Abstract
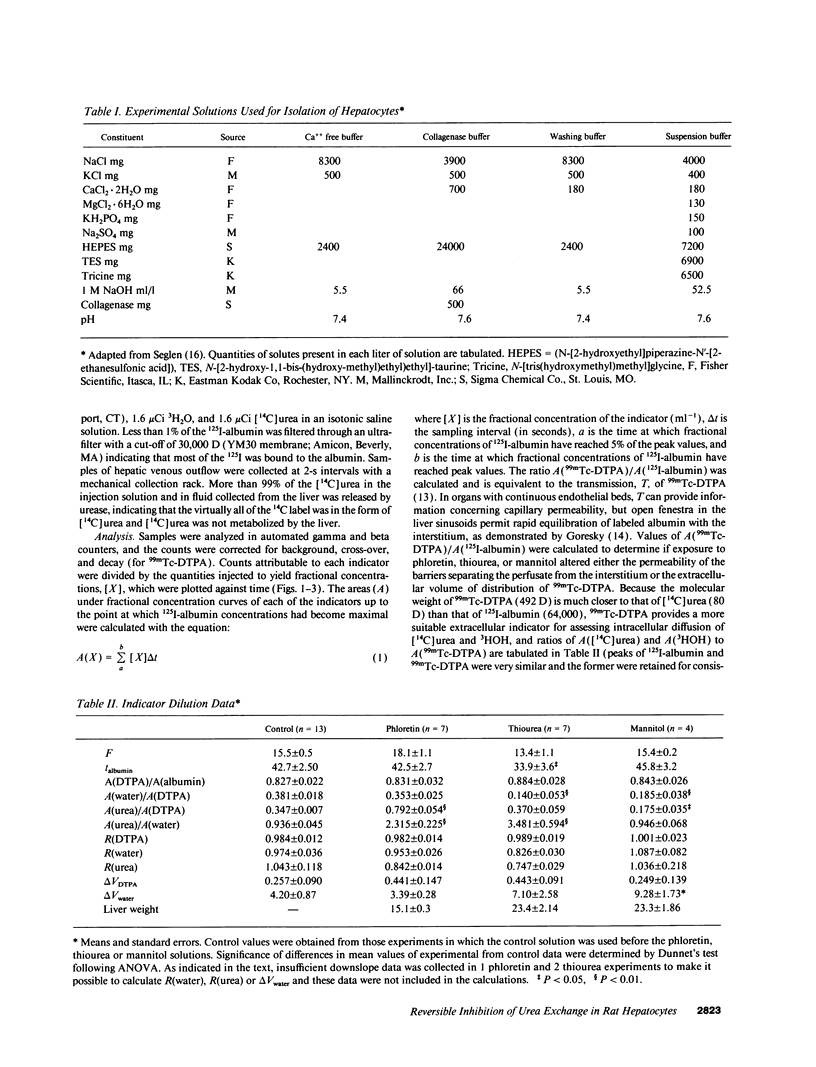
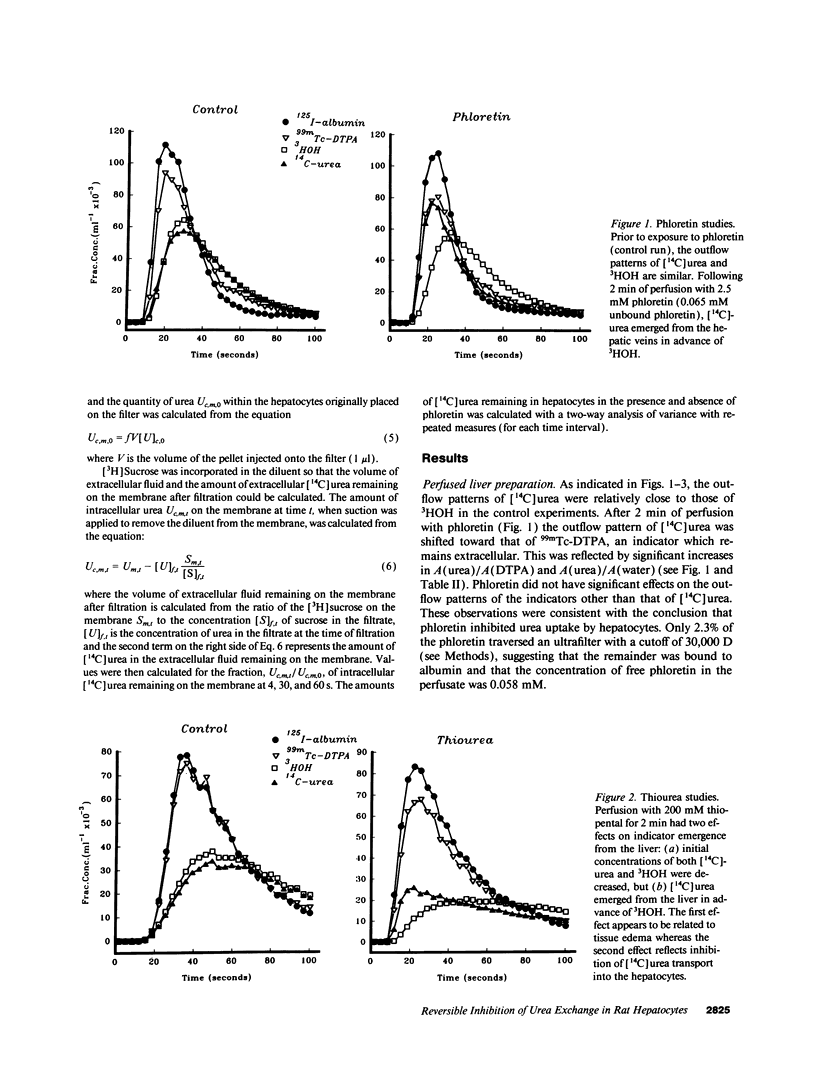
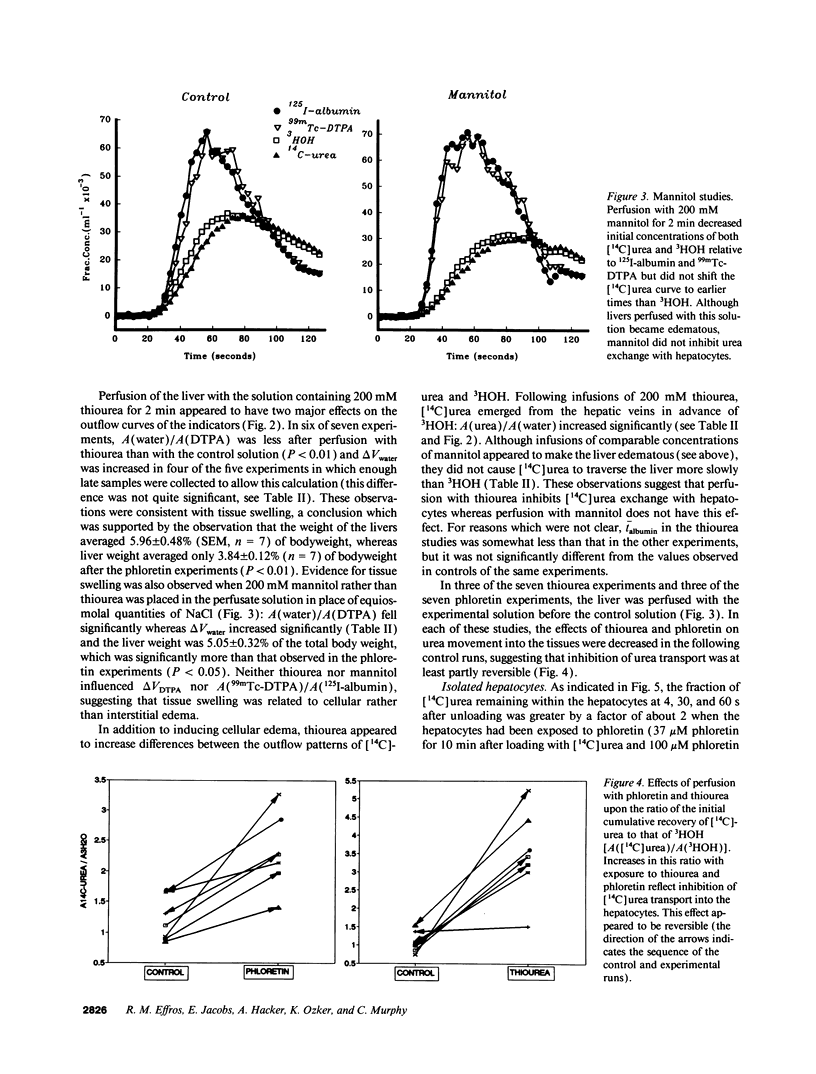
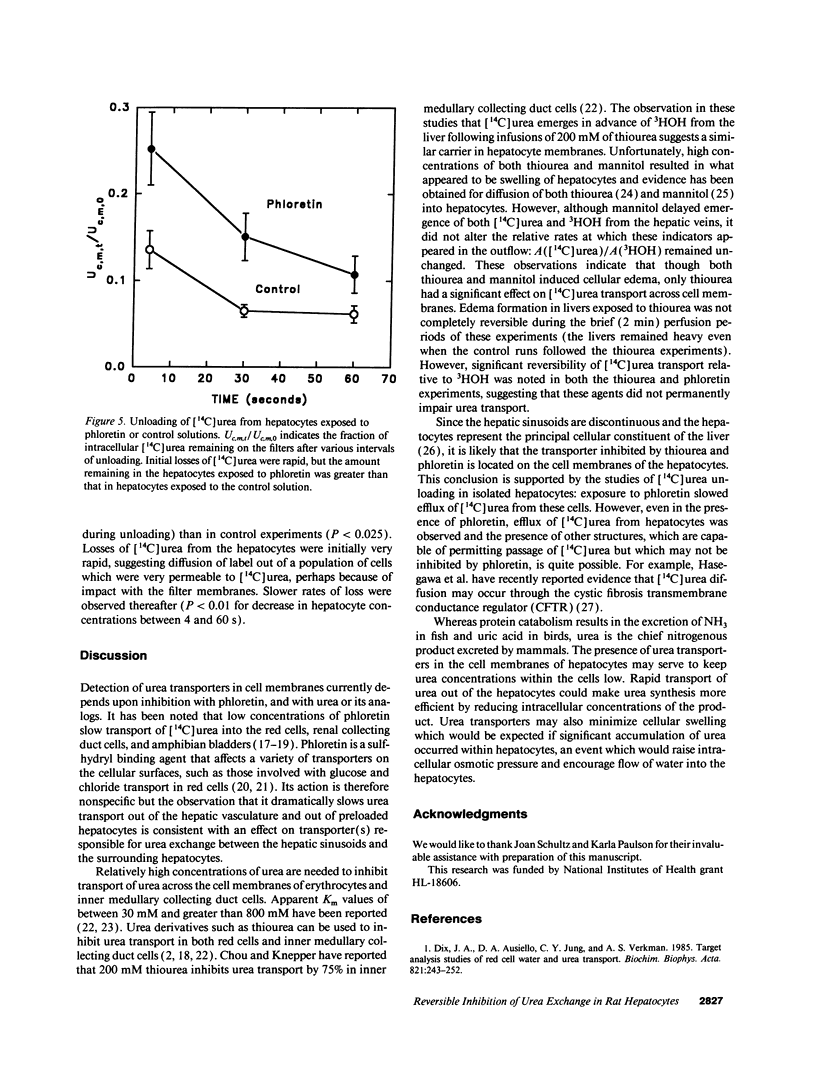
Urea exchange is enhanced in renal collecting duct cells and erythrocytes by transporters which can be inhibited by phloretin and urea analogs such as thiourea. In this study, evidence for a comparable transporter was found in rat livers perfused with solutions which contained no red cells and in suspensions of hepatocytes. Bolus injections containing 125I-albumin (intravascular indicator), 99mTc-DTPA (extracellular indicator), 3HOH (water indicator), and [14C]urea were administered into the portal vein and fluid was collected from the hepatic vein. Under control conditions, [14C]urea and 3HOH emerged from the hepatic vein at nearly the same rate. However when the perfusate contained 2.5 mM phloretin (equivalent to 0.058 mM phloretin not bound to albumin), the amount of [14C]urea which had been recovered in the hepatic venous outflow by the time of peak 125I-albumin concentrations exceeded 3HOH recovery by a factor of 2.31 +/- 0.23 (n = 7). When the perfusate contained 200 mM thiourea, the comparable recovery of [14C]urea from the hepatic veins exceeded that of 3HOH by a factor of 3.48 +/- 0.44 (n = 7). These effects were at least partially reversible and suggested inhibition of urea transporters in hepatocytes. This conclusion was supported by studies of unloading of [14C]urea from hepatocytes which were exposed to unlabeled solutions: in the presence of phloretin, the amount of [14C]urea remaining within hepatocytes at 4 s was approximately twice that remaining in hepatocytes which had not been exposed to phloretin. Rapid transport of urea out of hepatocytes may increase urea synthesis and minimize cellular swelling due to urea accumulation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arias I. M. The biology of hepatic endothelial cell fenestrae. Prog Liver Dis. 1990;9:11–26. [PubMed] [Google Scholar]
- Brahm J. Urea permeability of human red cells. J Gen Physiol. 1983 Jul;82(1):1–23. doi: 10.1085/jgp.82.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brigham K. L., Harris T. R., Owen P. J. [14C]urea and [14C]sucrose as permeability indicators in histamine pulmonary edema. J Appl Physiol Respir Environ Exerc Physiol. 1977 Jul;43(1):99–101. doi: 10.1152/jappl.1977.43.1.99. [DOI] [PubMed] [Google Scholar]
- Brigham K. L., Harris T. R., Rowlett R. D., Owen P. J. Comparisons of [14C]urea and [3H]mannitol as lung vascular permeability indicators in awake sheep: evidence against red cell urea trapping. Microvasc Res. 1977 Jan;13(1):97–105. doi: 10.1016/0026-2862(77)90118-2. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Knepper M. A. Inhibition of urea transport in inner medullary collecting duct by phloretin and urea analogues. Am J Physiol. 1989 Sep;257(3 Pt 2):F359–F365. doi: 10.1152/ajprenal.1989.257.3.F359. [DOI] [PubMed] [Google Scholar]
