skogseid B., Oberg K., Nakamura Y., Nordenskjöld M. Multiple endocrine neoplasia type 1 gene maps to chromosome 11 and is lost in insulinoma. Nature. 1988 Mar 3;332(6159):85–87. doi: 10.1038/332085a0. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lyons J., Landis C. A., Harsh G., Vallar L., Grünewald K., Feichtinger H., Duh Q. Y., Clark O. H., Kawasaki E., Bourne H. R. Two G protein oncogenes in human endocrine tumors. Science. 1990 Aug 10;249(4969):655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- McCarthy M. I., Noonan K., Wass J. A., Monson J. P. Familial acromegaly: studies in three families. Clin Endocrinol (Oxf) 1990 Jun;32(6):719–728. doi: 10.1111/j.1365-2265.1990.tb00918.x. [DOI] [PubMed] [Google Scholar]
- Parkinson D. B., Thakker R. V. A donor splice site mutation in the parathyroid hormone gene is associated with autosomal recessive hypoparathyroidism. Nat Genet. 1992 May;1(2):149–152. doi: 10.1038/ng0592-149. [DOI] [PubMed] [Google Scholar]
- Pestell R. G., Alford F. P., Best J. D. Familial acromegaly. Acta Endocrinol (Copenh) 1989 Aug;121(2):286–289. doi: 10.1530/acta.0.1210286. [DOI] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stanbridge E. J. Identifying tumor suppressor genes in human colorectal cancer. Science. 1990 Jan 5;247(4938):12–13. doi: 10.1126/science.2403692. [DOI] [PubMed] [Google Scholar]
- Thakker R. V., Bouloux P., Wooding C., Chotai K., Broad P. M., Spurr N. K., Besser G. M., O'Riordan J. L. Association of parathyroid tumors in multiple endocrine neoplasia type 1 with loss of alleles on chromosome 11. N Engl J Med. 1989 Jul 27;321(4):218–224. doi: 10.1056/NEJM198907273210403. [DOI] [PubMed] [Google Scholar]
- Thakker R. V., Davies K. E., Whyte M. P., Wooding C., O'Riordan J. L. Mapping the gene causing X-linked recessive idiopathic hypoparathyroidism to Xq26-Xq27 by linkage studies. J Clin Invest. 1990 Jul;86(1):40–45. doi: 10.1172/JCI114712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varmus H. E. The molecular genetics of cellular oncogenes. Annu Rev Genet. 1984;18:553–612. doi: 10.1146/annurev.ge.18.120184.003005. [DOI] [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Hamilton S. R., Kern S. E., Preisinger A. C., Leppert M., Nakamura Y., White R., Smits A. M., Bos J. L. Genetic alterations during colorectal-tumor development. N Engl J Med. 1988 Sep 1;319(9):525–532. doi: 10.1056/NEJM198809013190901. [DOI] [PubMed] [Google Scholar]
- Yoshimoto K., Iwahana H., Kubo K., Saito S., Itakura M. Allele loss on chromosome 11 in a pituitary tumor from a patient with multiple endocrine neoplasia type 1. Jpn J Cancer Res. 1991 Aug;82(8):886–889. doi: 10.1111/j.1349-7006.1991.tb01916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]