- Chou C. L., Sands J. M., Nonoguchi H., Knepper M. A. Concentration dependence of urea and thiourea transport in rat inner medullary collecting duct. Am J Physiol. 1990 Mar;258(3 Pt 2):F486–F494. doi: 10.1152/ajprenal.1990.258.3.F486. [DOI] [PubMed] [Google Scholar]
- Effros R. M., Haider B., Ettinger P. O., Ahmed Sultan S., Oldewurtel H. A., Marold K., Regan T. J. In vivo myocardial cell pH in the dog. Response to ischemia and infusion of alkali. J Clin Invest. 1975 May;55(5):1100–1110. doi: 10.1172/JCI108011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich O., Gunn R. B. Interactions of inhibitors on anion transporter of human erythrocyte. Am J Physiol. 1987 Feb;252(2 Pt 1):C153–C162. doi: 10.1152/ajpcell.1987.252.2.C153. [DOI] [PubMed] [Google Scholar]
- GORESKY C. A. A linear method for determining liver sinusoidal and extravascular volumes. Am J Physiol. 1963 Apr;204:626–640. doi: 10.1152/ajplegacy.1963.204.4.626. [DOI] [PubMed] [Google Scholar]
- Goresky C. A., Bach G. G., Nadeau B. E. On the uptake of materials by the intact liver. The transport and net removal of galactose. J Clin Invest. 1973 May;52(5):991–1009. doi: 10.1172/JCI107300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goresky C. A., Bach G. G., Nadeau B. E. Red cell carriage of label: its limiting effect on the exchange of materials in the liver. Circ Res. 1975 Feb;36(2):328–351. doi: 10.1161/01.res.36.2.328. [DOI] [PubMed] [Google Scholar]
- Hasegawa H., Skach W., Baker O., Calayag M. C., Lingappa V., Verkman A. S. A multifunctional aqueous channel formed by CFTR. Science. 1992 Nov 27;258(5087):1477–1479. doi: 10.1126/science.1279809. [DOI] [PubMed] [Google Scholar]
- Imai M., Taniguchi J., Yoshitomi K. Osmotic work across inner medullary collecting duct accomplished by difference in reflection coefficients for urea and NaCl. Pflugers Arch. 1988 Oct;412(6):557–567. doi: 10.1007/BF00583755. [DOI] [PubMed] [Google Scholar]
- Knepper M. A., Star R. A. The vasopressin-regulated urea transporter in renal inner medullary collecting duct. Am J Physiol. 1990 Sep;259(3 Pt 2):F393–F401. doi: 10.1152/ajprenal.1990.259.3.F393. [DOI] [PubMed] [Google Scholar]
- LEFEVRE P. G., MARSHALL J. K. The atachment of phloretin and analogues to human erythrocytes in connection with inhibition of sugar transport. J Biol Chem. 1959 Nov;234:3022–3026. [PubMed] [Google Scholar]
- Macey R. I., Farmer R. E. Inhibition of water and solute permeability in human red cells. Biochim Biophys Acta. 1970 Jul 7;211(1):104–106. doi: 10.1016/0005-2736(70)90130-6. [DOI] [PubMed] [Google Scholar]
- Macey R. I. Transport of water and urea in red blood cells. Am J Physiol. 1984 Mar;246(3 Pt 1):C195–C203. doi: 10.1152/ajpcell.1984.246.3.C195. [DOI] [PubMed] [Google Scholar]
- Mayrand R. R., Levitt D. G. Urea and ethylene glycol-facilitated transport systems in the human red cell membrane. Saturation, competition, and asymmetry. J Gen Physiol. 1983 Feb;81(2):221–237. doi: 10.1085/jgp.81.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M. M., Verkman A. S. Human platelet osmotic water and nonelectrolyte transport. Am J Physiol. 1986 Oct;251(4 Pt 1):C549–C557. doi: 10.1152/ajpcell.1986.251.4.C549. [DOI] [PubMed] [Google Scholar]
- Pang K. S., Barker F., 3rd, Schwab A. J., Goresky C. A. [14C]urea and 58Co-EDTA as reference indicators in hepatic multiple indicator dilution studies. Am J Physiol. 1990 Jul;259(1 Pt 1):G32–G40. doi: 10.1152/ajpgi.1990.259.1.G32. [DOI] [PubMed] [Google Scholar]
- Rasio E. A., Bendayan M., Goresky C. A. Diffusion permeability of an isolated rete mirabile. Circ Res. 1977 Dec;41(6):791–798. doi: 10.1161/01.res.41.6.791. [DOI] [PubMed] [Google Scholar]
- SCHANKER L. S., HOGBEN C. A. Biliary excretion of inulin, sucrose, and mannitol: analysis of bile formation. Am J Physiol. 1961 May;200:1087–1090. doi: 10.1152/ajplegacy.1961.200.5.1087. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Zhang R. B., Verkman A. S. Urea transport in freshly isolated and cultured cells from rat inner medullary collecting duct. J Membr Biol. 1990 Sep;117(3):253–261. doi: 10.1007/BF01868455. [DOI] [PubMed] [Google Scholar]
- Ziegler W. H., Goresky C. A. Transcapillary exchange in the working left ventricle of the dog. Circ Res. 1971 Aug;29(2):181–207. doi: 10.1161/01.res.29.2.181. [DOI] [PubMed] [Google Scholar]


